Abstract
We amplified gene sequences from Anaplasma phagocytophilum, Borrelia garinii, B. valaisiana, B. turdi, Rickettsia monacensis, R. helvetica, R. sibirica sibirica, and Rickettsia spp. (including Candidatus Rickettsia vini) in ticks removed from birds in Spain. The findings support the role of passerine birds as possible dispersers of these tick-borne pathogens.
Keywords: Anaplasma phagocytophilum, birds, ticks, Borrelia burgdorferi, Rickettsia spp., Borrelia turdi, Ixodes arboricola, Rickettsia sibirica, Rickettsia vini, Spain, Rickettsia, vector-borne infections, rickettsiae
Hard ticks are a major vector of infectious diseases in industrialized countries. Several tick-borne bacterial diseases, such as Lyme disease, Mediterranean spotted fever, and tick-borne lymphadenopathy (also called Dermacentor-borne necrosis erythema and lymphadenopathy), are endemic to Spain. Furthermore, a few cases of human anaplasmosis and Rickettsia monacensis infection in humans have been diagnosed in Spain (1–3).
Birds are the preferred host for some tick species. As carriers of infected ticks, birds could be responsible for the spread of tick-borne bacteria that cause human anaplasmosis, Lyme disease, rickettsioses, and other diseases (4). Multiple studies support the conclusion or propose the hypothesis that birds play a role as reservoirs of Anaplasma phagocytophilum, Borrelia burgdorferi, and Rickettsia spp (4–6). Because the Iberian Peninsula plays a major role in the migratory routes of birds, we aimed to determine the presence and prevalence of A. phagocytophilum, B. burgdorferi sensu lato, and Rickettsia spp. in ticks removed from birds captured in northern Spain.
The Study
During April–October 2009, bird bandings were conducted in the protected area of Finca Ribavellosa in La Rioja, Spain (42°14′N, 2°54′W). Ticks were collected from birds and classified through taxonomic keys (7) and molecular methods (8). DNA was individually extracted by using 2 incubations of 20 minutes each with ammonium hydroxide (1 mL of 25% ammonia and 19 mL of Milli-Q water that had been autoclaved) at 100°C and 90°C.
DNA extracts were used as templates for PCRs targeting fragment genes for tick classification and for bacteria detection (Table 1). Two negative controls, 1 containing water instead of template DNA and the other with template DNA but without primers, and a positive control (a tick extract, A. phagocytophilum, B. burgdorferi sensu stricto, or R. slovaca) were included in all PCRs. Amplification products were sequenced, and nucleotide sequences were compared with those available in GenBank by using a BLAST search (www.ncbi.nlm.nih.gov/blast/Blast.cgi). Phylogenetic and molecular evolutionary analyses were conducted by using MEGA4 (16 in Technical Appendix).
Table 1. PCR primer pairs used in study of the role of birds in dispersal of etiologic agents of tick-borne zoonoses, Spain, 2009*.
