Abstract
Low high-density lipoprotein (HDL)-cholesterol levels are associated with an increased risk of coronary artery disease (CAD) and myocardial infarction, which has triggered the hypothesis that HDL, in contrast to low-density lipoprotein (LDL), acts as an anti-atherogenic lipoprotein. Moreover, experimental studies have identified potential anti-atherogenic properties of HDL, including promotion of macrophage cholesterol efflux and direct endothelial-protective effects of HDL, such as stimulation of endothelial nitric oxide production and repair, anti-apoptotic, anti-inflammatory and anti-thrombotic properties. Studies in gene-targeted mice, however, have also indicated that increasing HDL-cholesterol plasma levels can either limit (e.g. apolipoprotein A-I) or accelerate (e.g. Scavenger receptor class B type I) atherosclerosis. Moreover, vascular effects of HDL have been observed to be heterogenous and are altered in patients with CAD or diabetes, a condition that has been termed ‘HDL dysfunction’. These alterations in biological functions of HDL may need to be taken into account for HDL-targeted therapies and considering raising of HDL-cholesterol levels alone is likely not sufficient in this respect. It will therefore be important to further determine, which biological functions of HDL are critical for its anti-atherosclerotic properties, as well as how these can be measured and targeted.
Keywords: coronary artery disease, endothelium, high-density lipoprotein, inflammation, nitric oxide
Introduction
Atherosclerotic coronary artery disease (CAD) and its complications remain the leading cause of death in industrialized countries (Lopez et al, 2006). In recent years, intensive lowering of low-density lipoprotein (LDL), cholesterol using statins has been established as an effective therapy to lower cardiovascular risk (Baigent et al, 2010). However, the risk of major cardiovascular events in patients with CAD on optimal medical therapy, including statins, remains in the range of 20% after 3 years of follow-up after an acute coronary syndrome (Stone et al, 2011). Several lines of evidence have suggested that high-density lipoprotein (HDL) may act as an anti-atherogenic lipoprotein, as discussed in detail in this Review article. HDL-targeted therapies are therefore intensely pursued as a potential novel anti-atherogenic strategy to reduce cardiovascular risk and are an important frontier of basic, translational and clinical cardiovascular research.
HDL cholesterol levels, atherosclerosis and cardiovascular risk—epidemiological and genetic studies
Initially, the concept that HDL may protect from CAD was suggested by numerous epidemiological studies, indicating that low plasma levels of HDL cholesterol or apoA1, the major protein component of HDL, are associated with an increased risk of CAD and CAD-related cardiovascular events in the general population (Castelli et al, 1986; Di Angelantonio et al, 2009; Gordon et al, 1977; Sharrett et al, 2001). Later studies have suggested that also in patients with CAD and very low LDL cholesterol levels on statin treatment, low plasma concentrations of HDL cholesterol remained associated with an increased risk of cardiovascular events (Barter et al, 2007a). Low HDL cholesterol levels have also been suggested as a primary lipid abnormality in patients with premature CAD (Genest et al, 1991).
However, low plasma HDL cholesterol levels may not generally be associated with accelerated atherosclerosis. Several studies have examined the prevalence of cardiovascular disease in subjects with monogenic disorders of HDL metabolism, i.e. HDL deficiency syndromes due to apolipoprotein A-I (apoA-I) mutations, ATP binding cassette transporter A-1 (ABCA1) or lecithin/cholesterol acyltransferase (LCAT) deficiency. As described below, apoA-I mutations may be associated with an accelerated development of atherosclerosis, which is less clear for mutations in the other two genes. More recently, genome-wide association studies (GWAS) have examined the relation of single-nucleotide polymorphisms (SNPs) associated with altered HDL cholesterol levels to changes in the risk of coronary disease as described below. In addition, several genetically modified mice have been investigated to gain further insights into the role of HDL metabolism and function in atherosclerotic cardiovascular disease.
Apolipoprotein A-I (apoA-I)
Apolipoprotein A-I is the main protein constituent of HDL in plasma (Fig 1). To date, more than 40 genetic defects of apoA-I have been described (Schaefer et al, 2010). However, the consequences of these defects with regard to cardiovascular risk have remained inconclusive, largely because of the limited number of carriers of apoA-I gene defects. Notably, in one of the larger studies involving 54 heterozygotes for the apoA-I mutation L178P, carriers of the apoA-I gene defect had lower plasma levels of HDL cholesterol, impaired endothelial function, and increased carotid intima-media thickness (IMT) when compared to non-affected family controls (Hovingh et al, 2004). Notably, however, several apoA-I variants with amino acid substitutions have also been associated with amyloidosis (Schaefer et al, 2010), and amyloidosis has also been suggested to lead to endothelial dysfunction and increased carotid IMT (Modesto et al, 2007).
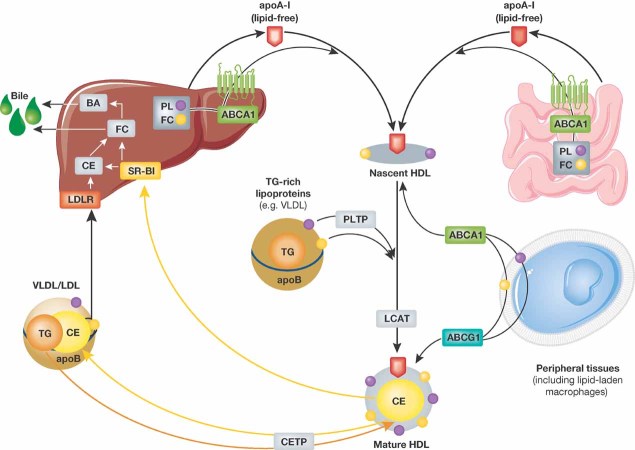
Figure 1. Molecular biosynthesis of HDL.
Lipid-free apoA-I is secreted by the liver and intestine and acquires phospholipids and free cholesterol via hepatic and intestinal ABCA-1. Nascent HDL takes up further phospholipids (via PLTP) as well as free cholesterol from peripheral tissues and triglyceride-rich lipoproteins. HDL-associated LCAT esterifies part of the free cholesterol to cholesterol esters, thereby forming the hydrophobic core of the HDL particle (‘HDL maturation’). HDL-associated cholesterol is either directly transferred to the liver via hepatic SR-BI or following CETP-mediated transfer to VLDL/LDL via the hepatic LDL receptor.
