Abstract
Nuclear factor of activated T cells (NFAT) comprises a family of transcription factors that regulate T cell development, activation and differentiation. NFAT signalling can also mediate granulocyte and dendritic cell (DC) activation, but it is unknown whether NFAT influences their development from progenitors. Here, we report a novel role for calcineurin/NFAT signalling as a negative regulator of myeloid haematopoiesis. Reconstituting lethally irradiated mice with haematopoietic stem cells expressing an NFAT-inhibitory peptide resulted in enhanced development of the myeloid compartment. Culturing bone marrow cells in media supplemented with Flt3-L in the presence of the calcineurin/NFAT inhibitor Cyclosporin A increased numbers of differentiated DC. Global gene expression analysis of untreated DC and NFAT-inhibited DC revealed differential expression of transcripts that regulate cell cycle and apoptosis. In conclusion, these results provide evidence that calcineurin/NFAT signalling negatively regulates myeloid lineage development. The finding that inhibition of NFAT enhances myeloid development provides a novel insight into understanding how the treatment with drugs targeting calcineurin/NFAT signalling influence the homeostasis of the innate immune system.
Keywords: Cyclosporin A, dendritic cell, haematopoiesis, myeloid lineage, NFAT
INTRODUCTION
Nuclear factor of activated T cells (NFAT) comprises a family of transcription factors originally described as regulators of interleukin-2 (IL-2) expression in T cells (Shaw et al, 1988). Subsequently, NFAT proteins have been shown to modulate the functions of a broad range of myeloid lineage cells including macrophages (Jennings et al, 2009; Yarilina et al, 2011), mast cells (Ulleras et al, 2008), megakaryocytes (Crist et al, 2008) and osteoclasts (Ishida et al, 2002) in addition to their profound effects on T cells. In mice, NFAT signalling is required for neutrophil-mediated resistance to infection with Candida albicans (Greenblatt et al, 2010), and our published data indicate that NFAT signalling is required for production of IL-2 by dendritic cells (DC) after bacterial encounter (Granucci et al, 2003) and can influence the early priming of natural killer (NK) cell responses (Granucci et al, 2004). DC longevity is also subject to regulation by NFAT-dependent genes, which can modulate the DC cell cycle in response to CD14-mediated lipopolysaccharide activation (Zanoni et al, 2009), leading to apoptosis of terminally differentiated DC and restricted T cell activation. These diverse functions depend on increases in intracellular calcium, which activate NFAT members 1–4 via the phosphatase calcineurin (Jain et al, 1993; McCaffrey et al, 1993), which is itself activated by the calcium-sensing protein calmodulin. Initiation of this signalling cascade permits NFAT translocation to the nucleus and induces gene transcription and leukocyte activation, thereby, driving production of the NFAT-dependent cytokines IL-2, IL-4, GM-CSF and tumour-necrosis factor (TNF)-α (Rao et al, 1997). Accordingly, mice deficient in NFAT signalling have been shown to exhibit severe immune defects (Crabtree & Olson, 2002; Horsley & Pavlath, 2002). Calcineurin/NFAT binding and NFAT translocation is efficiently inhibited by Cyclosporin A (CsA) and FK506 (Flanagan et al, 1991; McCaffrey et al, 1993), drugs broadly used in transplantation medicine and some autoimmune diseases.
In addition to potent effects on immune regulation, NFAT signalling also exerts pleiotropic effects on a variety of developmental and homeostatic processes. Hyperactivation of NFAT1 is deleterious in embryogenesis and restricts the development of lymphocytes and their progenitors (Muller et al, 2009). Calcineurin/NFAT signalling is also an important mediator of T cell selection in the thymus (Cante-Barrett et al, 2007; Gallo et al, 2008; Muller et al, 2009). NFAT2 has been shown to regulate the proliferation of stem cells in the skin by suppressing cyclin-dependent kinase 4 (CDK4) at the G0/G1 checkpoint and progression of cell cycle to S phase (Horsley et al, 2008), and NFAT1 was reported to control T cell proliferation by the same mechanism (Baksh et al, 2002). While NFAT expression in haematopoietic progenitor cells has been reported previously (Kiani et al, 2004, 2007) and appears to play a significant role in lymphopoiesis (Muller et al, 2009), it is unclear how far NFAT signalling regulates the development of other leukocyte populations particularly during myeloid differentiation.
Pluripotent haematopoietic stem cells (HSCs) give rise to common myeloid progenitors (CMP) and common lymphoid progenitors (CLP), which replenish the leukocyte populations of the blood. CMP-derived innate leukocytes are typically short-lived and do not proliferate extensively once released from bone marrow (BM). Development of these cells from their progenitors requires the orchestration of many different transcription factors and cytokines including fms-like tyrosine kinase receptor-3 ligand (Flt3-L; Naik et al, 2005), GM-, G-, M-CSF and their receptors (Sallusto & Lanzavecchia, 1994). In the steady-state, homeostasis of myeloid cells depends primarily on levels of the growth factor Flt3-L (McKenna et al, 2000), which can drive DC development through both lymphoid and myeloid differentiation pathways (Karsunky et al, 2003). Development of other myeloid lineages requires the activation of specific transcription factors such as C/EBP for the differentiation of neutrophils, Pu.1 (Sfpi1) for monocyte/macrophages and GATA-1 for erythrocytes, eosinophils and megakaryocytes (Shivdasani & Orkin, 1996; Tenen et al, 1997; Ward et al, 2000). However, it was recently shown that lineage differentiation requires multiple signals and that individual transcription factors are not definitive in lineage commitment. Indeed, Carotta et al. have demonstrated that, in addition to regulating B- and T-cell development, Pu.1 influences the differentiation of both major DC subsets: ‘conventional’ DC (cDC) and plasmacytoid (pDC) through dose-dependent effects on Flt3 expression (Carotta et al, 2010), and Pu.1 has also been implicated in osteoclast development (Crotti et al, 2008). While the role of calcineurin/NFAT signalling in the function of innate cells such as cDC and pDC (Feau et al, 2005; Granucci et al, 2001, 2004; Zanoni et al, 2009) as well as granulocytes (Greenblatt et al, 2010) is now well described, little is known about the role of this pathway in myeloid cell ontogeny.
In the current report, we have used inhibitors of calcineurin/NFAT binding CsA and VIVIT peptide (Aramburu et al, 1999) to investigate the role of NFAT in the development and homeostasis of the myeloid cell compartment. We have used genetically engineered HSC lines that express a potent inhibitory peptide of calcineurin/NFAT binding (VIVIT peptide) to attenuate NFAT signalling throughout myeloid cell development. We provide evidence that NFAT is expressed in HSC lines and is up-regulated upon Flt3-L stimulation, and that murine NFAT plays an important inhibitory role in the in vivo development of myeloid-lineage cells. These results suggest that the calcineurin/NFAT pathway is a potent negative regulator of myeloid cell development.
RESULTS
NFAT1 expression is up-regulated upon Flt3-L stimulation of haematopoietic stem cells lines
In order to achieve long-term, stable inhibition of calcineurin/NFAT signalling, we introduced the VIVIT peptide (Aramburu et al, 1999) into established, long-term haematopoietic ‘stem cell-like’ cell lines. These HSC lines were derived from BM cells transduced with constructs encoding the Nucleoporin 98 (NUP)-HOXB4 fusion protein (Ruedl et al, 2008; Sauvageau et al, 1995). We have previously shown that NUP-HOXB4 transduction of BM cells results in long-term stem cell-like lines, which can be further transduced and cultured for extended periods of time (Ruedl et al, 2008). HSC lines have all of the key characteristics of their in vivo HSC counterparts; they reconstitute haematopoiesis when transplanted into an irradiated host, they can be serially transplanted for many generations, they support self-renewal and generation of progenitor cells, and they can differentiate into DC in vitro in the presence of GM-CSF or Flt3-L (Ruedl et al, 2008) In the current study, HSC lines were transduced with constructs expressing either VIVIT-IRES-eGFP or control-IRES-tdTomato, and fluorescent cells were sorted for further analyses.