| Bacteria | Gene target | Primer name | Primer sequence, 5′ → 3′ | Amplified fragment, bp | Annealing temp., °C | Ref. |
|---|---|---|---|---|---|---|
| Anaplasma spp. | 16S rRNA, nested | ge3a | CACATGCAAGTCGAACGGATTATTC | 932 | 55 | (9) |
| ge10r | TTCCGTTAAGAAGGAT CTAATCTCC | |||||
| ge9f | AACGGATTATTCTTTATAGCTTGCT | 546 | 55 | (9) | ||
| ge2 | GGCAGTATTAAAAGCAGCTCCAGG | |||||
| msp | msp3F | CCAGCGTTTAGCAAGATAAGAG | 334 | 56 | (10) | |
| msp3R | GCCCAGTAACAACATCATAAGC | |||||
| Borrelia spp. | flaB, nested† | Outer 1 | AARGAATTGGCAGTTCAATC | 497 | 52 | (11) |
| Outer 2 | GCATTTTCWATTTTAGCAAGTGATG | |||||
| Inner 1 | ACATATTCAGATGCAGACAGAGGTTCTA | 389 | 55 | (11) | ||
| Inner 2 | GAAGGTGCTGTAGCAGGTGCTGGCTGT | |||||
| 5S-23S intergenic spacer, nested | 23SC1 | TAAGCTGACTAATACTAATTACCC | 380 | 52 | (12) | |
| 23SN1 | ACCATAGACTCTTATTACTTTGAC | |||||
| 5SCB | GAGAGTAGGTTATTGCCAGGG | 226 | 55 | (12) | ||
| 23SN2 | ACCATAGACTCTTATTACTTTGACCA | |||||
| Rickettsia spp. | ompA, seminested | Rr190.70p | ATGGCGAATATTTCTCCAAAA | 631 | 46 | (13,14) |
| Rr190.701n | GTTCCGTTAATGGCAGCATCT | |||||
| Rr190.70p | ATGGCGAATATTTCTCCAAAA | 532 | 48 | (14) | ||
| Rr190.602n | AGTGCAGCATTCGCTCCCCCT | |||||
| ompB, nested | rompB OF | GTAACCGGAAGTAATCGTTTCGTAA | 511 | 54 | (15) | |
| rompB OR | GCTTTATAACCAGCTAAACCACC | |||||
| rompB SFG IF | GTTTAATACGTGCTGCTAACCAA | 420 | 56 | (15) | ||
| rompB SFG/TG IR | GGTTTGGCCCATATACCATAAG | |||||
| gltA central region, nested | RpCS.877p | GGGGGCCTGCTCACGGCGG | 381 | 48 | (14) | |
| RpCS1258n | ATTGCAAAAAGTACAGTGAACA | |||||
| RpCS.896p | GGCTAATGAAGCAGTGATAA | 337 | 54 | (15) | ||
| RpCS.1233n | GCGACGGTATACCCATAGC |
*Temp., temperature; ref., reference; msp, p44 major surface protein gene; flaB, flagellin gene; ompB, 120-kDa genus common antigen gene; ompA, 190-kDa protein antigen gene; gltA, citrate synthase gene. †R = A/G; W = A/T.
A total of 222 ticks belonging to the species Haemaphysalis punctata (n = 1), Ixodes frontalis (n = 7), I. arboricola (n = 26), I. ricinus (n = 181), and other Ixodes spp. (n = 7) were collected from 97 passerine birds. Two nucleotide sequences for the 16S rRNA fragment gene of I. arboricola ticks were recorded (GenBank accession nos. JF791812 and JF791813) (Table 2).
Table 2. Anaplasma phagocytophilum, Borrelia burgdorferi s.l., and Rickettsia spp. detected in ticks removed from birds, Spain, 2009.
| Bacteria | Tick |
Bird species (no. specimens) | Gene targets | |
|---|---|---|---|---|
| Species | Stage | |||
| A. phagocytophilum | Ixodes ricinus | 1 L | Turdus merula (1) | msp |
| B. garinii | I. ricinus | 4 L, 2 N | T. merula (9) | flaB, 5–23S is |
| 3 L, 4 N | flaB or 5–23S is | |||
| 1 L | Erithacus rubecula (1) | flaB | ||
| 1 L | T. philomelos (1) | flaB, 5–23S is | ||
| 1 L | Troglodytes troglodytes (1) | flaB, 5–23S is | ||
| I. frontalis | 1 F | T. philomelos (1) | flaB, 5–23S is | |
| Ixodes spp. | 1 L | E. rubecula (1) | 5–23S is | |
| Haemaphysalis punctata | 1 L | T. merula (1) | flaB, 5–23S is | |
| B. valaisiana | Ixodes spp. | 1 L | T. merula (1) | flaB, 5–23S is |
| I. ricinus | 1 L, 1 N | T. merula (3) | flaB, 5–23S is | |
| 2 L | flaB | |||
| 1 L, 1 N | T. philomelos (2) | flaB, 5–23S is | ||
| 1 L | E. rubecula (1) | flaB, 5–23S is | ||
| 1 L | Garrulus glandarius (1) | flaB | ||
| B. turdi | I. frontalis | 1 F | T. merula (1) | flaB, 5–23S is |
| R. monacensis | I. ricinus | 1 N | Sylvia atricapilla (1) | ompA |
| R. helvetica | I. ricinus | 1 N | G. glandarius (1) | gltA |
| R. sibirica sibirica | I. ricinus | 1 L | S. atricapilla (1) | ompA |
| Rickettsia spp.† | I. ricinus | 1 N, 1 L | T. philomelos (1) | ompB or gltA |
| I. ricinus | 4 L | E. rubecula (4) | ompB or gltA | |
| 2 N | T. merula (2) | gltA | ||
| 1 L | Tr. troglodytes (1) | gltA | ||
| Candidatus Rickettsia vini | I. abroricola | 20 N | Cyanistes caeruleus (1) | ompA, ompB, gltA |
| 5 L | Parus major (1) | ompA, ompB, gltA | ||
| I. ricinus | 2 L | E. rubecula (2) | ompA, ompB, gltA | |
*L, larva; msp, p44 major surface protein gene; N, nymph; flaB, flagellin gene; 5S-23S is, 5S-23S rRNA intergenic spacer; ompB, 120-kDa genus common antigen gene; ompA, 190-kDa protein antigen gene; gltA, citrate synthase gene. †Same identity with >1 validly published Rickettsia species.
