Abstract
Cardiovascular diseases are the most common causes of human morbidity and mortality despite significant therapeutic improvements by surgical, interventional and pharmacological approaches in the last decade. MicroRNAs (miRNAs) are important and powerful mediators in a wide range of diseases and thus emerged as interesting new drug targets. An array of animal and even human miRNA-based therapeutic studies has been performed, which validate miRNAs as being successfully targetable to treat a wide range of diseases. Here, the current knowledge about miRNAs therapeutics in cardiovascular diseases on their way to clinical use are reviewed and discussed.
Keywords: antagomir, antimir, cardiovascular disease, microRNA, therapeutics
Introduction
Diseases of the cardiovascular system are the most common cause of human morbidity and mortality in the industrialized nations (European World Health Organisation, WHO/Europe). The costs of cardiovascular diseases to the European health care system are estimated to be as high as 200 billion Euros per year (according to http://www.cordis.europa.eu). Although classical pharmacologic treatment strategies have improved cardiovascular outcome and survival of heart failure patients [e.g. using beta-blockers and ACE (angiotensin (Ang)-converting enzyme) inhibitors], the prognosis of affected individuals remains poor with about 2 million cardiovascular deaths in the European Union per year. It is estimated that up to 50 million people are currently suffering from heart failure in the European Union.
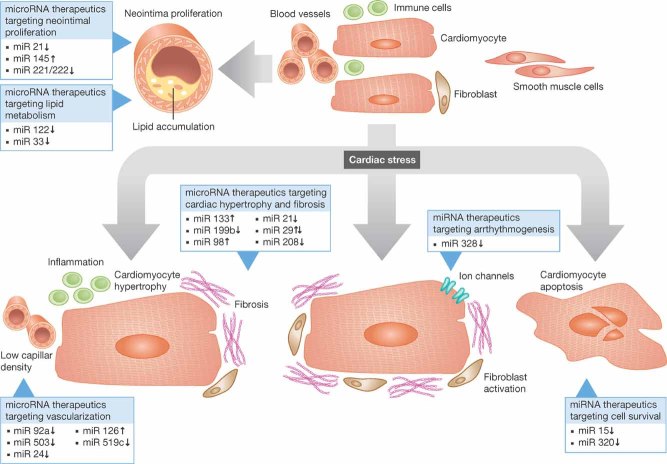
This highlights the need for further understanding of underlying mechanisms and development of innovative effective therapies for heart diseases. A variety of cardiovascular diseases, such as coronary artery disease, hypertension, myocardial infarction, valvular heart diseases, myocarditis and genetic forms of cardiomyopathies often result in a phenotypically similar endpoint, which is heart failure (Hill & Olson, 2008). Exposure of the heart to different stressors leads to cardiac remodelling with finally detrimental outcomes (Fig 1). At the cellular level, there is fibroblast activation and proliferation with subsequently increased growth factor secretion and extracellular matrix (ECM) production leading to fibrosis and further decline in cardiac function (Hill & Olson, 2008). Impaired vascularization and low capillary density as well as inflammatory processes further contribute to the development of heart failure (Fiedler et al, 2011; Heymans et al, 2009). Indeed, alterations of non-cardiomyocytes such as cardiac fibroblasts and endothelial cells strongly impact on cardiomyocyte and thus on general cardiac function.
Figure 1.
Identified miRNAs to be of therapeutic interest during cardiovascular disease.
Recent studies have uncovered important and unexpected roles for a family of small regulatory RNA molecules, known as microRNAs (miR; miRNAs) in the regulation of diverse aspects of cardiac function (Bonauer et al, 2009; Care et al, 2007; da Costa Martins et al, 2010; Fiedler et al, 2011; Thum et al, 2008b; van Rooij et al, 2007). MiRNAs are non-protein-coding, small RNAs of 20–23 nucleotides (nt) that exist in virtually all organisms and are highly evolutionary conserved (Ambros, 2001) suggesting a superior role in essential biological processes. Initially, primary miRNAs (pri-miRNA) are generated in the cellular nucleus by the transcription machinery and are then processed by the RNase-III-type enzyme Drosha to form so-called precursor miRNAs (pre-miRNAs; Lee et al, 2003; Thum et al, 2008a). Following exportation into the cytoplasm, miRNAs are processed by the ribonuclease Dicer into small 20–23 nt long miRNA duplexes. Finally, miRNAs are incorporated into RNA-induced silencing complexes (RISC) to silence gene expression at the post-transcriptional level by targeting messenger RNAs (mRNAs) with the result of mRNA degradation or by translational inhibition finally leading to target protein repression. Details about the biogenesis and regulation of cardiovascular miRNAs have recently been reviewed (Bauersachs & Thum, 2011). The miRNA expression patterns change in various cardiovascular diseases, such as myocardial infarction, cardiac hypertrophy and heart failure (Bonauer et al, 2009; Care et al, 2007; da Costa Martins et al, 2010; Fiedler et al, 2011; Thum et al, 2007, 2008b; van Rooij et al, 2007, 2008).
Surprisingly, also circulating extracellular miRNAs are present in body fluids of cardiovascular-diseased patients (Fichtlscherer et al, 2010; Gupta et al, 2010; Widera et al, 2011; Zampetaki et al, 2010). Despite the existence of ribonucleases, miRNAs remain stable in serum and other body fluids due to loading of the miRNAs into proteins, lipids or lipoprotein complexes such as exosomes or microvesicles. Thus, they may be used as biomarkers but may also function as mediators of disease (Gupta et al, 2010; Hunter et al, 2008; Valadi et al, 2007). As miRNAs target not only single mRNAs, but complete networks of often functionally related transcripts, they emerged as interesting novel candidates for the development of miRNA-based therapeutic strategies in cardiovascular disease. In the following, the current knowledge about the use of miRNA modulators as cardiovascular therapeutics is reviewed and discussed.
Historical perspective and chemical structures of miRNA modulators
To inhibit miRNAs in vivo, synthetic complementary oligonucleotides of 8–25 nt of length against the seed sequence and adjacent nucleotides of the miRNA of interest have been synthesized. Normally, nucleic acids are quickly degraded in biological compartments; thus various chemical modifications have been tested to stabilize their structure and to increase binding efficacy and cellular uptake. Chemical modifications mainly include 2-O-methyl-modified oligonucleotides and locked nucleic acid (LNA)-modified oligonucleotides, where the 2-oxygen is connected to the 4-position by a methylene linker to result in a tight bicycle and is locked into a C3-endo (RNA) sugar conformation (Elmen et al, 2008a; Krutzfeldt et al, 2007, 2005). In addition, the balance between phosphodiester- and phosphorothioate linkages between the nucleotides is important for the oligonucleotide stability as phosphorothioates show improved stability (Faria & Ulrich, 2008).