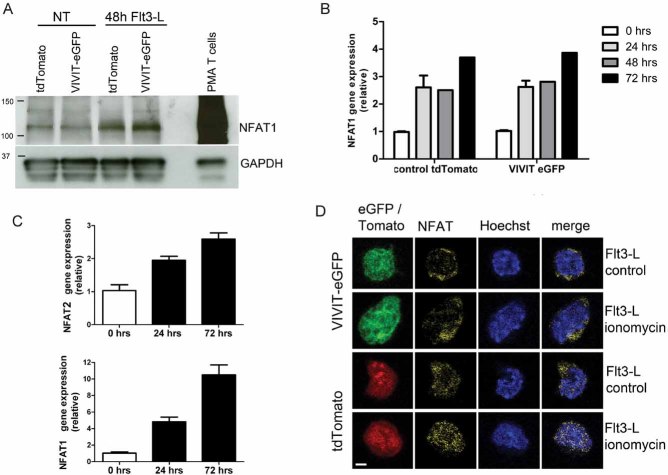
Given that NFAT1 is expressed in stem cells more strongly than other NFAT proteins (Kiani et al, 2004) and that Flt3-L is a key growth factor in early myeloid development, we initially analysed total expression of NFAT1 protein in control-tdTomato and VIVIT-eGFP expressing HSC, both in the steady-state and in response to Flt3-L stimulation, to determine whether Flt3-L influences NFAT expression. By Western blot analysis, NFAT1 expression could be detected in untreated HSC lines, and stimulation with Flt3-L for 48 h moderately up-regulated expression levels (Fig 1A). Notably, NFAT1 expression was far lower than was observed in PMA-stimulated T cells (Fig 1A; positive control) and in un-stimulated T cells (unpublished observations). Up-regulation of NFAT1 protein in response to Flt3-L was also detected at the mRNA level (Fig 1B). In further experiments, it was possible to demonstrate enhanced expression of NFAT1 and NFAT2 mRNA in BM cells treated with Flt3-L. Sorted lineage negative BM cells (lin−; CD3e−, B220−, CD19− CD11b−, Gr-1−, TER-119−, NK1.1− and CD127−) were used to exclude any contribution from differentiated cells and lymphoid progenitors (Fig 1C).
Figure 1. NFAT1 is expressed in HSC lines and increases following stimulation with Flt3-L.
- Western blot analysis of un-stimulated and Flt3-L stimulated VIVIT expressing and control HSC lines. PMA treated splenic T cells were used as a positive control.
- qPCR analysis of NFAT1 mRNA expression in HSC lines after stimulation with Flt3-L.
- qPCR analysis of NFAT2 and NFAT1 expression in freshly isolated lineage (lin−) negative cells from BM stimulated with Flt3-L.
- Confocal images of VIVIT-eGFP and control-tdTomato expressing HSC lines stimulated for 48 h with Flt3-L. NFAT translocation was induced by treatment with ionomycin for 2 h (original magnification, ×200, scale bar 2 µm). Data are representative of three (A and C) and five (B) independent experiments (mean ± SEM). Three mice per group for each time-point were used (C).
To confirm the inhibition of calcineurin/NFAT pathway in VIVIT-transduced cells, we stimulated VIVIT-eGFP and control-tdTomato expressing HSCs with Flt3-L for 48 h and induced NFAT translocation with ionomycin. While NFAT1 was partially present in the cytoplasm and nuclei of both VIVIT-eGFP and control tdTomato cells, increased translocation of NFAT1 was only detected in ionomycin-treated tdTomato cells (Fig 1D and Fig S1A and B of Supporting Information). Portion of NFAT signal in nuclei was quantified (Fig S1B of Supporting Information). Thus, VIVIT efficiently inhibits calcineurin/NFAT translocation in Flt3-L stimulated HSCs.
Taken together, these data confirm the expression of NFAT1 in HSC lines, as well as in lineage negative BM cells, and demonstrate impairment of NFAT translocation by over-expression of VIVIT peptide. We also detected up-regulation of NFAT1 mRNA and protein upon Flt3-L stimulation, suggesting that NFAT might exhibit Flt3-L-dependent roles in haematopoiesis. In subsequent experiments, we focused on the role played by calcineurin/NFAT signalling in the in vivo development of myeloid cells under non-inflammatory conditions.
Impaired calcineurin/NFAT signalling in haematopoietic stem cells preferentially expands myeloid cells in vivo
HSC lines were transplanted into irradiated mice to model the normal development of immunity and establishment of immune homeostasis. This system supports typical onset of haematopoiesis and representative differentiation of lineages (Ruedl et al, 2008). Mice were lethally irradiated (9Gy) and intravenously injected with 2–4 × 106 VIVIT-eGFP expressing HSC or control tdTomato cells. Haematopoietic reconstitution was analysed 8 weeks after injection of HSC and increased proportions of both BM and splenic DC (CD11c+MHCII+) as well as splenic monocytes (CD11b+Gr-1−) were detected in these mice (Fig S2A and B of Supporting Information). In contrast, the proportion of VIVIT-eGFP-expressing splenic CD3+ T cells present was moderately decreased compared with tdTomato controls. These data indicate that the level of calcineurin/NFAT signalling inhibition achieved in our model did not abolish T cell development, but rather enhanced the development of myeloid cells. To confirm inhibition of calcineurin/NFAT signalling in VIVIT-expressing cells, we next purified splenic CD11c+ DC from the VIVIT-eGFP and tdTomato-control reconstituted mice. Decreased IL-2 protein and mRNA levels were observed in splenic DC 24 h after lipopolysaccharide or curdlan treatment, consistent with inhibition of calcineurin/NFAT signalling in VIVIT-expressing DC (Fig S3A and B of Supporting Information). Comparable inhibition could also be achieved after curdlan stimulation of DC derived from HSC lines in Flt3-L supplemented medium in vitro (Fig S3C of Supporting Information).
To extend these observations, we performed competitive reconstitution of irradiated hosts using mixed HSC lines in a 1:1 ratio; VIVIT-eGFP expressing HSCs as competitors to control tdTomato HSC lines. The ratio of VIVIT-eGFP to control-tdTomato derived granulocytes (CD11b+Gr1+), monocytes (CD11b+Gr1−), cDC (CD11c+MHCII+) and pDC (CD11c+B220+) was analysed in BM, spleen, lymph nodes and peripheral blood 8 weeks after engraftment. We observed a selective advantage for calcineurin/NFAT impaired cells in the repopulation of pDC, cDC, granulocytic and monocytic compartments throughout the BM, spleen, lymph nodes and peripheral blood (shown as log ratio of engrafted NFAT-impaired competitors to controls, Fig 2A). Critically, the relative proportions of lymphoid T and B cells remained unchanged and simply reflected the ratio of injected HSC (Fig 2A), indicating comparable engraftment of both HSC lines and confirming that lymphopoiesis is not impaired by this level of calcineurin/NFAT inhibition. The unaffected ratio of lymphocytes indicates that self-renewal and maintenance of stem cell progenitors is not affected by impaired NFAT signalling.
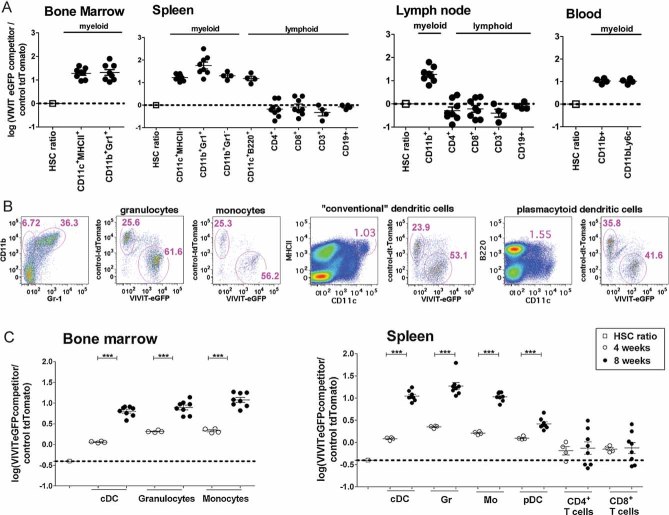
Figure 2. Calcineurin/NFAT inhibition in HSC confers a growth advantage to myeloid cells.
Host mice were lethally irradiated and rescued by intravenous injection of HSC. HSC populations comprised a mixture of cells expressing either VIVIT or a control plasmid with a fluorescent tag in the indicated ratios.
- Mice were reconstituted with a mixture of VIVIT-eGFP and control tdTomato HSC in a 1:1 ratio and analysed 8 weeks later. Subsets of DC (CD11c+MHCII+), granulocytes (CD11b+Gr-1+) and monocytes (CD11b+Gr1−) were gated. The ratio between VIVIT-eGFP and control tdTomato cells in each lineage is plotted as the logarithm of the percentage of competitor VIVIT-eGFP cells, divided by percentage of control tdTomato cells (value 0 reflects 1:1 ratio), for BM, spleen, lymph nodes and blood. Initial ratio of HSC used for reconstitution is shown.