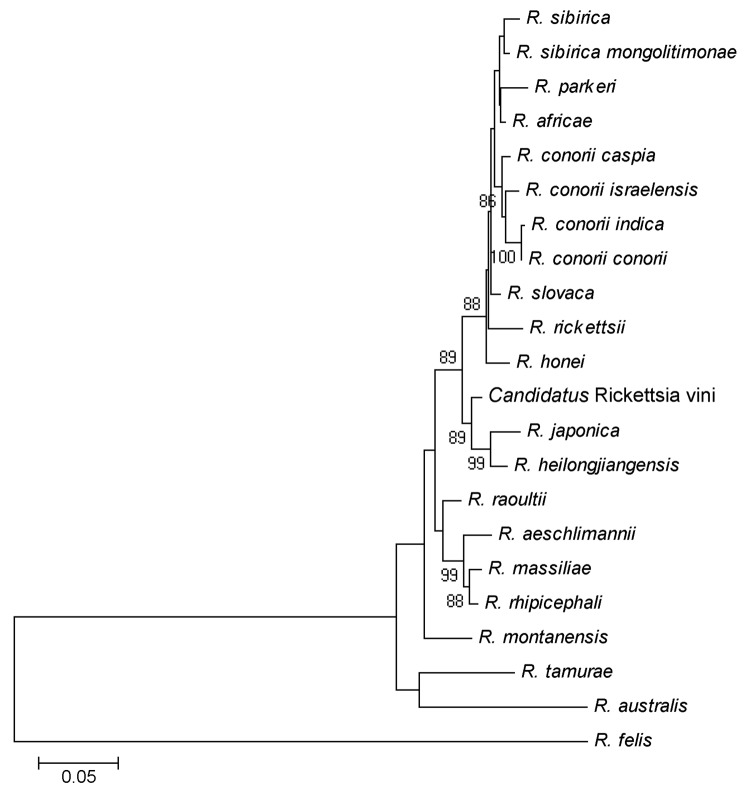
A. phagocytophilum was detected only in 1 larva of an I. ricinus tick (0.5%). Twenty-nine (13.1%) samples tested positive for B. burgdorferi s.l. The most prevalent genospecies was B. garinii (n = 19), which was detected in I. ricinus (n = 16), H. punctata (n = 1), I. frontalis (n = 1), and Ixodes sp. (n = 1) ticks. B. valaisiana was amplified in 9 samples (8 I. ricinus and 1 Ixodes sp. ticks). B. turdi was found in 1 I. frontalis tick. Rickettsia infection was detected in 39 (17.6%) ticks. R. monacensis (n = 1), R. helvetica (n = 1), R. sibirica sibirica (n = 1), and Rickettsia spp. (n = 9) were detected in 12 I. ricinus ticks. Furthermore, according to gltA, ompA, and ompB sequence analysis, a possible new Rickettsia sp. was found in 25 I. arboricola ticks and 2 I. ricinus ticks. For these 27 samples, highest identities with R. heilongjiangensis (97.1%) and R. japonica (99.1%) were found for ompA (GenBank accession no. JF758828) and ompB (GenBank accession no. JF758826) nucleotide sequences, respectively, whereas gltA nucleotide sequences were identical to those from both Rickettsia spp. According to multilocus sequence typing (data not shown) and genetic criteria agreed on by experts, a Candidatus status could be assigned. We named it Candidatus Rickettsia vini (17 in Technical Appendix) (Table 2). The phylogenetic tree based on ompA gene shows the nearest relationships among Rickettsia spp. (Figure).