As shown in Table 1, the 2-O-methyl modification has been used very often to achieve high oligonucleotide stability and efficacy. A cholesterol construct has been linked to improve cellular uptake (Krutzfeldt et al, 2005; Thum et al, 2008b) and this approach has successfully been used to target cardiac tissue in vivo (Table 1). Recently, an excellent historical description of miRNA therapeutic development was provided (van Rooij, 2011). The group of Stoffel was the first to report mammalian miRNA knockdown using cholesterol-conjugated antagomirs to inhibit a liver-specific miRNA, miR-122 (Krutzfeldt et al, 2005). In addition, this group investigated the knockdown efficacy of many other antagomirs and showed for the first time that the cholesterol-based chemistry was also able to knockdown miRNA expression in cardiac tissue after intravenous injection (Krutzfeldt et al, 2005). After this landmark study, Care et al employed a cholesterol-based antagomir against miR-133, which resulted in cardiac hypertrophy of mice (Care et al, 2007). Another group showed the first successful therapeutic approach using an antagomir against fibroblast-enriched miR-21 to prevent cardiac fibrosis (Thum et al, 2008b). This was followed by many other studies successfully using miRNA inhibitors to beneficially effect cardiovascular function (Table 1).
Table 1.
Cardiovascular therapeutic miRNA modulation in vivo
| Field of studies | Reference | miRNA | miRNA modulator chemistry | Company | Dose | Timing | Species/organ | Outcome |
|---|---|---|---|---|---|---|---|---|
| Early studies and hypertrophy and cardiac fibrosis | ||||||||
| Krutzfeldt et al (2005) | let7, 22 16, 122 192.194 | 2′-O-methyl + 3′cholesterol | Done by authors | 80 mg/kg body weight | Up to 22 days | Mice | Administration of antagomirs against miR-16, miR-122, miR-192 and miR-194 resulted in a marked reduction of corresponding miRNA levels. In mice treated with antagomir-16, miR-16 was efficiently silenced in all tissues tested (including heart) except brain | |
| Care et al (2007) | 133 | 2′-O-methyl + 3′cholesterol | Dharmacon | 80 mg/kg body weight continously via osmotic minipumps | 1 month | Mice | In vivo inhibition of miR-133 caused cardiac hypertrophy | |
| da Costa Martins et al (2010) | 199b | 2′-O-methyl + 3′cholesterol | n.a. | 80 mg kg body weight/day, i.p. | Three daily injections | Mice, heart | In vivo inhibition of miR-199b normalized significantly attenuated cardiac functional impairment, fibrosis and NFAT activity; Dyrk1 is a miR199b target | |
| Yang et al (2011) | 98 | Adenoviral miR-98 or anti-miR-98 contructs | Done by authors | 109 pfu, intracardial (LV) injection | 14 days | Mice | MiR-98 overexpression reduced AngII-mediated fibrosis and amount of apoptotic myocytes | |
| Thum et al (2008b) | 21 | 2′-O-methyl + 3′cholesterol | Regulus Therapeutics | 80 mg/kg body weight, jugular vein | Three daily injections | Mice | Antagomir-21 decreased development of cardiac fibrosis and improved cardiac function | |
| Thum et al (2011) | 21 | 2′-O-methyl + 3′cholesterol, F/MOE Modification, 8 mer LNAs | Regulus Therapeutics | 10–80mg/kg body weight | Two injections | Mice | Differential effects of miRNA inhibitor chemistries on cardiac fibrosis and heart function | |
| Patrick et al (2010) | 21 | LNA-modified, unconjugated and fully phosphorothiolated oligonucleotides, 8-mer | Santaris Pharma | 25 mg/kg | Three daily injections | Mice | No effect on cardiac remodeling and fibrosis | |
| van Rooij et al (2007) | 29b | 2′-O-methyl + 3′cholesterol, miR mimics | n.a. | 80 mg/kg body weight | Two injections, tail-vein | Mice | MiR-29 downregulation increases fibrosis | |
| Boon et al (2011) | 29 | LNA-modified oligonucleutides, 16-mer | Exiqon | 20 mg/kg | Intravenous injection | Mice | MiR-29 downregulation prevents aortic dilatation | |
| Neointima formation | ||||||||
| Cheng et al (2009) | 145 | Adenoviral miR-145 construct | Done by authors | 5 × 109 pfu/ml | One local injection | Mice | Inhibition of neointima formation | |
| Cordes et al (2009) | 145 | Pre-miR-145 lentivirus | System Biosciences | 1 × 107 infectious units | External gel application | Mice | Inhibition of neointima formation | |
| Ji et al (2007) | 21 | 2′-O-methyl | Integrated DNA Technologies | 30 µg/carotid artery | local Delivery at days 3 and 7 | Mice | Inhibition of neointima formation | |
| Liu et al (2010) | 221/222 | 2′-O-methyl | Integrated DNA Technologies | 10 µg/carotid artery | Local delivery at do + gel | Mice | Inhibition of neointima formation | |
| Lipid metabolism | ||||||||
| Krutzfeldt et al (2005) | 122 | 2′-O-methyl + 3′cholesterol | Done by authors | 80 mg/kg body weight | Three daily injections | Mice | Cholesterol reduction | |
| Marquart et al (2010) | 33 | MiR-33 inhibitor (no chemistry mentioned) | miRagen Therapeutics | 5 mg/kg/day | Three consecutive days | Mice | Increase in plasma HDL levels | |
| Najafi-Shoushtari et al (2010) | 33a | LNA-modified oligonucleutides | n.a. | 20 mg/kg/day i.v. | Three consecutive days | Mice | Increase in plasma HDL levels | |
| Rayner et al (2010) | 33 | Lentiviral anti-miR-33 construct | System Biosciences | 2 × 109 pfu/mouse retroorbitally | Single injection | Mice | Increase in plasma HDL levels | |
| Rayner et al (2011) | 33a/b | 2′-F/MOE modification | Regulus Therapeutics | 5 mg/kg s.c. | Twice weekly for 2 weeks, then weekly | Monkeys | Increase in plasma HDL levels Decrease in VLDL triglycerides | |
| Endothelial biology | ||||||||
| Bonauer et al (2009) | 92a | 2′-O-methyl + 3′cholesterol | VBC Biotech | 8 mg/kg body weight (5 injections) | 14 days | Mice | Inhibition of miR-92a led to enhanced blood vessel growth and functional recovery of damaged tissue | |
| Caporali et al (2011) | 503 | Adenoviral decoy-miR-503 | Invitrogen | 109 pfu, one muscular injection | 3–21 days | Mice | Inhibition of miR-503 normalizes post-ischemic flow | |