In experimental studies using atherosclerosis-susceptible mice (inbred C57BL/6), it was observed that transgenic overexpression of high amounts of human ApoA-I significantly protected from development of early atherosclerotic lesions, i.e. fatty streak lesions (Rubin et al, 1991). Similarly, overexpression of human ApoA-I in apoE-deficient mice on chow diet suppressed early atherosclerotic lesion formation (Plump et al, 1994). Moreover, overexpression of apoA-I in mice was associated with an increased reverse cholesterol transport (RCT) from macrophages to feces in vivo, which might, at least in part, account for the inhibition of atherosclerosis progression in mice after apoA-I gene transfer (Zhang et al, 2003).
Lack of apoA-I after targeted disruption of the apoA-I gene did not result in an increased atherosclerotic lesion formation in mice (inbred 129-strain) on atherogenic diet, despite lower HDL cholesterol levels (Li et al, 1993). In contrast, however, knocking out apoA-I in human apoB-transgenic female mice, LDL receptor-deficient and LDL receptor/apoE-deficient mice resulted in an accelerated atherosclerosis development (Moore et al, 2003, 2005; Voyiaziakis et al, 1998). Additional studies evaluating the effects of apoA-I at different stages of atherosclerosis development in mice will be further discussed below.
ATP binding cassette transporter A-1 (ABCA1)
ABCA1 mediates efflux of cholesterol and phospholipids to lipid-free or lipid-poor apoA-1, which leads to formation of nascent HDL (Fig 1). Studies by several groups in 1998 identified loss-of-function mutations in both alleles of the ABCA1 gene as a cause of the rare Tangier´s disease that presents with extremely low HDL cholesterol levels and tissue accumulation of macrophages due to decreased ABCA1-mediated efflux of cholesterol/phospholipids from peripheral cells (Rust et al, 1999). Some studies in these patients suggested an increased risk of premature CAD (Schaefer, 1984; Schaefer et al, 2010), however, the number of mutation carriers was very low, so that a definitive conclusion could not be drawn. When compared to subjects with heterozygous familial hypercholesterolemia (i.e. very high LDL cholesterol levels caused by mutations in the LDL receptor gene), the potential increase in cardiovascular risk in ABCA1-deficient subjects is not as profound as expected, which has also been ascribed to concomitantly decreased LDL cholesterol levels in these subjects (Schaefer et al, 1980). A recent population-based study from Denmark has demonstrated that lower plasma levels of HDL cholesterol in subjects heterozygous for four rare loss-of-function mutations in the ABCA1 gene were not associated with an increased risk of ischemic heart disease when compared to the general population (Frikke-Schmidt et al, 2008).
Glossary
Acute coronary syndrome
A spectrum of clinical presentations ranging from ST elevation myocardial infarction, non-ST elevation myocardial infarction to unstable angina. Any group of symptoms compatible with a sudden interruption of the blood flow to the heart.
Atherosclerotic coronary artery disease
Buildup of cholesterol and plaques on the inner walls of the coronary artery. These plaques can restrict blood flow to the heart muscle by physically clogging the artery or by causing abnormal artery tone and function.
Carotid intima media thickness
Thickness of the inner two layers of the carotid artery—the intima and media; a surrogate endpoint for evaluating the regression and/or progression of atherosclerotic cardiovascular disease.
Cholesterol
A steroid metabolite found in cell membranes. It is transported in the blood in lipoprotein particles and excess circulating cholesterol is associated with atherosclerosis.
Endothelial cells
Cells forming the endothelium, which lines the interior surface of blood vessels and lymphatic vessels.
Genome-wide association study (GWAS)
Examination of many common genetic variants in different individuals to evaluate if any variant is associated with a trait. Typically single-nucleotide polymorphisms (SNPs) are investigated and usually investigated traits include major diseases.
High-density lipoprotein (HDL)
Good cholesterol, high levels are thought to be protective against atherosclerosis.
Low-density lipoprotein (LDL)
Bad cholesterol, high circulating levels have been shown to correlate with atherosclerosis.
Macrophages
Large phagocytes developed from circulating monocytes that migrate to tissues to eliminate cellular debris and particulate antigens. They produce and respond to inflammatory cytokines.
Nitric oxide
NO, also called nitrogen monoxide, an important cellular signalling molecule involved in many physiological and pathological processes.
Plaque
The buildup of cells and cholesterol in the arterial wall. Severe plaque buildup can narrow the arterial lumen interfering with the flow of blood.
Moreover, several experimental studies have examined the effects of complete or bone-marrow-derived cell-specific deficiency of ABCA1 (or ABCA1 and ABCG-1) on development of atherosclerosis. Notably, complete deficiency of ABCA1 did not result in a measurable increase of atherosclerotic lesion formation in hypercholesterolemic apoE- or LDL receptor-deficient mice and was associated with reduced plasma cholesterol levels (Aiello et al, 2002). Similarly, a combined complete deficiency of ABCA1 and the ATP binding cassette transporter G-1 (ABCG1) had no significant effect on atherosclerotic lesion formation and was associated with hypocholesterolemia (Out et al, 2008). In contrast, in experimental studies in mice with a selective deficiency of ABCA1 or ABCA1/ABCG1 in bone-marrow-derived cells (i.e. macrophages, leukocytes) an accelerated atherosclerosis development was observed (Aiello et al, 2002; van Eck et al, 2002). Taken together, these findings suggest that complete absence of ABCA1 leads to marked alterations of plasma lipoprotein homeostasis, and a potential atherogenic effect from ABCA1 deficiency may be compensated for by a less atherogenic lipoprotein profile. In contrast, deletion of ABCA1 or both ABCA1 and ABCG1 only in bone-marrow-derived cells (macrophages, leukocytes) led to an accelerated atherosclerotic lesion formation, suggesting that the transporter activity in bone marrow-derived cells (macrophages, leukocytes) exerts an anti-atherogenic effect that is particularly visible when both transporters, i.e. ABCA1 and ABCG1, are deleted (Yvan-Charvet et al, 2007).
