See related article in EMBO Molecular Medicine http://dx.doi.org/10.1002/emmm.201100184
The clarity of the ocular lens stands in stark contrast to the obscurity of our understanding of lens physiology, but there are encouraging signs that the darkness may be lifting. There is increasing experimental support for the fluid circulation model (Fig 1) first proposed by Mathias & Rae, 1985 and more recently expanded by Mathias et al, 2007. The first experimentally verified predictions of the model were that as one approaches the centre of the lens the intracellular voltage should be increasingly positive and the extracellular voltage increasingly negative (Mathias & Rae, 1985). Much later diffusion tensor imaging and measurement of external circulating currents showed that both water entry (Vaghefi et al, 2011) and external circulating current (first measured by Robinson & Patterson, 1983) were eliminated by high external potassium. And in the past year Mathias and colleagues have shown that the internal pressure of the lens is about a third of an atmosphere at the centre of the lens, decreasing away from the centre until it reaches zero at the surface (Gao et al, 2011). This pressure is inversely proportional to the number of gap junctions distributed throughout the fibre cells in the interior of the lens, a finding that not only strongly supports the fluid circulation model but also provides the most direct evidence yet that water moves through gap junctions.
Figure 1. Elements of the fluid circulation model.
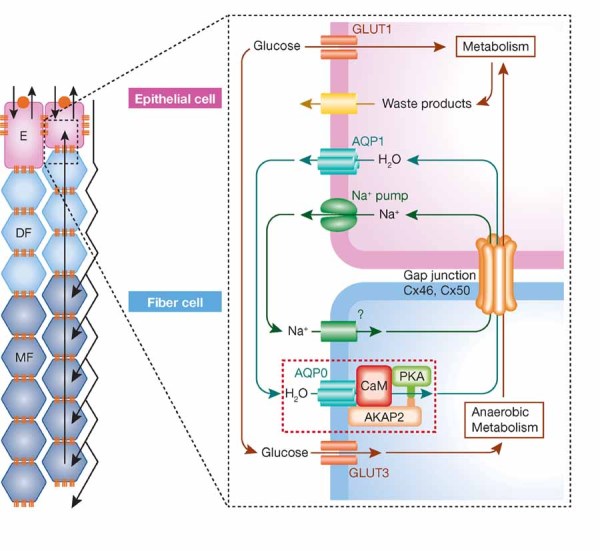
The regulatory complex described by Gold et al, 2011 is enclosed in a dashed rectangle. Transporters, channels, pumps and gap junctions are shown in the right hand panel that depicts the membranes of epithelial cells and cortical fibre cells. The left hand panel shows the proposed inward flow of ions, water and nutrients through the interstitium and the outward intracellular flow through gap junctions. While the regulation of AQP0 by the mechanism described by Gold et al, 2011 is essential for lens clarity, just how the feedback loops controlling the circulation described operate remains obscure. E, epithelial cells; DF, developing fibre cells; MF, mature fibre cells (Figure modified after Donaldson et al, 2001).
The essence of the fluid circulation model is that NaCl flows inward through interstitial space and enters cells driven by the electrochemical gradient of Na. This in turn increases intracellular osmotic pressure promoting an inward flow of water which increases intracellular hydrostatic pressure forcing water out through gap junctions. Thus, transmembrane flow is driven by osmotic pressure and depends on aquaporin zero (AQP0), and cell-to-cell flow is driven by hydrostatic pressure and depends on gap junctions. These water flows and the osmotic flow of NaCl must be matched and regulated to conform to the metabolic structures imposed on the lens by its lack of blood vessels and limited metabolic budget.
»…this paper establishes clearly a link between a regulatory pathway and maintenance of lens clarity.«
Gold et al, 2011 in their paper “AKAP2 anchors PKA with aquaporin-0 to support optical transparency” not only suggest a means by which one component of such regulation might be accomplished but also give a glimpse of the systems approach that will likely be required to lift the cloud of obscurity from our understanding of lens physiology. In showing first that AKAP2 lures PKA into close proximity with AQP0 and second that PKA from this association leads to loss of cortical transparency, this paper establishes clearly a link between a regulatory pathway and maintenance of lens clarity.