| van Solingen et al (2009) | 126 | 2′-O-methyl + 3′cholesterol | Dharmacon | 1.0 mg/mouse i.v. | 10 days | Mice | MiR-126 inhibition reduced angiogenic response | |
| McArthur et al (2011) | 200b | n.a. | Dharmacon | 1.4 µg/week | 4 weeks | Rat retina | Increased VEGF production | |
| Fiedler et al (2011) | 24 | 2′-O-methyl + 3′cholesterol | Regulus Therapeutics | 80 mg/kg body weight, retroorbital injection | 14 days | Mice | Endothelial miR-24 blockade improves capillary density, limits infarct size and improves heart function | |
| Cell survival | ||||||||
| Porrello et al (2011) | 15b,16 | Locked nucleic acid-modified anti-miRNAs | miRagen Therapeutics | 25 mg/kg body weight | Three injections | Mice | Knockdown of miR-15 family was associated with an increased number of mitotic cardiomyocytes | |
| Ren et al (2009) | 320 | 2′-O-methyl + 3′cholesterol | Dharmacon | 80 mg/kg body weight | Single tail vein injection, 3 days | Mice | In vivo treatment with antagomir-320 reduced infarction size and increased Hsp20 in the heart | |
| Cardiac rhythm | ||||||||
| Lu et al (2010) | 328 | Adenoviral miR-328 precursor; antagomir: 2′-O-methyl + 3′cholesterol | Overexpression GenScript and by authors Antagomir: Ribobio Co. | Overexpression: 109 pfu, multiple Intraatrial injections; antagomir 80 mg/kg/d i.v. for 3 days | Dogs: 12 h Mice: 14 days | Dogs (overexpression) Mice (antagomir) | Antagomir-328 abrogated or stopped atrial fibrillation and improved cardiac function post-myocardial infarction | |
n.a., not available.
Glossary
AAVs
Adeno-associated viruses, whose various serotypes have some cell-type tropism. Thus, AAVs may be used to improve cell-type enriched delivery of miRNAs.
Angiogenesis
Development of blood vessels in the embryo or in an adult tissue.
Antagomirs/antimirs
Different names for chemistries used for miRNA inhibitors that lead to miRNA silencing in vitro or in vivo.
Arrhythmogenesis
Development of arrhythmia, an irregular heartbeat or abnormal rhythm.
Atherosclerosis
A condition in which the artery wall thickens due to accumulation of fatty material, which can restrict blood flow.
Cardiac hypertrophy
Thickening of the heart muscle, which results in increased heart size and decreased chamber size.
Cardiomyocyte
Muscle cell of the heart.
Cardiovascular disease
A class of diseases that involves the heart and/or blood vessels.
Fibrosis
Development of excessive fibrous connective tissue in an organ.
microRNAs
Small RNA molecules, that regulate a large fraction of the genome by binding to complementary mRNA sequences leading to post-transcriptional gene silencing.
miRNA therapeutics
A new form of drugs leading to miRNA silencing (see antagomirs/antimirs description) or miRNA enrichment in individual cell types to counteract derailed gene expression and impaired function in cardiovascular and other diseases.
Morpholino
25-mer antisense oligonucleotide with modified bases containing morpholine ring, making them very stable.
Myocardial infarction
Commonly known as heart attack. Irreversible necrosis of the heart muscle caused by prolonged ischemia.
Neointima
New layer of arterial intima (inner lining of the artery) formed especially on a prosthesis and in atherosclerosis.
Vascular smooth muscle cell
The particular type of smooth muscle cell composing the majority of the wall of blood vessels.
Alternative modification strategies include use of LNA chemistries, which were shown to effectively knockdown target miRNA levels. LNA modification of oligonucleotides results in a thermodynamically very strong duplex formation with the complementary RNA (Grunweller & Hartmann, 2007) and thus is biologically highly effective as shown in many studies (Table 1). For instance, systemic delivery of unconjugated LNA-antimiR significantly silenced miR-122 both in mice and non-human primates (Elmen et al, 2008a, 2008b) and these antimiRs were successfully used in a disease model of hepatitis C in primates (Lanford et al, 2010). Importantly, use of this technique is currently under investigation in first phase I and II clinical trials (Santaris Pharma, http://www.ClinicalTrials.gov). Specifically, a clinical phase II trial treating patients with hepatitis C with an LNA-based specific inhibitor of miR-122 is ongoing and first results are expected soon.
Thus, both antagomir and LNA-modified oligonucleotides can effectively target miRNAs in vivo, although LNA-modified chemistries appear to require lower doses based on their high binding affinity. Recently, very short 8-mer fully modified LNA oligomers only directed against the seed region of a miRNA were introduced (Obad et al, 2011; Patrick et al, 2010). This approach might be helpful when targeting of multiple miRNA family members with the same seed sequence is desired. However, the pharmacokinetics of so-called tiny miRs appear to differ in various organ types; for instance, whereas, long-lasting high concentrations after application of tiny miR-21 were seen in organs such as liver and kidney, an extremely fast drop in tissue availability was seen in lung and heart (Obad et al, 2011) indicating that optimal targeting of different organs may need different chemistries. This may explain why treatment with 22 nt-long cholesterol- or (fluoro)O-methoxyethylphosphorothioate (F/MOE)-based antagomirs against miR-21 was successful to prevent pressure-overload-induced cardiac fibrosis in a mouse model (Thum et al, 2011, 2008b), whereas, application of LNA-based 8 nt-long antimiRs did not (Patrick et al, 2010).
Additional strategies to lower miRNA expression is to use miRNA erasers or sponges (Wang, 2011). The principle is to employ vector constructs that harbour multiple miRNA binding sites where the miRNA of interest can bind to and thus is not any more available to bind its targets.