The findings regarding the effect of ABCA1 overexpression on atherosclerosis development in mice are not entirely consistent, and there are likely important differences between systemic and hepatic overexpression of ABCA1. Human ABCA1-transgenic mice (overexpressing ABCA1 in multiple tissues) on an apoE-deficient background developed smaller and less complex atherosclerotic lesions compared to control mice (Singaraja et al, 2002). Increasing ABCA1 levels using anti-miR-33 treatment has recently been observed to reduce atherosclerotic plaque size and lipid content in LDL receptor-deficient mice (Rayner et al, 2011). On the contrary, however, ABCA1 overexpression in the liver of LDL receptor-deficient mice resulted in a pro-atherogenic lipoprotein profile and increased atherosclerotic lesion formation (Joyce et al, 2006). Moreover, hepatocyte-specific overexpression of murine ABCA1 in apoE-deficient mice has been shown to increase HDL cholesterol levels in plasma, but accelerated atherosclerosis development in these mice (Feng et al, 2010). HDL isolated from apoE-deficient mice overexpressing ABCA1 failed to stimulate migration of endothelial progenitor cells and to inhibit endothelial cell apoptosis in vitro (Feng et al, 2010). Interestingly, Yamamoto et al have recently reported that pharmacological inhibition of hepatic ABCA1 activity leads to reduced HDL cholesterol plasma levels and is associated with an augmented RCT in the bile, suggesting that hepatic inhibition of ABCA1 may promote reverse cholesterol transport (Yamamoto et al, 2011). Taken together, these findings suggest that increasing ABCA1 expression/activity systemically may exert anti-atherogenic effects, whereas a selective overexpression of ABCA1 in the liver may result in an accelerated development of atherosclerosis.
Lecithin/cholesterol acyltransferase (LCAT)
Lecithin/cholesterol acyltransferase mediates the conversion of free cholesterol into cholesteryl ester in HDL and thereby plays a role in the maturation of the HDL particle (Fig 1). The findings on carotid atherosclerosis in LCAT-deficient patients are heterogenous and may result from population differences. Hoving et al suggested that carriers of LCAT gene mutations have increased carotid IMT as assessed by B-mode ultrasound (Hovingh et al, 2005). In a study by Calabresi et al, subjects with low HDL cholesterol levels due to one or two loss-of-function mutations in the LCAT gene did not show an increased carotid IMT when compared to matched healthy subjects as examined by carotid ultrasound (Calabresi et al, 2009a). Similarly, there was no association between plasma LCAT concentration and carotid IMT observed by this group in subjects with a high-risk acute coronary syndrome that were not yet on drug treatment, indicating that LCAT deficiency may not increase the risk of carotid atherosclerosis (Calabresi et al, 2011). Moreover, it has been suggested that LCAT activity is not required for the ability of human plasma to promote macrophage cholesterol efflux in vitro (Calabresi et al, 2009b) and may have a minor role for transport of cholesterol from macrophages to the liver in human apoA-I transgenic mice (Tanigawa et al, 2009), which is consistent with the observation that HDL directly transfers a large amount of unesterified cholesterol to the liver for biliary cholesterol excretion (Schwartz et al, 1978, 1982). However, a more recent study using 3.0-T magnetic resonance imaging observed that carriers of LCAT gene mutations with decreased HDL cholesterol levels exhibited an increased carotid atherosclerosis (Duivenvoorden et al, 2011).
Scavenger receptor class B type I (SR-BI)
Hepatic SR-BI mediates the selective uptake of HDL cholesterol by the liver and is therefore considered a key receptor for HDL cholesterol clearance from plasma (Fig 1). Furthermore, endothelial SR-BI has been shown to mediate potentially important atheroprotective signalling in endothelial cells induced by HDL (see sections below). Overexpression of hepatic SR-BI increased transport of cholesterol from macrophages to the liver and its secretion into the bile in mice, although plasma levels of HDL cholesterol were reduced (Zhang et al, 2005). Conversely, SR-BI-deficient mice have increased HDL cholesterol levels, but increased atherosclerosis (Huby et al, 2006), suggesting that SR-BI expression is inversely related to plasma levels of HDL cholesterol and development of atherosclerosis.
Recently, Vergeer et al identified a novel missense mutation (P279S) of SR-BI in a Dutch family with markedly elevated plasma concentrations of HDL (i.e. above the 95th percentile of normal; Vergeer et al, 2011). Adenoviral expression of the P279S variant in SR-BI-deficient mice led to a reduction of the cholesterol ester uptake capacity of hepatocytes in these mice when compared to wild-type SR-BI, and the cholesterol efflux from monocyte–macrophages obtained from carriers of the P279S variant was lower, as compared to noncarriers (Vergeer et al, 2011).
The above findings further support the concept that HDL cholesterol plasma levels should not be considered in isolation as a therapeutic target or read-out for the capacity of macrophage RCT, since lower HDL cholesterol levels can also be associated with an enhanced RCT capacity (as described above) and higher HDL cholesterol levels after SR-BI knockout can be associated with accelerated atherosclerosis.
Genome-wide association studies (GWAS)
Recently, GWAS have emerged as a novel tool to identify common genetic variants that are associated with changes in blood lipids (Teslovich et al, 2010). In these studies, it can be determined whether SNPs associated with altered lipid levels are also associated with altered risk of CAD. Notably and in striking contrast to several SNPs associated with higher LDL cholesterol serum levels that were uniformly associated with increased risk of CAD, the findings for SNPs associated with increased HDL cholesterol levels revealed no clear association with CAD risk (Willer et al, 2008). Some SNPs associated with higher HDL cholesterol tended to be associated with an increased and others tended to be associated with a decreased risk of CAD (Willer et al, 2008). Interestingly, the above observations in gene-targeted mice suggest that increased HDL cholesterol levels may be indicative of a potential protective mechanism (i.e. increased apoA-I levels) or may reflect an alteration of an HDL-related pathway that may lead to potential pro-atherosclerotic effects (i.e. SR-BI mutation). Together, these studies further indicate that plasma concentrations of HDL cholesterol alone are likely not a sufficient marker to determine potential anti-atherogenic effects of HDL, an observation that is highly relevant for the design and evaluation of HDL-targeted therapies.