- Example of flow analysis of the ratio between VIVIT-eGFP and control tdTomato granulocytes, monocytes, cDC and pDC 4 weeks after reconstitution with mixture of HSC of 30% VIVIT-eGFP and 70% tdTomato control expressing HSC lines.
- Analysis of kinetic of reconstitution in mice injected with a mixture of 30% VIVIT-eGFP and 70% tdTomato control expressing HSC lines. Mice were analysed 4 and 8 weeks after reconstitution (value −0.36 reflects the injected 30:70 ratio). Granulocytes, monocytes and DC were analysed in BM and spleen, CD4+ and CD8+ T cells were analysed in spleen alone. Data (A) are summarized from two of three experiments with similar results (n = 8; mean ± SEM), (C) data are from one of 3 experiments (n = 4 for 4 weeks and n = 8 for 8 weeks time point). Changes in the ratios between two-time point have been analysed by Student t-test ***p ≤ 0.001.
To define the kinetics and efficiency of this specific myeloid repopulation advantage, we analysed reconstitution of haematopoiesis at two different time points post-engraftment of NFAT-impaired cells in the presence of excess control-Tomato HSC (30% VIVIT-eGFP competitors; 70% tdTomato control cells). Four weeks after transplantation (gating strategy shown in Fig 2B), the ratio of NFAT impaired granulocytes, monocytes, cDC and pDC starts to increase and continue to increase in time-dependent manner. This clearly indicates that impaired NFAT signalling did not influence HSC engraftment. Indeed, the ratio of VIVIT-expressing and control myeloid cells analysed after 4 weeks approximates the ratio of injected HSC. The myeloid repopulation advantage of NFAT-impaired HSC was sustained up to week 8 post-infusion, with expansion of both pDC and myeloid lineages increasing over time (Fig 2C). To confirm these findings, we repeated these experiments using independently transduced HSC lines. tdTomato-expressing HSC were transduced with VIVIT peptide and eGFP-expressing cells were used as controls. Lethally irradiated mice were then injected with the mixed HSC lines comprising 30% VIVIT-tdTomato competitors and 70% control-eGFP cells. Analysis of cell ratios 4 weeks after reconstitution clearly showed that engraftment and early onset of myeloid haematopoiesis occurred in the same ratio as the injected HSC. After 8 weeks, we observed preferential growth of granulocytes and DC in the calcineurin/NFAT-impaired condition (Fig S4 of Supporting Information).
In further studies, these data were confirmed using freshly transduced primary BM cells to replicate the results obtained with HSC lines. Freshly isolated BM cells were transduced with either VIVIT-eGFP or control tdTomato constructs before the cells were sorted and then injected into irradiated hosts in the ratio 30% VIVIT-eGFP competitors to 70% control tdTomato cells. Under these conditions using primary cells and excluding any possible interference of NUP-HOXB4 constructs, we still observed a preferential expansion of calcineurin/NFAT-impaired myeloid cells over controls. The ratio of granulocytes, DC and T cells was analysed at weeks 4–8 and 12 weeks post-engraftment. The competitive advantage of VIVIT-expressing myeloid cells over the mock-transduced controls was still evident (Fig 3).
Figure 3. Mouse reconstitution with primary BM cells expressing VIVIT recapitulates myeloid cell growth advantage.
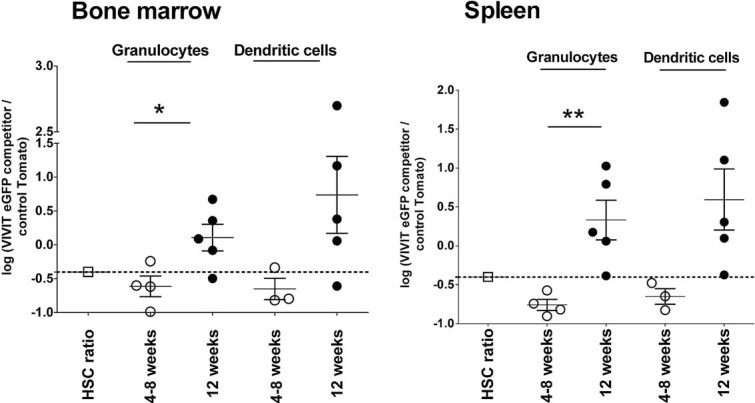
Freshly isolated BM cells were transduced with VIVIT-eGFP or tdTomato control, and used for reconstitution of lethally irradiated mice. The proportions of engrafted BM cells were 30% VIVIT-eGFP expressing and 70% control Tomato. Changes in the original ratio in BM and spleen were analysed 4, 8 and 12 weeks after reconstitution (n = 4–5). The initial ratio of HSC used for reconstitution is shown. The ratio between VIVIT-eGFP and control tdTomato cells in each lineage is plotted as the logarithm of the percentage of competitor VIVIT-eGFP cells, divided by percentage of control tdTomato cells (value 0.36 reflects 30:70 ratio). Data show one representative experiment of three. Changes in the ratios between two-time points have been analysed by Student t-test *p ≤ 0.05.
Taken together, these data indicate that inhibition of calcineurin/NFAT signalling in stem cells favours expansion of the myeloid cell compartment during reconstitution of the haematopoietic system. This observation was consistent across a variety of experimental conditions using different combinations of HSC lines as well as in freshly isolated and transduced BM cells.
Inhibition of calcineurin/NFAT signalling increases the number of myeloid progenitors in bone marrow and myeloid colony growth
To better understand the preference for in vivo expansion of myeloid cells, we analysed myeloid CFU obtained from reconstituted mice to determine the differentiation stage at which preferential expansion of VIVIT-expressing cells occurred. BM cells from reconstituted mice were cultured in methylcellulose media containing SCF, IL-3 and IL-6. Numbers of granulocytic and monocytic colony-forming units – granulocyte, macrophage (CFU-GM), corresponding to numbers of myeloid progenitors in BM, were determined after 4–7 days of culture (Fig 4A). Figure 4B shows numbers of CFU-GM from mice reconstituted with 30% VIVIT-eGFP competitors mixed with 70% control-tdTomato HSC and analysed 4 and 8 weeks later. Numbers of VIVIT-eGFP myeloid progenitors were significantly enriched over time compared with controls, consistent with the pattern of reconstitution achieved with NFAT-impaired myeloid cells in previous experiments (Fig 2B and C). After CFU-GM enumeration the colonies were harvested and the total number of CD11b+ cells was determined by flow cytometry. Figure 4C depicts average cell number per colony, which indicates significantly faster growth among VIVIT-eGFP expressing colonies. In mice singly engrafted with either VIVIT-eGFP or tdTomato control HSC alone, the respective progenitor populations remained detectable in BM for 12 weeks (unpublished observation).
Figure 4. Impaired calcineurin/NFAT signalling increases the number and proliferation of myeloid progenitors.