In addition to silencing of miRNAs, their upregulation and/or enhancement may be of therapeutic interest. However, the approaches to optimize miR mimics in terms of stabilization and efficacy of miRNA overexpression have been rather disappointing so far. A miR-mimic consists of a double-stranded oligonucleotide including the mature miRNA sequence and the complementary passenger strand. In a first attempt, van Rooij and colleagues locally injected miR-29 mimics into the heart after myocardial infarction and observed modest miR upregulation by this approach (van Rooij et al, 2008). Here, clearly improved chemical stabilization and/or conjugation to delivery molecules is needed to result in superior biological effects. A further strategy to augment miRNA levels is to use miRNA delivery through adeno-associated viruses (AAVs; Hinkel et al, 2011). Certain AAV serotypes have the unique feature of cell-type favouring tropism without leading to significant inflammatory responses (Wang et al, 2011). An example is AAV9, which is enriched in the heart after administration (Bish et al, 2008). Thus, upon viral delivery via specific AAV serotypes selected miRNAs can be enriched in target tissues, which may be important for such miRNAs that are downregulated during cardiovascular disease. A cell-type specific homing of such miRNA delivery tools may be achieved by use of tissue-specific promoters for expression.
Delivery of miRNA therapeutics, pharmacokinetics and costs
Most oligonucleotide chemistries are soluble in water and/or saline and have been injected into animals by various approaches and application routes (see Table 1). Intravenous delivery including the tail vein, jugular vein or the retro-orbital vein plexus has been the most commonly used route of administration. However, recent studies showed that also either intraperitoneal injections of cholesterol-based antagomirs (da Costa Martins et al, 2010) or LNA-based chemistries can be used to silence miRNAs within the heart (Table 1). A recent study compared intravenous, intraperitoneal or subcutaneous delivery routes and here 25 mg/kg antimiR-208a were injected (Montgomery et al, 2011). All three administration routes of showed robust inhibition of miR-208a at days 1, 4, 7 and 14 with no significant differences in antimiR-208a detection between the different delivery methods for plasma, heart, liver or kidney. Less experiences to target, for instance, the aorta are available but it seems that higher concentrations are needed (Boon et al, 2011). Another study compared the effects of different doses of cholesterol-based antagomirs on cell-type specific uptake and silencing of miR-24 (Fiedler et al, 2011). In this study, dosing with 80 mg/kg of an antagomir against miR-24 led to the silencing of miR-24 expression in both cardiomyocytes and cardiac endothelial cells, whereas, lower dosing with 5 mg/kg reduced miR-24 expression selectively in endothelial cells but not cardiomyocytes based on Cy3-labelling and cell fractionation studies (Fiedler et al, 2011). As endothelial cells that form cardiac capillaries are the first cells where antagomirs are delivered via the blood stream, low doses may be preferably used to target this cell compartment, whereas, higher concentrations subsequently affect capillary surrounding cells. An alternative explanation is altered cholesterol- and/or oligonucleotide uptake mechanisms within different cardiovascular cells, but scientific insight into this issue is limited so far.
Dosing is obviously dependent on the used chemistry and the abundance of the miRNA in the target organ. Thus, the optimal concentrations need to be determined individually for various miRNAs. Cell-type specific delivery may also be achievable in the future by linking the miRNA inhibitors and/or miR-mimics to cell-type specific antibodies, peptides, lipids or other cell-type-specific pro-trophic molecules.
Information about tissue distribution and pharmacokinetics of miRNA modulators are presently scarce. As mentioned above, Obad et al compared tissue distribution and effects of various doses of 8 nt-long LNA-based antimiR-21 (Obad et al, 2011). Another study showed significant differences in cell-type specific silencing based on the used dose of cholesterol-based antagomirs, with endothelial cells more specifically targeted by lower doses (Fiedler et al, 2011). Using an LNA-based antagonist of miR-208a, van Rooij's group recently showed progressive reduction in rat cardiac miR-208a levels using concentrations ranging from 0.0625 to 4 mg/kg body weight with only minor further miR reductions at higher doses (Montgomery et al, 2011). Thus, there are (probably chemistry-depended) dose/effect-relationships that need to be addressed with high accuracy in future studies in the field, as currently the range of used doses of miRNA modulators is large and only little direct comparisons have been made (see Table 1).
Initial studies employing miRNA modulating substances such as antimiRs or antagomirs were only possible by interaction of university-based laboratories with pharmaceutical industry and miRNA start-up companies and in 2005/2006, the costs per injection per mouse were in the several thousand Euros (personal experience). Today, many groups still have cooperations with miRNA companies and thus receive miRNA inhibitors for free pending on material transfer agreements and intellectual property discussions. However, it is nowadays also possible to purchase LNA-based antimiRs, cholesterol-based antagomirs or others (although the used detailed chemistry from such companies may differ from that of big miRNA therapeutic companies) and costs differ between 100 and 600 Euros per injection per mouse pending on the used dose (personal experience). Thus, as frequently observed for successful compounds in pharmaceutical development, the costs for miRNA-modulating chemistries have been reduced significantly in the past (which probably will continue) allowing more research group to enter this exciting field and paving the way for large animal and finally clinical studies where higher amounts of miRNA chemistries will be needed.
miRNA modulation by oligonucleotides in zebrafish
As described above, delivery of oligonucleotide chemistries to target miRNAs in organisms results in a better understanding of miRNA function and potential therapeutic effects. The zebrafish model has been used in the past to test effects of various miRNA modulators (Table 2). Modulation of miRNAs in zebrafish either by downregulation using morpholinos or by upregulation through injection of specific pre-miRNAs is an elegant approach to identify miRNAs with importance for the cardiovascular system.
Table 2.
microRNA modulation in zebrafish leading to cardiovascular phenotypes
| Field of studies | Reference | miRNA | miRNA modulator chemistry | Company | Dose | Time | Species | Results |
|---|---|---|---|---|---|---|---|---|
| Zebrafish | ||||||||
| Bonauer et al (2009) | 92a | miR mimics | Ambion | 2 nl of 20 µM stock solution | 30–48 hpf | Zebrafish | Overexpression of miR-92a induced defects in vessel formation | |
| Lagendijk et al (2011) | 23 | Morpholinos, miR mimics | Gene Tools | 1 nl of 0.5 mmol/L; 200 ng/µl | 2 days, 2 days | Zebrafish | Loss of miR-23 results in endocardial defects | |
| Fish et al (2011) | 218 | Morpholinos | Gene Tools/Open Biosystems | morpholino 8–12 ng | 2 days | Zebrafish | Loss of miR-218 results in cardiovascular defects and impaired circulation | |
| Fiedler et al (2011) | 24 | miR mimics | Ambion | 2 nl of 25 µM stock solution | 2 days | Zebrafish | miR-24 overexpression leads to blood accumulation and abnormal intersegmental vessel formation | |
The injection of miR-218 morpholinos resulted in cardiac oedema at 48 h post-fertilization, although gross vascular patterning appeared normal (Fish et al, 2011). The observation of a dysmorphic heart and pericardial oedema as well as the absence of severe vascular defects suggested that miR-218 might be directly required for cardiogenesis. Indeed, the authors showed that miR-218 and multiple Slit/Robo signalling components are required for heart tube formation in zebrafish and that this network modulates the previously unappreciated function of Vegf signalling in this process.