In a more recent GWAS, several loci were identified that were specifically related to HDL cholesterol or triglycerides, but not LDL cholesterol, and were associated with the risk of CAD (Teslovich et al, 2010). These findings support the concept that it is possible to modulate HDL cholesterol in a way congruently with a reduced risk of CAD, however, these studies cannot yet prove that the change in HDL cholesterol is causal for the change in CAD risk.
Biosynthesis of HDL
Secretion and initial lipidation of apoA-I
The first step of the biosynthesis of HDL entails the synthesis and secretion of apoA-I, the major protein constituent of HDL, by the liver and intestine (Rader, 2006). The second most abundant HDL protein, apoA-II, is synthesized only in the liver and its secretion results in the formation of a subclass of HDL particles containing apoA-I and apoA-II (Tailleux et al, 2002).
Following secretion in the liver and intestine, lipid-poor apoA-I immediately acquires cholesterol and phospholipids, in particular from hepatocytes and enterocytes (Brunham et al, 2006; Timmins et al, 2005). The initial lipidation of apoA-I is primarily mediated by ABCA-1 and results in the formation of nascent HDL (Fig 1). Nascent HDL acquires additional phospholipids and free cholesterol from extrahepatic tissues. The relative contribution of different organs for extrahepatic lipidation of HDL remains largely unknown. However, transplantation of bone marrow from wild-type mice into ABCA-1-deficient mice resulted only in a very modest increase in HDL cholesterol levels, suggesting that macrophages are likely not a major source of HDL cholesterol mass in mice (Haghpassand et al, 2001).
Phospholipidation of HDL
In addition, HDL acquires phospholipids and potentially apolipoproteins (such as apoC-III) during hydrolysis of triglyceride-rich lipoproteins (Fig 1). The transfer of phospholipid surface remnants from trigylceride-rich lipoproteins to HDL is mediated via the phospholipid transfer protein (PLTP) (Masson et al, 2009). Phospholipids transferred via PLTP are not only an important structural component of the HDL surface, but also serve as substrate for the esterification of HDL-associated cholesterol. Moreover, PLTP promotes the fusion of smaller HDL3 particles and subsequent generation of larger HDL2 particles (a process termed ‘HDL conversion’; Lagor & Rader, 2011). During this process, lipid-poor apoA-I is shed off and can again undergo lipidation for regeneration of preβ-HDL. In transgenic animal models, increased systemic levels of PLTP have been shown to promote atherosclerosis in mice (van Haperen et al, 2002; Yang et al, 2003) and rabbits (Masson et al, 2011). PLTP deficiency in atherosclerosis-prone mice results in decreased atherosclerotic plaque formation (Jiang et al, 2001). These findings have been attributed, at least in part, to changes in apolipoprotein B (apoB) production and plasma cholesterol levels in these animals, since PLTP also stimulates hepatic secretion of VLDL, possibly by phospholipidation of nascent apoB (Lie et al, 2002).
Studies on the association of PLTP activity and cardiovascular risk in humans have been complicated by the fact that PLTP activity and PLTP mass are affected by various cardiovascular risk factors, and poorly correlate with each other (Lagor & Rader, 2011). Interestingly, a recent study demonstrated that two SNPs at the PLTP locus that showed decreased PLTP mass and activity were strongly associated with a lower risk of cardiovascular disease, despite reduced plasma concentrations of HDL (Vergeer et al, 2010). Notably, in this study there was no association between the SNPs at the PLTP locus and plasma concentrations of LDL, suggesting an apoB-independent effect. In a smaller case control study, four SNPs in the PLTP locus were shown to be associated with the presence of carotid atherosclerosis (Jarvik et al, 2010). Among these, individuals with the rs6065904 allele showed elevated PLTP activity and this was associated with increased carotid atherosclerosis.
These studies suggest that decreased PLTP activity due to genetic variants at the PLTP locus are associated with a reduced cardiovascular risk in humans, indicating that increased phospholipidation of HDL does not necessarily lead to cardiovascular risk reduction, although it may raise HDL cholesterol levels.
Esterification of HDL-associated cholesterol by LCAT
Following efflux of cholesterol and phospholipid transfer from peripheral tissues, a proportion of HDL-associated free cholesterol in plasma is esterified to cholesteryl ester by LCAT (Fig 1). Although LCAT activity results in the formation of a hydrophic core in HDL and thus is important for maturation of the HDL particle, recent studies in subjects with LCAT mutations and LCAT-transgenic mice have suggested that LCAT likely does not play a major role for efficient transport of cholesterol from peripheral tissues back to the liver (Rader, 2009). As described above, the findings with respect to the vascular phenotype of subjects with LCAT mutations are heterogenous.
HDL cholesterol uptake by the liver
Hepatic SR-BI can selectively take up cholesteryl ester and unesterified cholesterol and thus directly deliver HDL-associated cholesterol to the liver (Cuchel & Rader, 2006; Ji et al, 1999). Alternatively, in humans and some other species, HDL cholesterol can be transferred to apoB-containing lipoproteins in exchange for triglycerides via the cholesteryl ester transfer protein (CETP) and subsequently be cleared by LDL receptor-mediated uptake of apoB-containing lipoproteins to the liver (Fig 1). In transgenic mice (that normally lack CETP) with CETP gene transfer, the transport of cholesterol from macrophages to the liver was increased (Tanigawa et al, 2007). However, studies on the effect of CETP on development of atherosclerosis in mice have yielded mixed results. Whereas overexpression of CETP increased atherosclerosis in wild-type and hypercholesterolemic mouse models (de Vries-van der Weij et al, 2009; Plump et al, 1999), a decreased atherosclerotic plaque burden was observed in CETP transgenic hypertriglyceridemic mice and in CETP transgenic mice overexpressing LCAT, despite lower plasma HDL cholesterol levels in these animals (Foger et al, 1999; Hayek et al, 1995). In rabbits (that are expressing CETP), inhibition of CETP by JTT-705 (today called dalcetrapib) attenuated atherosclerosis (Okamoto et al, 2000).