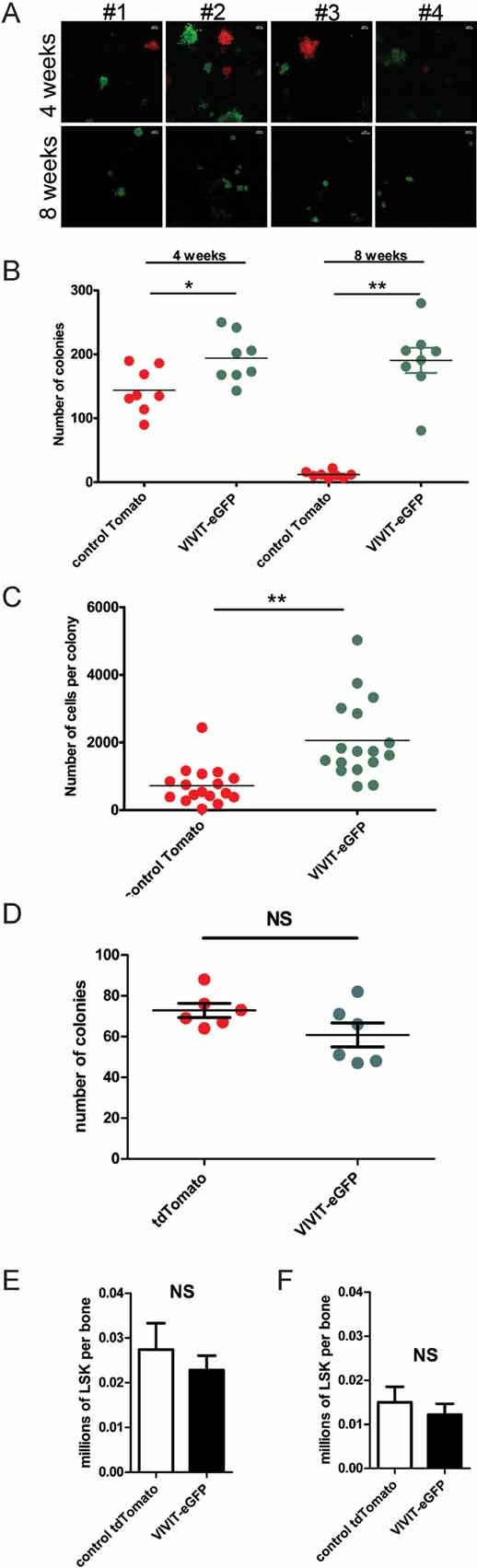
- CFU in methyl cellulose from BM of mice reconstituted with a mixture of VIVIT-eGFP expressing and control-tdTomato expressing HSC, injected in the proportions of 30% VIVIT-eGFP to 70% tdTomato control, the methylcellulose cultures were set 4 weeks (upper panel) and 8 weeks (lower panel) after reconstitution and counted after 4–7 days of culture. Images of colonies from eight individual representative mice are shown as example (original magnification, ×10, scale bar 200 µm).
- Numbers of VIVIT-eGFP and control tdTomato myeloid colonies (CFU) generated from the BM of mice 4 and 8 weeks after reconstitution with a mixture of HSC consisting of 30% VIVIT-eGFP and 70% tdTomato control expressing cells.
- Analysis of average cell numbers per colony. After the ratio between VIVIT-eGFP and control tdTomato colonies was determined, cultures were harvested and labelled with antibodies against CD11b+. Differentiated cells (over 90% of total) were counted by flow cytometry with four replicates per mouse. Average number of cells per colony is plotted. Data are summarized from three (B and C) independent experiments with at least three mice per group in each experiment.
- Numbers of VIVIT-eGFP and control tdTomato myeloid colonies (CFU) generated from HSC lines used for reconstitution of mice.
- Total numbers of LSK (lin−, Sca-1+ and c-Kit+) in BM of mice reconstituted with control tdTomato or VIVIT-eGFP HSC.
- Total numbers of VIVIT-eGFP and Tomato control LSK (lin−, Sca-1+ and c-Kit+) in mice reconstituted with mixture of control tdTomato or VIVITeGFP HSC in initial ratio 1:1. (E and F) Analysis was performed 6 weeks after reconstitution with five mice per group. Changes in total numbers of DCs, in IFN-β production and changes in percentage of proliferating cells have been analysed by Student t-test *p ≤ 0.05 and ***p ≤ 0.001.
In order to investigate the number of myeloid progenitors in HSC lines, we plated the same amount of control tdTomato or VIVIT-eGFP HSC lines in methylcellulose. Figure 4D shows that similar numbers of colonies were produced in both lines. Furthermore, to dissect the cell subsets, which are influenced by impaired NFAT signalling, we have analysed the total numbers of LSK (Lin−, Sca-1+ and c-Kit+) progenitors in BM of reconstituted mice 6 weeks after engraftment. Figure 4E shows the total number of LSK per femur in control or VIVIT-GFP reconstituted mice is similar (Fig 4E). In addition, we show that the total number per femur of control and VIVIT-eGFP progenitors in mice reconstituted with a 1:1 ratio of both HSC types is similar even in competitive experimental setting (Fig 4F).
Together with the in vivo data, these results indicate that calcineurin/NFAT signalling negatively regulates the total number of myeloid progenitors and their expansion during the growth of myeloid colonies. Moreover, the results confirm that both control and VIVIT-eGFP HSC lines contain similar numbers of myeloid progenitors before engraftment and also that the total number of short-term progenitors is not influenced by the expression of the VIVIT peptide.
Calcineurin/NFAT signalling antagonizes Flt3-L-driven development of bone marrow-derived dendritic cells
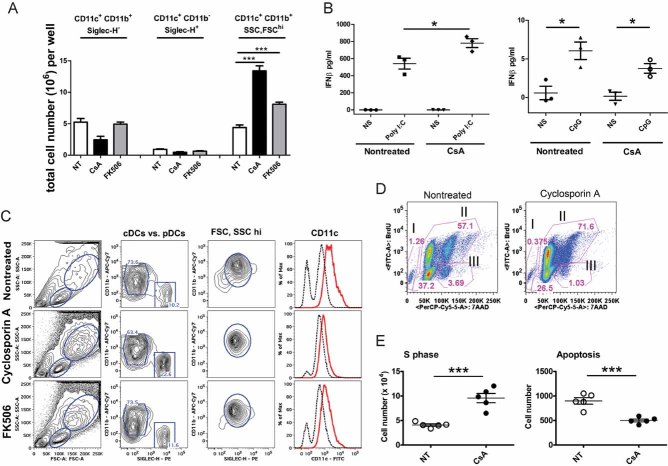
To clarify the processes by which myeloid cell enrichment occurs following impairment of calcineurin/NFAT signalling, we studied BM-DC differentiation in vitro in medium supplemented with Flt3-L (200 ng/ml) in the presence or absence of CsA (2 µg/ml) or FK506 (0.2 ug/ml). To achieve efficient inhibition of calcineurin/NFAT signalling, inhibitors were added to the cells 30 min prior to Flt3-L administration and were maintained throughout the 9–11 days culture period, at which time cell subset frequency and total number were analysed by flow cytometry. Part of the generated DC exhibited a pDC phenotype and expressed Siglec-H, B220 and PDCA-1 on surface of CD11c+ cells, also cDC were present in the culture as CD11c+CD11b+ cells. The addition of CsA or FK506 to the cultures induced significantly higher numbers of CD11c+ DC with higher SSC and FSC, while other DC subsets were not affected by treatment (Fig 5A and C). Both controls and CsA-inhibited cultures gave rise to DC that are able to produce interferon (IFN)-β, detected 18 h after stimulation with Poly I:C or CpG (Fig 5B). The basis of this DC population increase was investigated using BrdU incorporation to determine proliferation and apoptosis levels in the cultures. DC were pulsed with BrdU for the last 72 h of culture before analysis by flow cytometry (Fig 5D). DC treated with CsA included higher numbers of proliferating cells in the S phase, but decreased apoptosis (Fig 5E). Data from the in vitro Flt3-L/CsA-treated BM-DC cultures confirm our in vivo findings for DC expansion. Since we observed a strong impact of calcineurin/NFAT impairment on Flt3-L-derived DC development, we further investigated the possible mechanism of action by global gene transcription analysis.
Figure 5. Calcineurin/NFAT inhibition enhances the development of DCs in FLT3-L derived BM cultures.
BM derived DC cultivated in the presence of Flt3-L were harvested on day 11 of culture and labelled with antibodies against CD11c, Siglec-H and CD11b.
- Total numbers of cDC (CD11c+CD11b+Siglec-H−), pDCs (CD11c+CD11b−Siglec-H+) and SSC, FCShi CD11c+ DCs in FLT3-L derived BM-DC cultures with and without CsA and FK506. Representative of of five experiment (n = 6; mean ± SEM). Changes in the cell number were analysed by Student t-test, ***p ≤ 0.001.
- IFN-β production in FLT3-L derived DC grown in presence or absence of CsA. Protein levels IFN-β in supernatants were analysed 18 h after Poly I:C or CpG stimulation. Differences in IFN-β were analysed by Student t-test, *p ≤ 0.05 (mean ± SEM).
- Percentage of cDC (CD11c+CD11b+Siglec-H−), pDCs (CD11c+CD11b−Siglec-H+) and SSC, FCShi CD11c+ cells in FLT3-L derived BM-DC cultures with and without CsA and FK506. Example of flow analysis of experiment presented in (A).
- Representative example of changes in apoptosis (gate I), proliferation (gate II for S phase) and M + G2 phase (gate III), measured by BrdU incorporation in CsA-treated cells (right) compared to not-treated controls (left).