Overexpression of miR-92a leads to disturbed vessel growth and patterning in zebrafish (Bonauer et al, 2009). Quantification of the number of intersegmental vessels, which are properly connected to the aorta or cardinal vein on the ventral side and to the dorsal longitudinal anastomotic vessel on the dorsal side, revealed a significant reduction in vessel growth after miR-92a overexpression.
A significant contribution to our understanding of miRNAs involved in valve formation using the zebrafish as a model organism was made recently. Dicer null mutants showed excessive endocardial cushion formation accompanied by a strong increase in cardiac jelly (Lagendijk et al, 2011). This was mimicked by silencing miR-23 using morpholino injection. In vitro miR-23 upregulation prevented TGF-beta induced endothelial–mesenchymal transition. These studies suggest a dominant role of miR-23 during valve formation (see Table 2). Whether this mechanism is involved in other animal models and humans and may help to develop therapeutic strategies to target valve diseases such as development of aortic valve stenosis remains to be determined.
miR-24 is also enriched in cardiac endothelial cells and its overexpression results in endothelial apoptosis and impaired angiogenesis (Fiedler et al, 2011). Overexpression of miR-24 in zebrafish embryos resulted in pericardial oedema as well as blood accumulation. Studies in transgenic zebrafish that overexpress GFP in the vasculature showed that miR-24 overexpression impaired intersegmental vessel formation and resulted in impaired blood transportation (Fiedler et al, 2011). Morpholino-mediated silencing of the miR-24 targets gata2 and pak4 in zebrafish mimicked the effects of miR-24 overexpression showing an involvement and potential therapeutic importance of those targets in angiogenesis (Fiedler et al, 2011).
miRNA modulation by oligonucleotides in mice and large animals
Modulation of cardiovascular miRNA expression was achieved by many different approaches (see Fig 1 and Table 1). Most of the studies used pharmacological interventions such as antisense oligonucleotide-mediated knockdown or miR-mimic based overexpression techniques. Although mechanistic details are not clear, many different miRNA therapeutics can be taken up by cardiovascular cells including cardiomyocytes, fibroblasts, endothelial cells, smooth muscle cells and inflammatory cells. Upon cellular uptake such miRNA inhibitors bind to their target miRNAs resulting in a functional deactivation, which also can be seen by upregulation of the respective miRNA targets. In the following, miRNA therapeutics targeting different features of cardiovascular disease are summarized.
miRNA therapeutics targeting cardiac hypertrophy and fibrosis
One of the first studies that used an antagomir to inhibit a miRNA involved in cardiac hypertrophy was performed by Care and coworkers (Care et al, 2007). This group implanted mice subcutaneously with osmotic minipumps for a continuous delivery of a cholesterol-based antagomir targeting miR-133. After 1 month of infusion, echocardiographic analysis showed a marked increase in cardiac hypertrophy suggesting that miR-133 mimics may be of therapeutic relevance.
Another group showed involvement of miRNAs in cardiac hypertrophy; miR-199b is a direct calcineurin/nuclear factor of activated T cells (NFAT) target that increases in expression during mouse and human heart failure thus being a potential interesting target for therapies (da Costa Martins et al, 2010). Indeed, in vivo inhibition of miR-199b by a specific intraperitoneally administered antagomir normalized reduced nuclear NFAT activity and caused inhibition and even reversal of cardiac hypertrophy and fibrosis in mouse models of heart failure.
Thioredoxin, which regulates in part cardiomyocyte hypertrophy, leads to an increase in expression of the let-7 family including miR-98 (Yang et al, 2011). To evaluate the function of miR-98 in the heart in vivo, adenoviral miR-98 or antimiR-98 was injected into mouse hearts. Ang II-induced increases in hypertrophy, cardiomyoctye apoptosis and fibrosis were attenuated by Ad-miR-98 and significantly enhanced by Ad-antimiR-98. MiR-98 negatively regulates Ang II-induced cardiac hypertrophy and the accompanying histopathologic changes in vivo and thus is an attractive target for the treatment of cardiac remodelling.
Cardiac fibrosis development is often linked to cardiac stress including pathological cardiomyocyte hypertrophy. Cardiac fibroblasts are enriched with certain miRNAs, such as miR-21 (Liu et al, 2010; Roy et al, 2009; Thum et al, 2011, 2008b), which regulates the ERK–MAP kinase signalling pathway via targeting sprouty-1. By this mechanism, fibroblast survival and growth factor secretion, apparently regulating the extent of interstitial fibrosis and cardiac hypertrophy, is controlled. In vivo silencing of miR-21 by a specific cholesterol-based antagomir in a mouse pressure-overload-induced disease model reduced cardiac ERK–MAP kinase activity, inhibited interstitial fibrosis and attenuated cardiac dysfunction. In addition, recently it has been shown that miR-21 antagonism blocks endothelial–mesenchymal transition in TGF-beta-treated endothelial cells as well as in vivo in a cardiac hypertrophy model resulting in reduced fibrosis development (Kumarswamy et al, 2011). Strikingly, the effects of miR-21 inhibition on fibrosis prevention were validated in other organs often affected by pathological fibrosis during diseases such as the lung (Liu et al, 2010) and the kidney (Zhong et al, 2011). Thus, miR-21 inhibition may represent a general approach to inhibit exaggerated organ fibrosis.