Because CETP inhibition may change the composition of HDL particles and give rise to large HDL particles enriched in cholesterol esters, another study sought to characterize the ability of HDL from CETP-deficient subjects to mediate cholesterol efflux from macrophage foam cells (Matsuura et al, 2006). However, the cholesterol efflux potential of HDL isolated from human subjects with homozygous CETP deficiency was rather increased compared to normolipidemic control subjects. Importantly, whether pharmacological CETP inhibition by different CETP inhibitors affects other anti-atherogenic properties of HDL in patients with CAD remains to be tested in future studies.
Molecular mechanisms mediating potential atheroprotective functions of HDL: alterations in cardiovascular disease
In recent years, several properties or functions of HDL have been identified that could exert anti-atherosclerotic effects. Besides promoting macrophage cholesterol efflux and RCT, HDL has more recently been shown to exert direct potentially anti-atherosclerotic effects on endothelial cells, such as the direct stimulation of endothelial nitric oxide production by HDL or endothelial anti-inflammatory effects and anti-oxidant effects that will be discussed in more detail below (Fig 2). Notably, however, recent evidence suggests that vascular effects of HDL can be highly heterogeneous and are altered in patients with cardiovascular disease.
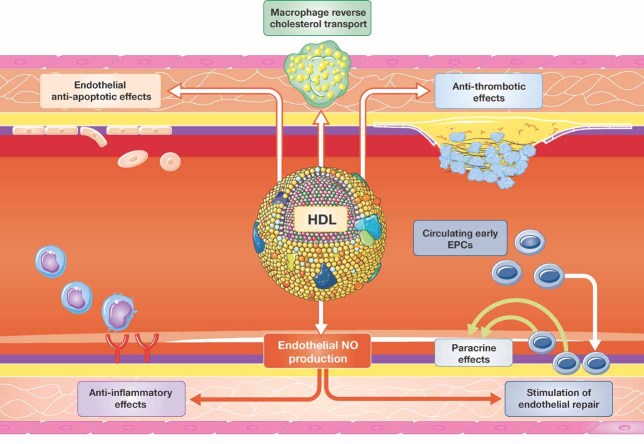
Figure 2. Proposed anti-atherogenic effects of HDL in the endothelium.
Besides stimulation of macrophage cholesterol transport, HDL has been shown to inhibit endothelial inflammatory activation and to promote endothelial repair. Both effects have been observed to be dependent on endothelial NO production. Furthermore, HDL exerts anti-apoptotic and anti-thrombotic effects that may contribute to its anti-atherogenic capacity.
Reverse cholesterol transport
Physiological mechanisms
Early on, potential anti-atherogenic effects of HDL were mainly attributed to its function in macrophage RCT, i.e. the removal of excess cholesterol from lipid-laden macrophage foam cells in the atherosclerotic plaque and its transport to the liver for excretion in the bile (Rader, 2006; Ross & Glomset, 1973).
Hydrolysis of cytoplasmatic cholesteryl esters to free cholesterol and the subsequent efflux of the free cholsterol to mature HDL or extracellular lipid-poor apoA-I is the first step of macrophage RCT. While it was initially thought that efflux of cholesterol from macrophages was primarily mediated by passive diffusion (Glomset, 1968), it has become clear that macrophage cholesterol efflux is facilitated by active transport systems, including the ABCA1 and ATP binding cassette transporter G1 (ABCG1) transporters (Rader, 2006; Tall et al, 2008). ABCA-1 primarily mediates the cholesterol efflux to lipid-poor apoA-I as studies in macrophages from ABCA1-knockout or -overexpressing mice have indicated (Bortnick et al, 2000; Haghpassand et al, 2001; Van Eck et al, 2006). In contrast, ABCG1 largely mediates cholesterol efflux from macrophages to mature HDL (Tall et al, 2008).
Interestingly, a recent study has quantitatively assessed the roles of SR-BI, ABCA1 and ABCG1 in macrophage RCT in mice in vivo (Wang et al, 2007). Using primary macrophages lacking SR-BI, the authors demonstrated that SR-BI did not promote macrophage RCT in vivo after intraperitoneal injection. In contrast, ABCA1 and ABCG1 contributed in an additive fashion to stimulation of macrophage RCT in vivo, and transplantation of bone marrow from ABCA1/ABCG1-deficient mice accelerated atherosclerotic lesion formation in LDL receptor-deficient mice (Wang et al, 2007). Consistently, Yvan-Charvet et al (Yvan-Charvet et al, 2008) demonstrated that SR-BI failed to stimulate net cholesterol efflux from HEK293 cells to plasma HDL and inhibited ABCG1-mediated cholesterol efflux, which was at least in part due to an increased uptake of HDL cholesteryl esters into these cells.
Following efflux from macrophages, HDL-associated cholesterol can get esterified by LCAT that transfers a fatty acyl residue from phospholipids to the 3-beta-hydroxy group of cholesterol (Rader, 2009). The final steps of RCT include the uptake of HDL cholesterol by the liver and its excretion in the bile. As described above, cholesterol esters and free cholesterol in HDL can either be directly taken up by the liver via SR-BI or transferred to apoB-containing lipoproteins in a process mediated by CETP (Cuchel & Rader, 2006). In the latter pathway, cholesterol esters are taken up by the liver via the LDL receptor. Hepatic cholesterol esters are then hydrolysed to free cholesterol that can be excreted into the bile either directly or following conversion to bile acid.
Alterations in cardiovascular disease
Of note, a recent study indicated that the cholesterol efflux capacity of apoB-depleted serum (as a measure of the capacity of HDL to accept cholesterol from macrophages) was inversely related to carotid IMT in healthy volunteers and to the likelihood of angiographic CAD in a case-control study (Khera et al, 2011). Importantly, these associations persisted after adjustment for HDL cholesterol and apoA-I levels. A reduced cholesterol efflux capacity via SR-BI- and ABCG-1-dependent pathways was also observed for large HDL2 particles in patients with familial hypercholesterolemia (Bellanger et al, 2011). Furthermore, the ability of HDL to deliver cholesterol esters to the liver was impaired in these patients, as detected using an in vitro model with HepG2 cells. However, a critical open question remains whether macrophage cholesterol efflux capacity, or any other atheroprotective function of HDL, is associated with clinical cardiovascular disease progression and outcome.