Figure.
The phylogenetic position of Candidatus Rickettsia vini based on the ompA nucleotide sequences in a study of the role of birds in dispersal of etiologic agents of tick-borne zoonoses, Spain, 2009. The evolutionary history was inferred by using the neighbor-joining method. The optimal tree with the sum of branch length = 1.09961140 is shown. The percentage of replicate trees in which the associated taxa clustered in the bootstrap test (1,000 replicates) is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed by using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. Codon positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated from the dataset. A total of 563 positions were in the final dataset. Phylogenetic analyses were conducted in MEGA4 (16 in Technical Appendix).
Two I. ricinus larvae showed co-infection with B. garinii and Rickettsia sp. One nymph was co-infected with B. valaisiana and Rickettsia sp.
Conclusions
The presence of Anaplasma, Borrelia, and Rickettsia species in ticks removed from passerine birds corroborates the role of these vertebrates in the epidemiology and dispersion of tick-borne pathogens in Spain and in other zones of the planet. Some of the parasitized birds in our study, such as the European robin (Erithacus rubecula) or Eurasian blackcap (Sylvia atricapilla), are considered migratory or partial migratory birds. In addition, these species share an ecologic niche and ectoparasites (horizontal transmission) with other migratory birds that cover long distances from Africa to the Eurasian region.
Except for I. arboricola, the tick species captured in this study previously had been found on birds in Spain (18 in Technical Appendix). Nevertheless, I. arboricola ticks are commonly hosted by birds. The high prevalence of I. ricinus ticks was expected because it is the most frequent tick in this area, and the immature stages of this tick frequently parasitize birds.
I. ricinus ticks are the main vectors of A. phagocytophilum in Europe, and this microorganism has been detected on vegetation in the studied area (1). However, the low prevalence (0.5%) of A. phagocytophilum in the ticks in our study corroborates data from other studies (19,20 in Technical Appendix). The presence of A. phagocytophilum in a larva in our study supports the role of birds as reservoirs of A. phagocytophilum.
The prevalence (13.1%) of B. burgdorferi in our samples is similar to prevalences reported in other studies in Europe in which I. ricinus is the main species of tick captured from birds (19 in Technical Appendix). In Spain, B. garinii, B. valaisiana, and B. afzelii have been detected in ticks from birds (18 in Technical Appendix). According to our data, the human pathogen B. garinii was the most prevalent species, as reported in birds from Europe (21 in Technical Appendix). B. turdi was discovered in Asia. Although it has been recently detected in ticks from birds in Norway (22 in Technical Appendix), its finding in Spain was unexpected.
Regarding Rickettsia species, R. monacensis and R. helvetica are among the human pathogens detected in our study. Both species have been identified in ticks from birds in Europe (19,20,23 in Technical Appendix). On the contrary, Candidatus Rickettsia vini, a potential new Rickettsia species, also detected in our study, has not been related to human disease (17 in Technical Appendix). Several genospecies closely related to R. heilongjiangensis and R. japonica have been identified in Ixodes spp. ticks removed from birds (23 in Technical Appendix). R. sibirica sibirica, responsible for Siberian tick typhus in western People’s Republic of China and in Siberia, was also amplified in an I. ricinus larva in this study.
Our data confirm the involvement of birds in the cycle of human tick-borne diseases. The findings confirm that birds can disperse vectors and microorganisms.
Supplementary Material
Additional references.
Acknowledgments
We thank Agustín Estrada-Peña for classifying the I. arboricola ticks and Óscar Gutiérrez for providing tick samples.