Another miRNA implicated in fibrosis development is miR-29. Van Rooij et al found that members of the miR-29 family are downregulated in the region of the heart adjacent to the infarct. Interestingly, the miR-29 family targets mRNAs that encode proteins involved in fibrosis, such as multiple collagens, fibrillins and elastin. Indeed, overexpression of miR-29 in fibroblasts reduced collagen expression (van Rooij et al, 2008). In contrast to a downregulation, during aging, an upregulation of miR-29 was seen in the aorta of mice and the increased expression of miR-29 family members was associated with a profound downregulation of numerous ECM components in aortas of aged mice and during aneurysm formation (Boon et al, 2011). Of clinical relevance, miR-29b levels were also profoundly increased in human thoracic aneurysms (Boon et al, 2011). The same group showed that LNA-modified antisense oligonucleotide-mediated silencing of miR-29 led to induction of ECM expression and inhibition of Ang II-induced dilation of the aorta in mice. This could be a therapeutic entry point for aneurysm treatment and/or stabilization also for clinical applications.
miRNA therapeutics targeting angiogenesis and vascularization
The Dimmeler group was the first to employ miRNA inhibitors to increase neovascularization after hindlimb ischemia and myocardial infarction (Bonauer et al, 2009). Indeed, hypoxia and tissue ischemia lead to upregulation of several miR-17-92 cluster members including miR-92a although the underlying detailed regulatory mechanisms are not entirely clear (Bonauer et al, 2009). Mice systemically injected with a cholesterol-based antagomir-92a exhibited a significant reduction in toe necrosis in comparison with mice treated with a control antagomir. Recovery of blood flow was increased, as was the number of capillaries and smooth muscle actin-positive arterioles after antagomir-92a treatment. To test the effects after myocardial infarction, antagomir-92a or control was intravenously injected at days 0, 2, 4, 7 and 9 after occlusion of the left coronary artery. Antagomir-92a treatment improved left ventricle systolic and diastolic function, reduced the infarct size, suppressed the number of apoptotic cells and augmented the number of in vivo perfused lectin-positive vessels, particularly in the infarct border zone. Whether the positive effects were mediated only by inhibition of miR-92a in endothelial cells remains to be determined, as it may be that there are additional, direct effects on cardiomyocytes or smooth muscle cells.
Another group found miR-503 expression to be enriched in endothelial cells isolated from ischemic hind limbs especially in a diabetic setting (Caporali et al, 2011). They tested whether local miR-503 inhibition would improve post-ischemic reparative neovascularization and blood flow recovery in diabetic mice using a decoy strategy to lower miR-503 availability in the muscle during ischemia. Indeed, post-ischemic foot blood flow recovery was impaired in diabetic mice and local injections of Ad decoy-miR-503 completely normalized blood flow recovery in diabetic mice. In addition, this therapy increased capillary and arteriolar densities in ischemic muscles of diabetic mice. Thus, manipulation of miR-503 may represent a novel molecular approach to foster reparative angiogenesis in diabetic patients.
MiR-126 is an endothelial cell-enriched abundant miRNA and miR-126 knockout mice die early due to impaired angiogenesis (Wang et al, 2008). To study the consequences of miR-126 silencing on vascular regeneration, van Solingen and colleagues injected mice with a single dose of antagomir-126 and exposed them to ischemia of the left hindlimb (van Solingen et al, 2009). Quantification of the capillary density in the ischemic hindlimb of mice after miR-126 knockdown revealed that they showed a markedly reduced angiogenic response suggesting that miR-126 mimics may be an appropriate strategy to increase vascularization in ischemic tissues. However, this awaits further experimentation and validation.
Angiogenic events are partly controlled by the hypoxia-inducible factor-1α (HIF-1α), which is regulated by miR-519c through direct binding to the HIF-1α 3′ untranslated region (Cha et al, 2010). Antagomir-mediated inhibition of miR-519c increased HIF-1α protein levels and enhanced angiogenic activity in tumors. This is interesting as blockade of miR-519c may represent an alternative strategy to stimulate angiogenesis in ischemic tissues such as after myocardial infarction although this remains to be tested.
In contrast to ischemic tissues, there is a need to block exaggerated angiogenesis in diabetic retinopathy, which is a leading cause of blindness. In the retina of diabetic rats, downregulation of miR-200b occurs, whereas, levels of its target VEGF are increased (McArthur et al, 2011). Local intravitreal injection of miR-200b mimics prevented diabetes-induced increased VEGF levels and prevented glucose-induced increased permeability and angiogenesis. This may be a very interesting therapeutic approach in affected patients due to its local and easy administration.
miRNA therapeutics targeting cardiovascular cell survival
Mammalian cardiomyocytes are prevented from cell cycling during early post-natal development, which limits the capacity of the adult heart to regenerate after injury. During early post-natal cardiac development, miR-195, which is a member of the miR-15 family, is highly upregulated and drives the expression of a number of cell cycle genes (Porrello et al, 2011). Importantly, knockdown of the miR-15 family in neonatal mice with LNA-modified antimiRNAs was associated with an increased number of mitotic cardiomyocytes and de-repression of the cell cycle protein Chek. Upregulation of the miR-15 family during the neonatal period may be an important regulatory mechanism governing cardiomyocyte cell cycle withdrawal and thus may be an interesting therapeutic entry point to re-stimulate cardiomyocyte division for regenerative purposes (Porrello et al, 2011).
Following ischemia/reperfusion (I/R) injury in mice, miR-320 expression is significantly decreased in the heart (Ren et al, 2009). In vitro overexpression of miR-320 enhanced cardiomyocyte apoptosis, whereas, its knockdown appeared cytoprotective. Indeed, in vivo treatment with cholesterol-modified antagomir-320 prevented cardiomyocyte apoptosis and reduced infarction size relative to the administration of mutant antagomir-320 and saline controls.
miRNA therapeutics targeting arrhythmogenesis
miRNAs may also regulate ion channels including the L-type calcium current and thus impact on action potential duration (Latronico & Condorelli, 2010; Thum et al, 2008a). miR-328 was upregulated in atria from dogs with induced atrial fibrillation and targets the L-type calcium channel (Lu et al, 2010). Strikingly, normalization of miR-328 levels with an antagomir reversed the conditions, and also a genetic knockdown of endogenous miR-328 dampened atrial fibrillation vulnerability (Lu et al, 2010). This suggests the potential of miR-328 as a target for atrial fibrillation treatment with normalization of miR-328 level for converting fibrillation to sinus rhythm and the prevention of arrhythmogenesis in a clinical setting.
miRNA therapeutics targeting atherosclerosis
Atherosclerosis is a pathological multifactorial process, which very often starts with endothelial dysfunction and at later stages results in the deposition of fatty acid compounds, cholesterol, calcium, waste products and other substances that progressively accumulate within arteries, finally leading to the formation of plaques. In addition, there is often activation of smooth muscle cells, which contribute to the pathologic arterial remodelling processes, for example during neointima formation of injured vessels. The general role of miRNAs in the process of atherosclerosis and inflammation has recently been reviewed (Weber et al, 2010). Accordingly, I only briefly summerize miRNA-based therapeutic strategies successfully affecting smooth muscle cell function, neointima formation and lipid metabolism here (Fig 1 and Table 1).