Stimulation of endothelial cell nitric oxide (NO) production
Physiological mechanisms
In recent years it has become clear that HDL from healthy subjects can exert direct potential atheroprotective effects on endothelial cells and the understanding of the vascular effects of HDL considerably changed with the important observation that HDL may directly stimulate endothelial NO synthase-mediated NO production as well as induce endothelium-dependent, NO-mediated vasodilation via endothelial SR-BI (Yuhanna et al, 2001). Endothelial NO plays a crucial role in the regulation of vascular tone and structure (Landmesser et al, 2004), and, importantly, endothelial NO has been shown to exert a variety of atheroprotective effects in the vasculature, such as anti-thrombotic, anti-coagulant, anti-inflammatory and pro-fibrinolytic effects.
In addition, experimental studies have consistently demonstrated the capacity of HDL to modify eNOS expression as well as activity and to stimulate endothelial NO production in vitro and in vivo (Besler et al, 2011; Kuvin et al, 2002; Mineo et al, 2003; Nofer et al, 2004; Ramet et al, 2003; Sorrentino et al, 2010). Moreover, in humans, administration of reconstituted HDL has been shown to improve endothelial function in subjects with hypercholesterolemia and in subjects with isolated low HDL due to heterozygous loss-of-function mutations in the ABCA-1 gene locus (Bisoendial et al, 2003; Spieker et al, 2002).
Several different mechanisms have been proposed to account for the endothelial NO-stimulating capacity of HDL (Fig 3). Early studies have suggested that HDL acts by preventing the detrimental effects of oxidized LDL on endothelial NO-synthase (Uittenbogaard et al, 2000) while a subsequent study by Yuhanna et al (Yuhanna et al, 2001) suggested that HDL can bind to endothelial SR-BI and thus directly stimulate eNOS-mediated NO production. Mechanistically, binding of HDL to SR-BI initially leads to tyrosine kinase Src-mediated activation of phosphoinositide (PI) 3-kinase, which in turn activates Akt and the MAP kinase/extracellular signal-regulated kinase pathway (Mineo et al, 2003). This activation of endothelial Akt has been shown to stimulate phosphorylation of eNOS at serine residue 1177 (Mineo et al, 2003; Nofer et al, 2004), known to be an important regulatory mechanism leading to eNOS activation (Dimmeler et al, 1999). In contrast, the mechanism through which the MAP kinase/extracellular signal-regulated kinase pathway activates eNOS in endothelial cells stimulated with HDL remains to be further elucidated.
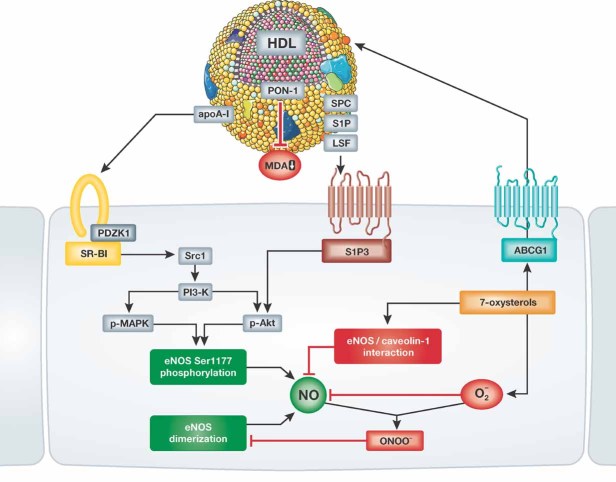
Figure 3. Signalling pathways mediating the effects of HDL on endothelial NO production.
HDL has been shown to stimulate endothelial NO synthase phosphorylation at serine residue 1177 via binding of apoA-I to SR-BI and binding of HDL-associated lysophospholipids to the S1P3 receptor. In addition, HDL-mediated efflux of 7-oxysterols via endothelial ABCG-1 has been observed to inhibit the interaction between eNOS and caveolin, and to prevent the loss of eNOS dimerization induced by reactive oxygen species in the endothelium.
However, other HDL components besides apoA-I are likely to be important for the eNOS-stimulating capacity of HDL since isolated apoA-I, the major SR-BI ligand of HDL, failed to activate eNOS in these studies. Interestingly, stimulation of eNOS-mediated NO production is also induced by binding of HDL-associated lysophospholipids (i.e. sphingosylphosphorylcholine, sphingosine-1-phosphate, lysosulfatide) to the lysophospholipid receptor S1P3 (Fig 3) that is expressed in endothelial cells and may partially mediate HDL- and lysophospholipid-induced vasodilation (Nofer et al, 2004). In this respect, an interaction between S1P3 and SR-BI to induce HDL signalling in endothelial cells has been suggested in which binding of HDL with SR-BI could provide the necessary spatial proximity for lysophospholipids to effectively stimulate S1P3 (Nofer et al, 2004).
Recently, Terasaka et al (Terasaka et al, 2008) identified a novel mechanism whereby HDL may maintain endothelial cell NO production and availability in mice fed a high-cholesterol diet. These authors suggested that ABCG1-mediated efflux of oxysterols from endothelial cells plays a role since 7-ketosterol, a dietary oxysterol, accumulated in endothelial cells of ABCG1-deficient mice on a western diet (Terasaka et al, 2008). Interestingly, incubation of human aortic endothelial cells with HDL prevented 7-ketosterol-induced production of reactive oxygen species and disruption of the active eNOS dimer. Furthermore, HDL-mediated cholesterol efflux via ABCG-1 reduced the inhibitory interaction of eNOS with caveolin-1 and thereby restored eNOS activity in cholesterol-loaded endothelial cells (Terasaka et al, 2010). These data suggest that the ability of HDL to preserve endothelial function in the presence of hypercholesterolemia may, at least in part, relate to an increased endothelial efflux of oxysterols (Fig 3).