- Analysis of cell cycle and proliferation. Non-treated and CsA DC cultures were pulsed with BrdU for the last 72 h of culture. The total number of cells in S phases (left) and undergoing apoptosis (right) is presented. Data are representative of three experiments (n = 3–5; mean ± SEM). Changes in the cell number were analysed by Student t-test, ***p ≤ 0.001.
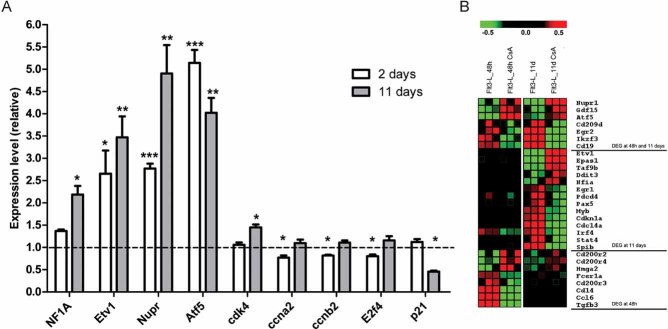
Microarray analysis of gene expression in BM cells was performed at 12, 24 and 48 h after Flt3-L stimulation, and in fully differentiated Flt3-L-treated DC cultivated in the presence or absence of CsA. Differential gene expression in CsA-treated BM cells began 48 h after stimulation (Fig S5A of Supporting Information). At this time point, 35 transcripts were up-regulated and 131 transcripts were decreased in Flt3-L-stimulated BM cells treated with CsA. On day 11 of the culture, fully differentiated Flt3-L/CsA-treated DC displayed 110 up-regulated transcripts and 571 down-regulated transcripts compared with untreated controls (Fig S5B of Supporting Information). Ingenuity Systems Pathway Analysis of these transcripts showed enrichment for genes involved in ‘Haematological System Development and Function’, and ‘Cellular Growth and Proliferation’ (Fig S5C of Supporting Information). Some of the differentially expressed genes in these subsets encode known crucial regulators of myeloid development and cell cycle (Fig 6A and B and Table 1). Genes important in DC development (Ikzf3, Spib and Stat4) were down-regulated, while genes responsible for cell cycle progression (Etv1, Epas1, Nupr1, Nfia and Atf5) were up-regulated. Critically, down-regulated transcription of the cell cycle repressor Cdkn1a (p21) was also observed (Fig 6A and Table 1), and was confirmed by qPCR (Fig 6A). Furthermore, increased levels of Cdk4 mRNA at 11 days time-point were detected in CsA treated cells (Fig 6A).
Figure 6. Calcineurin/NFAT inhibition during in vitro culture of Flt3-L derived DC leads to changes in expression of genes regulating cell cycle and differentiation.
- qPCR validation of selected DEG identified after 48 h and 11 days of differentiation of DC in Flt3-L-supplemented medium with or without CsA. Results represent triplicate measurements from three biological replicates (mean ± SEM); *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.
- Heat map of selected genes differentially expressed in BM cells after 48 h and 11 days stimulation with Flt3-L, in the presence or absence of CsA. Three biological replicates (n = 3) are shown for each condition and time point.
Table 1.
Microarray analysis of the expression of genes encoding proteins (in parentheses) involved in the biology of cells of the immune system and blood, presented as ‘fold change’ in CsA treated BM cultures relative to untreated controls, p ≤ 0.05
| A. Changes in gene expression in DC lineaged in presence of CsA (11 days) | ||
|---|---|---|
| Up-regulation | ||
| Etv1 | (Ets variant gene 1) | 1.33 |
| Epas1 | (Endothelial PAS domain protein 1) | 1.26 |
| Nupr1 | (Nuclear protein 1) | 1.13 |
| Taf9b | (TAF9B RNA polymerase II, TATA box binding protein (TBP)-associated factor) | 0.72 |
| Ddit3 | (DNA-damage inducible transcript 3) | 0.66 |
| Nfia | (Nuclear factor I/A) | 0.64 |
| Atf5 | (Activating transcription factor 5) | 0.60 |
| Down-regulation | ||
| Egr1 | (Early growth response 1) | −0.59 |
| Pdcd4 | (Programmed cell death 4) | −0.61 |
| Pax5 | (Paired box gene 5) | −0.63 |
| Myb | (Myeloblastosis oncogene) | −0.65 |
| Cdkn1a | (Cyclin-dependent kinase inhibitor 1A (P21)) | −0.68 |
| Cdc14a | (CDC14 cell division cycle 14 homolog A) | −0.91 |
| Irf4 | (Interferon regulatory factor 4) | −0.94 |
| Egr2 | (Early growth response 2) | −1.00 |
| Stat4 | (signal transducer and activator of transcription 4) | −1.00 |
| Naip3 | (NLR family, apoptosis inhibitory protein 3) | −1.01 |
| Ikzf3 | (IKAROS family zinc finger 3) | −1.48 |
| Spib | (Spi-B transcription factor (Spi-1/PU.1 related)) | −1.59 |
| B. Changes in gene expression in DC lineaged in presence of CsA (48 h) | ||
|---|---|---|
| Up-regulation | ||
| Gdf15 | (Growth differentiation factor 15) | 0.67 |
| Cd200r2 | (CD200 receptor 2) | 0.67 |
| Cd200r4 | (CD200 receptor 4) | 0.64 |
| Hmga2 | (High mobility group AT-hook 2) | 0.63 |
| Nupr1 | (Nuclear protein 1) | 0.61 |
| Down-regulation | ||
| Fcer1a | (Fc receptor, IgE, high affinity I, alpha polypeptide) | −0.59 |
| Cd209d | (CD209d antigen) | −0.59 |
| Egr2 | (Early growth response 2) | −0.59 |
| Cd19 | (CD19 antigen) | −0.62 |
| Cd200r3 | (CD200 receptor 3) | −0.64 |
| Ikzf3 | (IKAROS family zinc finger 3) | −0.64 |
| Ccl6 | (Chemokine (C–C motif) ligand 6) | −0.85 |
| Cd14 | (CD14 antigen) | −0.85 |
| Tgfb3 | (Transforming growth factor, beta 3) | −1.19 |
Data represent three biological replicates.
These differentially expressed genes in CsA-treated Flt3-L-supplemented cultures clarified the mechanism of action for NFAT inhibitors during pDC development: preferential expansion of the myeloid compartment, as shown in vivo, is due to the interaction of NFAT with cell cycle regulation genes, where NFAT acts as a suppressor. NFAT impairment, therefore, allows cells to proliferate faster and results in an expanded myeloid compartment both in vivo and in vitro.
DISCUSSION
In the current study, we report that repression of calcineurin/NFAT signalling drives enhanced development of the myeloid compartment in mice. The calcineurin/NFAT pathway acts as a negative regulator of myeloid development and contributes to the maintenance of innate immune homeostasis.
NFAT1-4 expression has previously been detected in human CD34+ cells mobilized with G-CSF and in particular, NFAT1 was present up to day 2 of culture in differentiating medium but decreased from day 4 onwards (Kiani et al, 2007). This substantiates our findings that NFAT1 was present in differentiating HSC after 2 days of culture in Flt3-L supplemented medium. An advantage of the HSC lines used in our study is the ability to expand and differentiate these cells with progenitor properties in steady-state conditions in vitro or directly in vivo, as no cytokine mobilization or sorting of progenitors is required to obtain the primary HCS. Other studies support the importance of NFAT in the homeostasis and function of myeloid cells including fully differentiated DC, neutrophils and megakaryocytes (Greenblatt et al, 2010; Kiani et al, 2007; Zanoni et al, 2009). Here, we provide evidence for the expression of NFAT1 in HSC lines and also in lineage negative progenitors from BM. Low, steady-state expression of NFAT1 was followed by up-regulation of the protein upon Flt3-L stimulation, suggesting a critical role for NFAT signalling in early lineage commitment. Balanced levels of intracellular calcium – an upstream signal for NFAT translocation – have already been reported as a key factor in haematopoiesis (Muller et al, 2009).