But much remains missing. Though Gold et al, 2011 and common sense strongly argue that regulation of water permeability (probably as one of multiple feedback loops in the control of the circulation of fluid) is essential for clarity, the nature of the feedback loops and how they are closed remains a mystery. Calcium is clearly a player. Its concentration increases toward the centre of the lens and increases markedly in cortical cataract (Duncan et al, 1984). But how is change in calcium concentration coupled to a need for increased or decreased water permeability, and how is the link between osmotically driven flow through AQP0 and hydrostatic pressure driven flow through gap junctions maintained? Is calcium concentration the link between regulation of both gap junction conductance (presumably closely related to their water permeability) and AQP0 water permeability? What about pH regulation of AQP0? It has been demonstrated in vitro (Németh-Cahalan & Hall, 2000), but does it have physiological importance? We know pH is more acidic by a pH unit or so in the interior than on the surface, but we do not know if this is used as a regulatory signal.
And what about the association of AQP0 with connexin 50 (Yu & Jiang, 2004; Zampighi et al, 2002)? Could the regulation of AQP0 by the complex described by Gold et al, 2011 be directly communicated to the gap junctional complex or does connexin 50 have its own suite of regulatory proteins?
The good news is that Gold et al, 2011 have shown us that a regulatory mechanism of one of the principal players in the fluid balance of the lens is essential for lens clarity, and they suggest that investigating other interactions of AKAP proteins in the lens may be fruitful. Their observations underscore the necessity of studying the lens as a system of interacting components not as a collection of independent proteins going their own way. So as always, the price paid for an increase in knowledge is an increase in the number of questions we never knew existed.
Acknowledgments
The author declares that he has no conflict of interest.
References
- Donaldson P, Kistler J, Mathias RT. Molecular solutions to mammalian lens transparency. News Physiol Sci. 2001;16:118–123. doi: 10.1152/physiologyonline.2001.16.3.118. [DOI] [PubMed] [Google Scholar]
- Duncan G, Jacob TJC, Nugent J, Whelan J. Human Cataract Formation. Bath, UK: Pitman Press; 1984. Calcium and the physiology of cataract; pp. 132–152. [Google Scholar]
- Gao J, Sun X, Moore LC, White TW, Brink PR, Mathias RT. Lens intracellular hydrostatic pressure is generated by the circulation of sodium and modulated by gap junction coupling. J Gen Physiol. 2011;137:507–520. doi: 10.1085/jgp.201010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MG, Reichow SL, O'Neill SE, Weisbrod CR, Langeberg LK, Bruce JE, Gonen T, Scott JD. AKAP2 anchors PKA with aquaporin-0 to support ocular lens transparency. EMBO Mol Med. 2011 doi: 10.1002/emmm.201100184. DOI: 10.1002/emmm.201100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias RT, Kistler J, Donaldson P. The lens circulation. J Membr Biol. 2007;216:1–16. doi: 10.1007/s00232-007-9019-y. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Rae JL. Transport properties of the lens. Am J Physiol. 1985;249:C181–C190. doi: 10.1152/ajpcell.1985.249.3.C181. [DOI] [PubMed] [Google Scholar]
- Németh-Cahalan KL, Hall JE. pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem. 2000;275:6777–6782. doi: 10.1074/jbc.275.10.6777. [DOI] [PubMed] [Google Scholar]
- Robinson KR, Patterson JW. Localization of steady currents in the lens. Curr Eye Res. 1983;2:843–847. doi: 10.3109/02713688209020020. [DOI] [PubMed] [Google Scholar]
- Vaghefi E, Pontre BP, Jacobs MD, Donaldson PJ. Visualizing ocular lens fluid dynamics using MRI: manipulation of steady state water content and water fluxes. Am J Physiol Regul Integr Comp Physiol. 2011;301:R335–R342. doi: 10.1152/ajpregu.00173.2011. [DOI] [PubMed] [Google Scholar]
- Yu XS, Jiang JX. Interaction of major intrinsic protein (aquaporin-0) with fiber connexins in lens development. J Cell Sci. 2004;117:871–880. doi: 10.1242/jcs.00945. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Eskandari S, Hall JE, Zampighi L, Kreman M. Micro-domains of AQP0 in lens equatorial fibers. Exp Eye Res. 2002;75:505–519. doi: 10.1006/exer.2002.2041. [DOI] [PubMed] [Google Scholar]