Notably, a recent study from our group has identified the HDL-associated antioxidant enzyme paraoxonase (PON) 1 as an important determinant of the capacity of HDL to stimulate endothelial NO production and to exert NO-dependent endothelial-atheroprotective effects (Besler et al, 2011). Inhibition of PON1 in HDL from healthy subjects impaired the capacity of HDL to stimulate endothelial NO production and HDL isolated from PON1-deficient mice failed to stimulate NO production in mouse aortic endothelial cells. Furthermore, inhibition of eNOS-mediated NO production prevented the inhibitory effects of HDL from healthy subjects on nuclear factor κB (NF-κB) activity, vascular cell adhesion molecule (VCAM)-1 expression and endothelial monocyte adhesion, suggesting that the capacity of HDL to stimulate endothelial NO production is important for these endothelial anti-inflammatory effects of HDL (Besler et al, 2011).
Alterations in cardiovascular disease
We and others have recently shown that the direct endothelial effects of HDL from patients with CAD and diabetes are markedly altered when compared to HDL from healthy subjects. In marked contrast to HDL from healthy subjects, HDL from patients with diabetes and low levels of HDL cholesterol failed to stimulate endothelial cell NO production and to promote endothelial repair in a carotid artery injury model in mice (Sorrentino et al, 2010). Moreover, HDL from patients with either stable CAD or an acute coronary syndrome—in contrast to HDL from age- and gender-matched healthy subjects—inhibited rather than stimulated endothelial cell NO production and lost the capacity to limit endothelial inflammatory activation as well as to promote endothelial repair in vivo (Besler et al, 2011).
Inhibition of lipid oxidation by HDL
Basic mechanisms
In the initiation and progression of atherosclerotic vascular disease, accumulation and subsequent oxidation of LDL in the subendothelial space is thought to play an important role. Oxidized LDL, a pro-atherogenic form of LDL, is taken up by scavenger receptors of macrophages and several studies have demonstrated that oxidatively modified LDL promotes endothelial cell inflammatory activation (Navab et al, 2004). Modification of LDL by 1-electron oxidants, such as tyrosyl radical or nitrogen dioxide radical, leads to the formation of lipid hydroperoxides and advanced products of oxidation, i.e. alkanes, aldehydes and isoprostanes. Of note, it has been suggested that HDL is major carrier of both early and late products of lipid oxidation (Bowry et al, 1992; Proudfoot et al, 2009) and that apoA-I, the main protein constituent of HDL, is capable of binding and removing lipid hydroperoxides of LDL in vitro and after injection into mice as well as after infusion in humans in vivo (Navab et al, 2000). In addition, HDL contains several anti-oxidant enzymes that may be involved in prevention of lipid oxidation or degradation of lipid hydroperoxides, such as LCAT, platelet-activating factor acetylhydrolase (PAF-AH), reduced glutathione selenoperoxidase and PON1. In particular, mice deficient in PON1 displayed significantly larger aortic atherosclerotic lesions as compared to their wild type controls and HDL isolated from PON1-deficient mice was unable to prevent LDL oxidation in a cell co-culture model of the arterial wall (Shih et al, 1998).
However, others have questioned the hypothesis that HDL directly acquires lipid hydroperoxides from LDL or is oxidized in plasma, since the transfer of lipid hydroperoxides between LDL and HDL appears to be slow and the plasma contains several anti-oxidant defense mechanisms (Shao & Heinecke, 2009). Another explanation for the enrichment of lipid hydroperoxides in HDL could be the fact that HDL binds lipid oxidation products, such as 7-ketocholesterol (Terasaka et al, 2008) at sites of inflammation and transports them back to the plasma, thereby protecting endothelial cells against inflammatory activation (Shao & Heinecke, 2009). Of interest in this respect, Nicholls and colleagues (Nicholls et al, 2005) have reported that reconstituted HDL inhibits superoxide production and vascular inflammation induced by a non-occlusive carotid periarterial collar in normocholesterolemic rabbits.
Alterations of antioxidant effects of HDL in acute phase response and cardiovascular disease
In 1995, Van Lenten et al demonstrated that HDL obtained during an acute phase response (i.e. within 2–3 days after cardiac surgery) promoted rather than inhibited LDL-induced endothelial monocyte chemoattractant protein (MCP)-1 expression and monocyte adhesion, indicating that the anti-inflammatory properties of HDL can be highly heterogenous (Van Lenten et al, 1995). The capacity of HDL to inhibit oxidative LDL modification induced in a reconstituted artery wall model (i.e. a coculture of smooth muscle and endothelial cells) was markedly impaired during the acute phase response. Similarly, an acute Influenza A infection in wild type mice progressively impaired the ability of HDL to inhibit LDL oxidation and LDL-induced monocyte chemotactic activity in human artery wall cell cocultures up to 9 days after inoculation (Van Lenten et al, 2001).
More recent studies addressed the question whether HDL also has altered effects on LDL oxidation in patients with cardiovascular disease. In marked contrast to the anti-oxidative and anti-inflammatory effects of HDL from healthy subjects, HDL from normolipidemic patients with angiographically confirmed CAD (i.e. ≥50% narrowing of at least one coronary artery) increased LDL-induced monocyte chemotactic activity by human artery wall cells and promoted LDL oxidation (Navab et al, 2001). A subsequent study by Ansell et al suggested that the capacity of HDL to alter LDL-induced monocyte chemotactic activity in patients with CAD was somewhat improved after 6 weeks of simvastatin therapy (Ansell et al, 2003). However, HDL from patients with CAD on statin therapy remained pro-inflammatory in contrast to HDL from age- and sex-matched healthy subjects.
Other potential anti-atherogenic properties of HDL
HDL isolated from healthy subjects or reconstituted HDL has also been shown to stimulate endothelial repair mechanisms and to inhibit endothelial cell apoptosis, platelet aggregation and expression of prothrombotic factors in endothelial cells, that may contribute to anti-atherogenic properties of HDL (Barter et al, 2004; Mineo et al, 2006; Mineo & Shaul, 2007).