This study was presented in part at the XIV Congreso SEIMC (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica) (Spain) in May 2010, the GEPE Scientific Meeting from the XV Congreso SEIMC (Spain) in June 2011, and the 6th International Meeting on Rickettsiae and Rickettsial Diseases (Greece) in June 2011.
Fundación Rioja Salud awarded a grant (FRS/PIF-01/10) to A.M.P. Financial support was provided in part by a grant from “Instituto de Salud Carlos III” (EMER 07/033), Ministerio de Ciencia e Innovación (Spain).
Biography
Dr Palomar has worked in the Center of Rickettsiosis and Arthropod-borne Diseases at the Infectious Diseases Area, Hospital San Pedro–Center for Biomedical Research of La Rioja since March 2009. Her research interests are the taxonomy of ticks and their associated pathogens.
Footnotes
Suggested citation for this article: Palomar AM, Santibáñez P, Mazuelas D, Roncero L, Santibáñez S, Portillo A, et al. Role of birds in dispersal of etiologic agents of tick-borne zoonoses, Spain, 2009. Emerg Infect Dis [serial on the Internet] 2012 Jul [date cited]. http://dx.doi.org/10.3201/eid1807.111777
References
- 1.Blanco JR, Oteo JA. Human granulocytic ehrlichiosis in Europe. Clin Microbiol Infect. 2002;8:763–72. 10.1046/j.1469-0691.2002.00557.x [DOI] [PubMed] [Google Scholar]
- 2.Oteo JA, Backenson PB, del Mar Vitutia M, García Moncó JC, Rodríguez I, Escudero R, et al. Use of the C3H/He Lyme disease mouse model for the recovery of a Spanish isolate of Borrelia garinii from erythema migrans lesions. Res Microbiol. 1998;149:39–46. 10.1016/S0923-2508(97)83622-4 [DOI] [PubMed] [Google Scholar]
- 3.Oteo JA, Portillo A. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis. 2012. In press. [DOI] [PubMed] [Google Scholar]
- 4.Hubálek Z. An annotated checklist of pathogenic microorganisms associated with migratory birds. J Wildl Dis. 2004;40:639–59. [DOI] [PubMed] [Google Scholar]
- 5.Hulinska D, Votypka J, Plch J, Vlcek E, Valesová M, Bojar M, et al. Molecular and microscopical evidence of Ehrlichia spp. and Borrelia burgdorferi sensu lato in patients, animals and ticks in the Czech Republic. New Microbiol. 2002;25:437–48. [PubMed] [Google Scholar]
- 6.Humair PF. Birds and Borrelia. Int J Med Microbiol. 2002;291:70–4. 10.1016/S1438-4221(02)80015-7 [DOI] [PubMed] [Google Scholar]
- 7.Manilla G. Fauna D’Italia Ixodida. Bologna (Italy): Calderini; 1998. [Google Scholar]
- 8.Black WC, Piesman J. Phylogeny of hard and soft tick taxa (Acari:Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci U S A. 1994;91:10034–8. 10.1073/pnas.91.21.10034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massung RF, Slater K, Owens JH, Nicholson WL, Mather TN, Solberg VB, et al. Nested PCR assay for detection of granulocytic ehrlichiae. J Clin Microbiol. 1998;36:1090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeidner NS, Burkot TR, Massung R. Transmission of the agent of human granulocytic ehrlichiosis by Ixodes spinipalpis ticks: evidence of an enzootic cycle of dual infection with Borrelia burgdorferi in northern Colorado. J Infect Dis. 2000;182:616–9. 10.1086/315715 [DOI] [PubMed] [Google Scholar]
- 11.Clark K, Hendricks A, Burge D. Molecular identification and analysis of Borrelia burgdorferi sensu lato in lizards in the southeastern United States. Appl Environ Microbiol. 2005;71:2616–25. 10.1128/AEM.71.5.2616-2625.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rijpkema SG, Molkenboer MJ, Schouls LM, Jongejan F, Schellekens JF. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J Clin Microbiol. 1995;33:3091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roux V, Fournier PE, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol. 1996;34:2058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi YJ, Jang WJ, Kim JY, Lee SH, Park KH, Paik HS, et al. Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg Infect Dis. 2005;11:237–44. 10.3201/eid1102.040603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional references.