We investigated the role of NFAT signalling in myeloid lineage ontogeny using Nup98HoxB4-expressing HSC lines (Ruedl et al, 2008; Sauvageau et al, 1995) co-transduced with VIVIT, a peptide inhibitor of NFAT (Aramburu et al, 1999). VIVIT-expressing and control HSC lines fully reconstituted the immune system when injected into irradiated hosts. Moderate enrichment of the myeloid compartment in BM, spleen was consistently observed in mice reconstituted NFAT-impaired HSC line comparing to control HSC line. Interestingly, when the mice were engrafted with a mixture of both NFAT impaired and control HSC, 8 weeks later the vast majority of myeloid cells consisted from VIVIT expressing HSC line. The weak effect of NFAT inhibition after reconstitution with single type HSC was probably due to stringent control of total myeloid cell numbers. In contrast, VIVIT provided a significant advantage when HSC impaired in NFAT signalling were engrafted in combination with control cells. The competitive reconstitution approach allowed us to observe the function of modulated NFAT signalling under more physiological conditions, since a portion of control unimpaired cells is present to maintain the cytokine environment. This may better reflect in vivo mechanisms of homeostasis. In mice reconstituted with both HSC lines in competitive setting, VIVIT-expressing myeloid cells and pDC progressively became the dominant population in all primary and secondary lymphoid organs over time. Interestingly, the level of calcineurin/NFAT inhibition achieved by VIVIT expression did not influence the development of T cells: while calcium/NFAT signalling is important for T cell selection in the thymus (Muller et al, 2009), the much higher levels of NFAT signalling present in T cells compared to myeloid cells was unlikely to be significantly impaired by our experimental approach. In order to exclude the effect of NUP-HOXB4 constructs present in HCS lines, we have confirmed the experimental data using freshly isolated, transduced BM cells.
Gallo et al. reported that calcineurin/NFAT signalling was dispensable, without any significant role for the development of granulocytes and DC in mice lacking the calcineurin subunit B1 (CNB1; Gallo et al, 2008) and, moreover, NFAT signalling has not been observed as leading to the inhibition of myeloid development in NFAT knockouts. Nevertheless, a very recent study suggested a role of NFAT1 in the maintenance of haematopoiesis in aged NFAT1 knockout mice (Bauer et al, 2011). In the present study, we have shown that NFAT is a negative regulator of myeloid lineage development as revealed by partial inhibition of calcineurin/NFAT signalling. The level of NFAT inhibition achieved by VIVIT peptide did not lead to impairment of T and B cell development as seen in the inducible CNB1 knockout (Gallo et al, 2008). Indeed we demonstrated that NFAT1 is expressed at an early stage of differentiation to favour Flt3-L-driven DCs development in vitro. This finding may explain the increased numbers of in vivo myeloid progenitors and pDC detected since HSC as well as CMP express Flt-3 receptor (Karsunky et al, 2003). VIVIT expression in progenitors resulted in increased numbers of granulocytes, monocytes and both cDC and pDC, indicating that the calcineurin/NFAT pathway is important for developmental processes shared among these cell types, or directly influences their common progenitors. The observed co-regulation of pDC development with cells comprising the myeloid compartment may be explained by pleiotropy of the transcription factors which act in early lineage commitment.
Steady-state development of myeloid cells is strongly dependent on Flt3-L levels and receptor expression (McKenna et al, 2000), while under inflammatory conditions, GM-CSF, G-CSF and M-CSF play increasingly important roles. The receptor for Flt3-L is expressed on DC, and common DC progenitors are Flt3-dependent. Thus Flt-3 plays a non-redundant role in steady-state development of all DC subsets (Naik et al, 2007; Onai et al, 2007). Clearly, the development of cDC and pDC is critically dependent on levels of Flt3-L (Karsunky et al, 2003; McKenna et al, 2000), but Flt3 is also expressed at a variable levels by HSC (D'Amico & Wu, 2003; Karsunky et al, 2003). This may explain why impaired NFAT signalling influences not only the entire myeloid compartment, but also pDC, during haematopoietic reconstitution. In previous studies, inhibition of STAT3 (a transcription factor activated by Flt3 signalling) decreased DC numbers in vivo (Laouar & Welte, 2003; McKenna et al, 2000; Onai et al, 2006) and Flt3-L stimulation gave rise to pDC as well as cDC (Shortman & Naik, 2007). Our experiments suggest the role of NFAT during cell development is similarly dependent on Flt3-L stimulation. In the steady state, Flt3 has also been reported to influence the expansion and maturation of neutrophils from CD34+ progenitors (Tura et al, 2007). In the current study, we prepared BM-DC in vitro in the presence of Flt3-L, and DC in these cultures differentiated mainly into cDC (CD11c+CD11b+), pDC (CD11c+B220+, Siglec-H+, PDCL-1+) and FSC,SSChi CD11c+ DCs after 9–11 days of culture. To test the role of calcineurin/NFAT signalling during the development of DC, we blocked NFAT translocation using CsA and FK506. Surprisingly, we found that CsA enhanced the development of DCs of FSC,SSChi CD11c+ phenotype, which were able to produce IFN-β in response to Poly I:C or CpG stimulation.
The developmental advantage provided by VIVIT peptide-expression was evident in all major subsets of myeloid cells, including monocytes, neutrophils, cDC and pDC, hence we may postulate that calcineurin/NFAT plays a role in regulating early progenitors of myeloid cells. The kinetics of enhanced myeloid differentiation indicate that calcineurin/NFAT signalling influences long-term maintenance of homeostasis, but clearly does not influence the engraftment itself. This was confirmed by testing myeloid colony frequency in BM from reconstituted mice. These data suggested that the competitive advantage of VIVIT-expressing HSC is already active in myeloid progenitors, as shown by the increased number of myeloid colonies. Interestingly, we also observed that inhibition of calcineurin/NFAT results in higher proliferation of progenitors after lineage commitment, since VIVIT-expressing colonies contained higher numbers of cells. In addition we have shown that impaired calcineurin/NFAT signalling does not influence the levels of LSK, and hence specifically regulates myelopoiesis. This corroborates with an earlier report that human CD34+ HSC differentiating into neutrophils show increased proliferation while NFAT1 is down-regulated (Kiani et al, 2007), thus myeloid cells proliferate more as the role of NFAT diminishes. In the skin, proliferation of hair follicle stem cells is also associated with suppression of NFAT signalling (Horsley et al, 2008) again suggesting a regulatory role for NFAT in early progenitors.
Innate leukocytes are key players in the immediate immune response to pathogens and are also important regulators of immune homeostasis. Since they are often short-lived cells that do not usually divide after lineage commitment, differentiation of these populations from multipotent stem cells in the BM is the key regulatory checkpoint for homeostasis of these leukocytes. Accordingly, this process is controlled by a complex interplay of various different transcription factors (Geissmann et al, 2010). Whether NFAT can directly modulate transcription factors involved in myeloid haematopoiesis was previously unknown, but our data provide novel evidence that NFAT can influence lineage commitment and proliferation of myeloid progenitors. Indeed, we have shown that CsA inhibition during BM-DC development enhances DC growth, resulting in higher numbers of DC in culture with Flt3-L. Indeed, our microarray data identified differential expression of genes involved in cell cycle regulation upon CsA treatment (supported by analyses of BrdU incorporation) confirming that inhibition of calcineurin/NFAT signalling enhances the growth of myeloid cells and their progenitors.
The paper explained
PROBLEM
Homeostasis of myeloid cells is a key factor for maintenance of innate immune responses. Myeloid leukocytes provide the first line of immune protection by recognition and elimination of pathogens. Regulation of homeostasis as well as fast and continuous self-renewal of these short-lived cells from BM progenitors is a complex process regulated by many factors. The role of NFAT signalling in T cell differentiation and function has been extensively described. In contrast, its role in myeloid differentiation is unknown and only recently, a new function of NFAT regulating the fate of dendritic cells (DCs) has been described by us (Nature, p. 264–268, 460, 2009). Here, we studied the possible role of NFAT during the development of innate immune cells in vivo.
RESULTS
NFAT expression is increased after stimulation of myeloid progenitors with the grow factor Flt3-L. Such activation leads to the differentiation of myeloid progenitors into DCs. Here, we show that specific inhibition of NFAT signalling during the myeloid differentiation of BM progenitors in vivo results in preferential expansion of myeloid cells over control cells. We also show an increased expansion of myeloid progenitor colonies in semi-solid methylcellulose. Gene expression analysis of cells impaired in NFAT signalling and untreated controls clearly showed that the inhibition of NFAT signalling interferes with cell cycle control, indicating that NFAT acts as a negative regulator of myeloid cells development.