MiR-21 has been shown to activate and to induce a contractile phenotype in human vascular smooth muscle cells by affecting TGF-beta and bone morphogenic proteins (BMPs; Davis et al, 2008). Indeed, a miR-21 antisense strategy prevented restenosis by blocking smooth muscle cell proliferation in a balloon-injured rat carotid artery model (Ji et al, 2007). MiR-145 and miR-143 are smooth muscle cell-enriched miRNAs that regulate crucial functions in this cell type and miR-143/145-knockout mice show decreased number of contractile smooth muscle cells and increased cell proliferation (Boettger et al, 2009; Cordes et al, 2009; Elia et al, 2009). In vivo, virus-mediated upregulation of miR-143/145 in injured rat carotid arteries and other mouse models of vascular injury resulted in a pronounced reduction of neointima lesion formation (Cheng et al, 2009; Elia et al, 2009; Xin et al, 2009) showing the general potential of those miRNAs for many vascular diseases such as restenosis prevention (Table 1). Finally, miR-221 and miR-222 have been described to be activated in vascular smooth muscle cells from injured carotid arteries (Liu et al, 2009). The same authors showed that in vivo silencing of miR-221 and miR-222 inhibited smooth muscle cell proliferation and thus neointima thickening after vascular injury (see Fig 1 and Table 1).
A dysbalanced metabolic state facilitates atherosclerosis development and recently, miRNAs have been implicated in the regulation of lipid metabolism [reviewed in (Fernandez-Hernando et al, 2011)]. Here, I briefly summarize the recent miRNA-based therapeutic trials that have been performed to target lipid metabolism both in small and large animals. Until now, several different miRNAs have been studied for their properties to regulate lipid metabolism and these include miR-122, miR-370, miR-378/378*, miR-335, miR-125a-5p and miR-33 [reviewed in (Fernandez-Hernando et al, 2011)]. I focus on therapeutic trials using inhibitors against miR-122 and miR-33.
MiR-122 is enriched in the liver and is involved in regulation of cholesterol metabolism (Krutzfeldt et al, 2005) and hepatitis C infection (Lanford et al, 2010). Indeed, the group of Stoffel was the first showing that plasma cholesterol levels are reduced in antagomir-122-treated mice (Fig 1 and Table 1). MiR-122 modulation in vitro and in vivo let to alterations of many genes implicated in liver metabolism, fatty acid synthesis and oxidation (Elmen et al, 2008a; Esau et al, 2006; Krutzfeldt et al, 2005).
The other miRNA I would like to mention here because of its intriguing translational potential is miR-33. The intronic miR-33a is located in the human sterol regulatory element-binding protein 2 (SREBF-2), which controls several genes involved in cholesterol uptake and synthesis (Horie et al, 2010). An important miR-33 target gene is the transporter responsible for shifting cholesterol out of the cell, ABCA1 (Marquart et al, 2010; Najafi-Shoushtari et al, 2010). Of great therapeutic relevance was the investigation that inhibition of endogenous miR-33 in vivo increased ABCA1 protein expression resulting in increased cholesterol efflux to apoA1 (Marquart et al, 2010; Najafi-Shoushtari et al, 2010; Rayner et al, 2010). There are several other miR-33 targets involved in lipid metabolism, which have been reviewed recently (Fernandez-Hernando et al, 2011) and thus are not focus of this Review. MiR-33 additionally regulates high-density lipoprotein (HDL) levels in in vivo-based studies with viral and LNA-stabilized miRNA antagonists (Marquart et al, 2010; Najafi-Shoushtari et al, 2010; Rayner et al, 2010; Table 1). This is important as low HDL levels are a hallmark of patients with coronary artery disease. An exciting translational study was now performed in non-human primates where therapeutic silencing of miR-33 by 2′F/MOE-modified antagomirs resulted in raised high-density lipoprotein (HDL) and reduced plasma very low-density lipoprotein (VLDL) triglyceride levels (Rayner et al, 2011). It will be interesting to see whether this promising approach will develop soon into first clinical phase I/II trials.
Pending issues.
Better identification and characterization of miRNAs important in cardiovascular function and disease.
Comparison of existing, development of new miRNA inhibitors and miR-mimics and optimization with respect to specificity, efficacy and toxicity.
Development of optimized delivery strategies for miRNA inhibitors and miR-mimics. This may be achieved by combining miRNA modulators with virus, lipids, proteins, peptides, etc. that allow organ/cell type-specific targeting.
Translation of early therapeutic findings in zebrafish and mouse models to larger animal models of cardiovascular disease. This is needed for the successful development of first cardiovascular therapeutic trials in patients.
Future developments and concluding remarks
In the next years, we likely will see further breakthroughs in cardiovascular miRNA research. First, large animal data also in the cardiovascular field will probably appear as this is worldwide currently under investigation in several laboratories. This will not only result in more insight into their therapeutic potential in larger species but also lead to important information about pharmacokinetics of such drugs and safety data. Second, we will see first detailed phase I and II data using miRNA therapeutics in non-cardiovascular but also cardiovascular medicine. This may pave the way for large-scale mechanism orientated miRNA-based therapeutic trials in cardiovascular medicine.
Acknowledgments
This work was funded by grants of the German Ministry for Education and Research (IFB-Tx to T. T., 01EO0802) and the German Research Foundation (TH903/10-1 to T. T.). The Editorial help of Yvonne Görzig (IMTTS) is highly appreciated. I apology to those whose work could not be cited owing to space limitations.
Conflict of interest statement: T. T. has filed and licensed patents concerning the use of miRNAs as cardiovascular diagnostics and therapeutics.