IMPACT
Our data provide the first evidence of the new role of calcineurin/NFAT signalling during the renewal of myeloid cells in steady-state conditions, highlighting a new function of NFAT as a negative regulator of myeloid development. These findings will certainly impact the field of immune suppression since immunosuppressive drugs such as Cyclosporin A specifically target the calcineurin/NFAT pathway inhibiting T cells functions. Here, we show an effect in the regulation of myeloid cells homeostasis.
As p21 is the main inhibitor of cellular progression through the cell cycle, down-regulation of p21 in conjunction with up-regulation of other genes (notably Cdk4, Etv1, Nupr1, Nfia and Atf5), which we have validated by qPCR, could drive proliferation of CsA-treated cells. Intriguingly, it has already been reported that NFAT is an important regulator of cyclins which drive the cell cycle during lymphocyte activation (Baksh et al, 2002; Caetano et al, 2002). Moreover, the critical role of p21, which was down-regulated by CsA in our experiments, has been reported in homeostatic regulation of stem cells quiescence through governing the entry to the cell cycle (Cheng et al, 2000). Finally, up-regulation of Nupr1, which activates Atf5 has been shown to enhance cell growth and confer a survival advantage (Clark et al, 2008).
In conclusion, we report a novel role for NFAT in the regulation of myeloid haematopoiesis. These data bear upon the management of a wide variety of immune disorders and in organ and BM transplantations where therapeutic inhibition of NFAT is a common treatment regimen. In the clinical, the administration of CsA is used to target T cell functions in order to induce immune-suppression. Our findings demonstrate that the myeloid compartment is also affected, which may offer new interpretations of variable CsA treatment outcomes mainly during infections where the response of myeloid cells is necessary.
MATERIALS AND METHODS
Haematopoietic stem cell lines and mouse reconstitutions
The pMyc-NUP98-HOXB4-IP-expressing HSC lines have been previously described (Ruedl et al, 2008). HSC lines were co-transduced with VIVIT peptide-encoding vector (gift from A. Rao) or control pMYc-IG vector (Kitamura et al, 2003) carrying eGFP or tdTomato tags.
C57BL/6(B6) Ly5.1 and Ly5.2 mice (6–10 weeks) were used for transplantation. The B6-Ly5.2 recipients were lethally irradiated with 9 Gy in two doses separated by 4 h. Twelve to 24 h later, 2–4 × 106 VIVIT-eGFP-expressing and/or tdTomato control HSC were administered intravenously. Mice were maintained under specific pathogen-free conditions in the NTU animal facility. All animal experiments were carried out within institutional guidelines.
DC cultures
BM cells from femurs of C57BL/6 mice (8–12 week) were cultured in IMDM (containing 10% heat-inactivated FCS (Life Technologies), penicillin 100 U/ml, streptomycin 100 µg/ml and Flt3-L 200 ng/ml (from transfected B16 tumor cells; Dranoff et al, 1993) at 2.5 × 106 cells/ml. CsA and FK506 (Cell signalling Technology) was added to cultures at 2 and 0.2 µg/ml, respectively, for 30 min prior to addition of Flt3-L and was maintained throughout the culture. For in vitro BrdU proliferation assays, cells were harvested after 4 or 10 days.
Western blotting
HSC were stimulated with Flt3-L (400 ng/ml) for 48 h then lysed on ice for 10 min using buffer containing 0.15 M NaCl, 5 mM EDTA pH 8.0, 10 mM Tris–Cl pH 7.4, 0.5% NP-40, 1 mM β-mercaptoethanol (all Sigma) and protease-inhibitor (Roche). Supernatants were collected and boiled for 5 min in 3× SDS sample buffer. Ninety micrograms of protein extract was separated by SDS–PAGE, transferred onto nitrocellulose membranes and analysed by immunoblotting with mAbs anti-NFAT1 (1:1000, Thermo-Scientific MA1-025) and anti-GAPDH (1:10,000, Millipore MAB374).
Immunofluorescent labelling
Cells were cultured for 48 h in Flt3-L supplemented medium, followed by stimulation with ionomycin (500 ng/ml) for 2 h. The detailed method can be found in the Supporting Information.
Flow cytometry and cell separations
Cell suspensions were prepared from spleen, thymus, BM and 200 µl of blood was obtained by cardiac puncture and the mononuclear cells were separated by Ficoll centrifugation (Sigma). Cells were incubated for 20 min with antibodies (for clones, conjugates and suppliers see Supporting Information). Anti-mouse CD11c, CD11b, I-A/I-E, CD45R (B220), CD8a, Gr-1 and F4/80 mAbs were used to label DC, granulocytes, monocytes and macrophages. Anti-mouse CD3e, CD4, CD8a, CD45R (B220) and NK1.1 mAbs were used to label T, B and NK cell populations. Anti-mouse CD135, Ly-6A/E (Sca-1), CD117 (c-kit), CD34 and CD16/32 mAbs were used to label progenitors and stem cells. Samples were acquired on a BD LSRII flow cytometer (BD Biosciences) and analysed using FlowJo software (Treestar Inc.) Biotinylated mAbs and streptavidin-microbeads were used for depletion of lineage marker positive BM cells: CD3e, CD19, CD45R, TER-119, Gr-1, CD11b, NK1.1 and CD127 (IL-7Ra) by magnetic separation on AutoMACS (Miltenyi). Purity of lineage negative cells was verified by flow cytometry before use for quantitative RT-PCR analysis.
ELISA
IL-2 ELISA was performed using the DuoSet kit (R&D Systems) following the manufacturer's recommendations. IFN-β ELISA was performed using the VeriKineTM Mouse Interferon-Beta ELISA Kit following the manufacturer's recommendations.
Colony-forming unit assay
BM cells were cultured in MethoCult M3534 (StemCell Technologies) at a concentration 104 cells per/ml for BM or 500 cell/ml for HSC lines. Five hundred microliters of MethoCult were plated per well of a 12-well suspension culture plate (Greiner) and incubated at 37°C in 5% CO2. Colonies were counted after 4–7 days. In some experiments colonies were washed in PBS and stained for FACS analysis.
Proliferation assays and cell cycle analysis
For in vitro BrdU assays, BM Flt3-L cultures were pulsed with 1 mM BrdU for 8 h (for cells harvested after day 4 of culture) or for 72 h (for cells harvested after 10 days of culture). Using the BrdU flow kit (BD Biosciences) the surface stained and fixed cells were labelled with anti-BrdU mAb for 20 min at room temperature and incubated with 7-AAD before acquisition on the LSRII.
Quantitative real-time PCR
Total RNA was extracted by Trizol (Invitrogen) phase separation and isolated using the RNeasy Mini kit (Qiagen). Six hundred nanograms of RNA (determined using Nanodrop 1000, Thermo-Scientific) was reverse transcribed using high-capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems). Real-time PCR was carried out with primers as described in the Supporting Information, using QuantiTect SYBRGreen Master Mix (Qiagen) on a Stratagene Mx3005P QPCR machine. Relative gene expression was calculated using the comparative Ct method (2−ΔΔCt).
Array hybridization and analysis
The detailed method can be found in the Supporting Information. All microarray data files are available for free download at the Gene Expression Omnibus (GEO accession number: GSE29094; http://www.ncbi.nlm.nih.gov/geo).
Statistical methods
Data were analysed with Student's paired or unpaired, two tailed, t-test using GraphPad Prism 5 (GraphPad Software). p-Values of less than 0.05 were considered statistically significant. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.
Acknowledgments
We wish to thank Prof. A. Rao for providing the VIVIT peptide construct; J. Lum and F. Zolezzi for microarray processing and data generation; C. Phua for animal handling; K. Karjalainen for HSC lines and critical discussion; and L. Robinson with N. McCarthy and A. Mertes for review of the paper. This research was funded by the Biomedical Research Council, A*STAR, Singapore and Grant (#08-019) from the Singapore Immunology Network, A*STAR, Biomedical Research Council.
Supporting information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
JF designed and conducted the majority of the experiments and wrote the manuscript; CXFL performed and designed some experiments, contributed to the manuscript; EGLK carried out confocal imaging and some of CFU experiments and contributed to the manuscript; BH and THS contributed to some experiments; SABMI reconstituted the mice; CR organized the in vivo experiments and provided HSC cells; JC did microarray data analysis; AM supported the design of the study; PRC conceived and supervised the scientific project.
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- Baksh S, Widlund HR, Frazer-Abel AA, Du J, Fosmire S, Fisher DE, DeCaprio JA, Modiano JF, Burakoff SJ. NFATc2-mediated repression of cyclin-dependent kinase 4 expression. Mol Cell. 2002;10:1071–1081. doi: 10.1016/s1097-2765(02)00701-3. [DOI] [PubMed] [Google Scholar]
- Bauer W, Rauner M, Haase M, Kujawski S, Arabanian LS, Habermann I, Hofbauer L, Ehninger G, Kiani A. Osteomyelosclerosis, anemia and extramedullary hematopoiesis in mice lacking the transcription factor NFATc2. Haematologica. 2011;96:1580–1588. doi: 10.3324/haematol.2011.042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano MS, Vieira-de-Abreu A, Teixeira LK, Werneck MB, Barcinski MA, Viola JP. NFATC2 transcription factor regulates cell cycle progression during lymphocyte activation: evidence of its involvement in the control of cyclin gene expression. FASEB J. 2002;16:1940–1942. doi: 10.1096/fj.02-0282fje. [DOI] [PubMed] [Google Scholar]
- Cante-Barrett K, Winslow MM, Crabtree GR. Selective role of NFATc3 in positive selection of thymocytes. J Immunol. 2007;179:103–110. doi: 10.4049/jimmunol.179.1.103. [DOI] [PubMed] [Google Scholar]
- Carotta S, Dakic A, D'Amico A, Pang SH, Greig KT, Nutt SL, Wu L. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity. 2010;32:628–641. doi: 10.1016/j.immuni.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- Clark DW, Mitra A, Fillmore RA, Jiang WG, Samant RS, Fodstad O, Shevde LA. NUPR1 interacts with p53, transcriptionally regulates p21 and rescues breast epithelial cells from doxorubicin-induced genotoxic stress. Curr Cancer Drug Targets. 2008;8:421–430. doi: 10.2174/156800908785133196. [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Crist SA, Sprague DL, Ratliff TL. Nuclear factor of activated T cells (NFAT) mediates CD154 expression in megakaryocytes. Blood. 2008;111:3553–3561. doi: 10.1182/blood-2007-05-088161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotti TN, Sharma SM, Fleming JD, Flannery MR, Ostrowski MC, Goldring SR, McHugh KP. PU.1 and NFATc1 mediate osteoclastic induction of the mouse beta3 integrin promoter. J Cell Physiol. 2008;215:636–644. doi: 10.1002/jcp.21344. [DOI] [PubMed] [Google Scholar]
- D'Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte–macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feau S, Facchinetti V, Granucci F, Citterio S, Jarrossay D, Seresini S, Protti MP, Lanzavecchia A, Ricciardi-Castagnoli P. Dendritic cell-derived IL-2 production is regulated by IL-15 in humans and in mice. Blood. 2005;105:697–702. doi: 10.1182/blood-2004-03-1059. [DOI] [PubMed] [Google Scholar]
- Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Gallo EM, Ho L, Winslow MM, Staton TL, Crabtree GR. Selective role of calcineurin in haematopoiesis and lymphopoiesis. EMBO Rep. 2008;9:1141–1148. doi: 10.1038/embor.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granucci F, Vizzardelli C, Pavelka N, Feau S, Persico M, Virzi E, Rescigno M, Moro G, Ricciardi-Castagnoli P. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol. 2001;2:882–888. doi: 10.1038/ni0901-882. [DOI] [PubMed] [Google Scholar]
- Granucci F, Feau S, Angeli V, Trottein F, Ricciardi-Castagnoli P. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. J Immunol. 2003;170:5075–5081. doi: 10.4049/jimmunol.170.10.5075. [DOI] [PubMed] [Google Scholar]
- Granucci F, Zanoni I, Pavelka N, Van Dommelen SL, Andoniou CE, Belardelli F, Degli Esposti MA, Ricciardi-Castagnoli P. A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J Exp Med. 2004;200:287–295. doi: 10.1084/jem.20040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt MB, Aliprantis A, Hu B, Glimcher LH. Calcineurin regulates innate antifungal immunity in neutrophils. J Exp Med. 2010;207:923–931. doi: 10.1084/jem.20092531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Pavlath GK. NFAT: ubiquitous regulator of cell differentiation and adaptation. J Cell Biol. 2002;156:771–774. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N, Hayashi K, Hoshijima M, Ogawa T, Koga S, Miyatake Y, Kumegawa M, Kimura T, Takeya T. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem. 2002;277:41147–41156. doi: 10.1074/jbc.M205063200. [DOI] [PubMed] [Google Scholar]
- Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- Jennings C, Kusler B, Jones PP. Calcineurin inactivation leads to decreased responsiveness to LPS in macrophages and dendritic cells and protects against LPS-induced toxicity in vivo. Innate Immun. 2009;15:109–120. doi: 10.1177/1753425908100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani A, Habermann I, Haase M, Feldmann S, Boxberger S, Sanchez-Fernandez MA, Thiede C, Bornhauser M, Ehninger G. Expression and regulation of NFAT (nuclear factors of activated T cells) in human CD34+ cells: down-regulation upon myeloid differentiation. J Leukoc Biol. 2004;76:1057–1065. doi: 10.1189/jlb.0404259. [DOI] [PubMed] [Google Scholar]
- Kiani A, Kuithan H, Kuithan F, Kyttala S, Habermann I, Temme A, Bornhauser M, Ehninger G. Expression analysis of nuclear factor of activated T cells (NFAT) during myeloid differentiation of CD34+ cells: regulation of Fas ligand gene expression in megakaryocytes. Exp Hematol. 2007;35:757–770. doi: 10.1016/j.exphem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T, Kumagai H. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31:1007–1014. [PubMed] [Google Scholar]
- Laouar Y, Welte T, Fu XY, Flavell RA. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity. 2003;19:903–912. doi: 10.1016/s1074-7613(03)00332-7. [DOI] [PubMed] [Google Scholar]
- McCaffrey PG, Luo C, Kerppola TK, Jain J, Badalian TM, Ho AM, Burgeon E, Lane WS, Lambert JN, Curran T, et al. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993;262:750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- Muller MR, Sasaki Y, Stevanovic I, Lamperti ED, Ghosh S, Sharma S, Gelinas C, Rossi DJ, Pipkin ME, Rajewsky K, et al. Requirement for balanced Ca/NFAT signaling in hematopoietic and embryonic development. Proc Natl Acad Sci USA. 2009;106:7034–7039. doi: 10.1073/pnas.0813296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O'Keeffe M, Shao QX, Chen WF, et al. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Tussiwand R, Lanzavecchia A, Manz MG. Activation of the Flt3 signal transduction cascade rescues and enhances type I interferon-producing and dendritic cell development. J Exp Med. 2006;203:227–238. doi: 10.1084/jem.20051645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Ruedl C, Khameneh HJ, Karjalainen K. Manipulation of immune system via immortal bone marrow stem cells. Int Immunol. 2008;20:1211–1218. doi: 10.1093/intimm/dxn079. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HJ, Largman C, Lansdorp PM, Humphries RK. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9:1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. [PubMed] [Google Scholar]
- Shivdasani RA, Orkin SH. The transcriptional control of hematopoiesis. Blood. 1996;87:4025–4039. [PubMed] [Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- Tenen DG, Hromas R, Licht JD, Zhang DE. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90:489–519. [PubMed] [Google Scholar]
- Tura O, Barclay GR, Roddie H, Davies J, Turner ML. Optimal ex vivo expansion of neutrophils from PBSC CD34+ cells by a combination of SCF, Flt3-L and G-CSF and its inhibition by further addition of TPO. J Transl Med. 2007;5:53. doi: 10.1186/1479-5876-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulleras E, Karlberg M, Moller Westerberg C, Alfredsson J, Gerondakis S, Strasser A, Nilsson G. NFAT but not NF-kappaB is critical for transcriptional induction of the prosurvival gene A1 after IgE receptor activation in mast cells. Blood. 2008;111:3081–3089. doi: 10.1182/blood-2006-10-053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AC, Loeb DM, Soede-Bobok AA, Touw IP, Friedman AD. Regulation of granulopoiesis by transcription factors and cytokine signals. Leukemia. 2000;14:973–990. doi: 10.1038/sj.leu.2401808. [DOI] [PubMed] [Google Scholar]
- Yarilina A, Xu K, Chen J, Ivashkiv LB. TNF activates calcium-nuclear factor of activated T cells (NFAT)c1 signaling pathways in human macrophages. Proc Natl Acad Sci USA. 2011;108:1573–1578. doi: 10.1073/pnas.1010030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.