For more information
Clinical trials including miRNA therapeutic studies:
microRNA data base:
References
- Ambros V. MicroRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- Bauersachs J, Thum T. Biogenesis and regulation of cardiovascular microRNAs. Circ Res. 2011;109:334–347. doi: 10.1161/CIRCRESAHA.110.228676. [DOI] [PubMed] [Google Scholar]
- Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, Gao G, Wilson JM, Sweeney HL. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther. 2008;19:1359–1368. doi: 10.1089/hum.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, Vinciguerra M, Rosenthal N, Sciacca S, Pilato M, et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011;109:1115–1119. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- Caporali A, Meloni M, Vollenkle C, Bonci D, Sala-Newby GB, Addis R, Spinetti G, Losa S, Masson R, Baker AH, et al. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- Cha ST, Chen PS, Johansson G, Chu CY, Wang MY, Jeng YM, Yu SL, Chen JS, Chang KJ, Jee SH, et al. MicroRNA-519c suppresses hypoxia-inducible factor-1alpha expression and tumor angiogenesis. Cancer Res. 2010;70:2675–2685. doi: 10.1158/0008-5472.CAN-09-2448. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa Martins PA, Salic K, Gladka MM, Armand AS, Leptidis S, el Azzouzi H, Hansen A, Coenen-de Roo CJ, Bierhuizen MF, van der Nagel R, et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol. 2010;12:1220–1227. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjarn M, Hansen HF, Berger U, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008a;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- Elmen J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjarn M, Hansen JB, Hansen HF, Straarup EM, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008b;36:1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Faria M, Ulrich H. Sugar boost: when ribose modifications improve oligonucleotide performance. Curr Opin Mol Ther. 2008;10:168–175. [PubMed] [Google Scholar]
- Fernandez-Hernando C, Suarez Y, Rayner KJ, Moore KJ. MicroRNAs in lipid metabolism. Curr Opin Lipidol. 2011;22:86–92. doi: 10.1097/MOL.0b013e3283428d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, Galuppo P, Kneitz S, Pena JT, Sohn-Lee C, et al. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- Fish JE, Wythe JD, Xiao T, Bruneau BG, Stainier DY, Srivastava D, Woo S. A Slit/miR-218/Robo regulatory loop is required during heart tube formation in zebrafish. Development. 2011;138:1409–1419. doi: 10.1242/dev.060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunweller A, Hartmann RK. Locked nucleic acid oligonucleotides: The next generation of antisense agents. BioDrugs. 2007;21:235–243. doi: 10.2165/00063030-200721040-00004. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Bang C, Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet. 2010;3:484–488. doi: 10.1161/CIRCGENETICS.110.958363. [DOI] [PubMed] [Google Scholar]
- Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, et al. Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11:119–129. doi: 10.1093/eurjhf/hfn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- Hinkel R, Trenkwalder T, Kupatt C. Gene therapy for ischemic heart disease. Expert Opin Biol Ther. 2011;11:723–737. doi: 10.1517/14712598.2011.570749. [DOI] [PubMed] [Google Scholar]
- Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, Kinoshita M, Kuwabara Y, Marusawa H, Iwanaga Y, et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci USA. 2010;107:17321–17326. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee ML, Schmittgen TD, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- Krutzfeldt J, Kuwajima S, Braich R, Rajeev KG, Pena J, Tuschl T, Manoharan M, Stoffel M. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 2007;35:2885–2892. doi: 10.1093/nar/gkm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park D-H, Thum T. TGF-beta-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.234286. DOI 10.1161/ATVBAHA.111.234286. [DOI] [PubMed] [Google Scholar]
- Lagendijk AK, Goumans MJ, Burkhard SB, Bakkers J. MicroRNA-23 restricts cardiac valve formation by inhibiting Has2 and extracellular hyaluronic acid production. Circ Res. 2011;109:649–657. doi: 10.1161/CIRCRESAHA.111.247635. [DOI] [PubMed] [Google Scholar]
- Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latronico MV, Condorelli G. MicroRNAs and cardiac conduction. Curr Drug Targets. 2010;11:907–912. doi: 10.2174/138945010791591340. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. MiR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang Y, Wang N, Pan Z, Gao X, Zhang F, Zhang Y, Shan H, Luo X, Bai Y, et al. MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation. 2010;122:2378–2387. doi: 10.1161/CIRCULATIONAHA.110.958967. [DOI] [PubMed] [Google Scholar]
- Marquart TJ, Allen RM, Ory DS, Baldan A. MiR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci USA. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur K, Feng B, Wu Y, Chen S, Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60:1314–1323. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124:1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, Straarup EM, et al. Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet. 2011;43:371–378. doi: 10.1038/ng.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, van Rooij E, Olson EN. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120:3912–3916. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, Dorn GW, II, van Rooij E, Olson EN. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan GC. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- Thum T, Catalucci D, Bauersachs J. MicroRNAs: novel regulators in cardiac development and disease. Cardiovasc Res. 2008a;79:562–570. doi: 10.1093/cvr/cvn137. [DOI] [PubMed] [Google Scholar]
- Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008b;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- Thum T, Chau N, Bhat B, Gupta SK, Linsley PS, Bauersachs J, Engelhardt S. Comparison of different miR-21 inhibitor chemistries in a cardiac disease model. J Clin Invest. 2011;121:461–462. doi: 10.1172/JCI45938. author reply 462–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- van Rooij E. The art of microRNA research. Circ Res. 2011;108:219–234. doi: 10.1161/CIRCRESAHA.110.227496. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Solingen C, Seghers L, Bijkerk R, Duijs JM, Roeten MK, van Oeveren-Rietdijk AM, Baelde HJ, Monge M, Vos JB, de Boer HC, et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med. 2009;13:1577–1585. doi: 10.1111/j.1582-4934.2008.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. The concept of multiple-target anti-miRNA antisense oligonucleotide technology. Methods Mol Biol. 2011;676:51–57. doi: 10.1007/978-1-60761-863-8_4. [DOI] [PubMed] [Google Scholar]
- Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Faust SM, Rabinowitz JE. The next step in gene delivery: molecular engineering of adeno-associated virus serotypes. J Mol Cell Cardiol. 2011;50:793–802. doi: 10.1016/j.yjmcc.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Weber C, Schober A, Zernecke A. MicroRNAs in arterial remodelling, inflammation and atherosclerosis. Curr Drug Targets. 2010;11:950–956. doi: 10.2174/138945010791591377. [DOI] [PubMed] [Google Scholar]
- Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K, Kempf T, Wollert KC, Thum T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol. 2011;51:872–875. doi: 10.1016/j.yjmcc.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ago T, Zhai P, Abdellatif M, Sadoshima J. Thioredoxin 1 negatively regulates angiotensin II-induced cardiac hypertrophy through upregulation of miR-98/let-7. Circ Res. 2011;108:305–313. doi: 10.1161/CIRCRESAHA.110.228437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- Zhong X, Chung AC, Chen HY, Meng XM, Lan HY. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol. 2011;22:1668–1681. doi: 10.1681/ASN.2010111168. [DOI] [PMC free article] [PubMed] [Google Scholar]