Effects of HDL on development and progression of atherosclerosis
Animal models of atherosclerosis
Important in vivo evidence demonstrating that HDL might protect against development and progression of atherosclerosis came from studies in cholesterol-fed rabbits. Badimon et al (Badimon et al, 1989) observed that administration of homologous HDL plasma fractions concomitantly with an atherogenic diet inhibited aortic fatty streak formation and cholesterol deposition in cholesterol-fed rabbits. Furthermore, in cholesterol-fed rabbits with pre-existing atherosclerosis administration of homologous HDL plasma fractions induced regression of established aortic fatty streaks and lipid deposits (Badimon et al, 1990). Similarly, liver-directed gene transfer of human apoA-I in LDL-receptor-deficient mice with pre-existing atherosclerotic lesions (induced by 5 weeks of western-type diet) led to regression of atherosclerotic lesion area (Tangirala et al, 1999). However, subsequent studies have questioned whether a selective increase of HDL can induce regression of atherosclerotic lesions as described below.
Transplantation of thoracic aortic segments from apoE-deficient mice containing advanced atherosclerotic lesions into syngeneic apoE-deficient mice expressing a human apoA-I transgene retarded progression of atherosclerosis and remodelled atherosclerotic plaques towards a more stable appearing phenotype (Rong et al, 2001). Likewise, adenoviral apoA-I transfer into LDL-receptor-deficient mice with pre-existing atherosclerotic lesions increased collagen content in lesions, but did not induce regression (Van Craeyveld et al, 2011). Furthermore, hepatocyte-directed adenoviral rabbit apoA-I or rabbit LCAT transfer inhibited progression of atherosclerosis and induced cholesterol unloading of pre-existing atherosclerotic plaques in LDL-receptor-deficient rabbits (Van Craeyveld et al, 2009). These findings suggest that HDL may induce regression of atherosclerosis only in early atherosclerotic lesions and not in advanced atherosclerotic lesions. However, HDL may lead to ‘remodelling’ of advanced atherosclerotic lesions as described above (Rong et al, 2001).
More recently, studies have examined the effect of recombinant apoA-I Milano/phospholipid complexes (also known as ETC-216) on development and progression of atherosclerosis in animal models. ApoA-I Milano is a naturally occurring variant of the major HDL protein apoA-I containing an arginine to cysteine substitution at amino acid 173. Infusion of recombinant apoA-I Milano reduced neointima formation after arterial injury in cholesterol-fed rabbits (Ameli et al, 1994) and prevented aortic atherosclerosis in apoE-deficient mice on a high-cholesterol diet (Shah et al, 1998). Notably, in these studies the prevention of neointimal thickening or atherosclerosis progression was accompanied by a decreased lipid and macrophage content in the arterial wall.
Human atherosclerosis
Given the above discussed results of recombinant apoA-I Milano/phospholipid complexes in animal models of experimental atherosclerosis, Nissen and colleagues (Nissen et al, 2003) conducted a prospective randomized placebo-controlled clinical trial that investigated the effect of 5 weekly infusions of two doses of recombinant apoA-I Milano/phospholipid complexes or placebo on coronary atheroma burden as measured by intravascular ultrasound (IVUS) in patients with an acute coronary syndrome. This study suggested that infusion of recombinant apoA-I Milano/phospholipid complexes results in a small, but significant regression of coronary atheroma volume as compared with no significant change from baseline in the placebo group. Forty-seven patients were included in the analysis. However, the dose of apoA-I Milano that was infused during the trial was high, making it an expensive treatment that is difficult to translate into the clinical setting. In one patient the treatment was terminated due to a possible hypersensitivity reaction (Nissen et al, 2003).
Notably, a second study characterized the effects of 4 weekly infusions of reconstituted HDL (CSL-111, i.e. apoA-I and phosphatidylcholine, 40 or 80 mg/kg) or placebo on coronary atheroma burden in 183 patients with a recent acute coronary syndrome (Tardif et al, 2007). The higher-dosage CSL-111 treatment group was discontinued early because of liver function test abnormalities. Two to three weeks after the last infusion, there was no significant difference in atheroma volume (% change in atheroma volume: −3.4% with CSL-111 and −1.6% with placebo) or nominal change in plaque volume between the low-dose CSL-111 and placebo group as assessed by IVUS. However, infusion of CSL-111 significantly improved the plaque characterization index, a secondary endpoint, on IVUS and coronary score on quantitative coronary angiography.
Additional larger studies using infusions of different forms of reconstituted HDL are currently under way and will shed more light into the potential effects of such an administration of HDL on coronary atherosclerosis.
Potential HDL-targeted therapies
HDL-targeted therapies are currently intensely evaluated as a potential novel treatment strategy to address the substantial residual risk in patients with atherosclerotic cardiovascular disease. Degoma and Rader have recently classified HDL-targeted drugs that are currently tested in preclinical or clinical studies into four groups (Degoma & Rader, 2011): drugs which are (i) directly augmenting apoA-I levels (apoA-I infusions, upregulators of endogenous apo A-I production), (ii) indirectly augmenting apoA-I and HDL cholesterol levels (CETP inhibitors, niacin, endothelial lipase inhibitors), (iii) mimicking the functionality of apoA-I (i.e. apoA-I mimetics) and (iv) enhancing RCT (liver × receptor agonists, LCAT activators). Most of these HDL-targeted drugs are still in preclinical or early clinical investigation. In the search for an HDL-raising, cardioprotective drug recent interest has primarily focused on niacin and CETP inhibitors, and the present review will provide a short update on recent clinical trials testing these compounds.
Niacin
Niacin (nicotinic acid) has been shown to increase HDL cholesterol levels dose-dependently by 15–35% and lowers plasma concentrations of other potential pro-atherogenic lipoproteins, i.e. LDL cholesterol, trigylcerides and lipoprotein (a) (Gille et al, 2008).
A recent experimental study in rabbits indicated that the effects of niacin on endothelial function and vascular inflammation are, at least in part, independent of changes in plasma lipid levels (Wu et al, 2010). Moreover, Lukasova et al have recently reported that niacin can reduce the progression of atherosclerosis in mice independently of its lipid-modifying effects through the activation of GPR109A on immune cells (Lukasova et al, 2011). We have observed that extended-release niacin treatment improved endothelial-protective functions of HDL in type 2 diabetics with low HDL-cholesterol levels (Sorrentino et al, 2010).
In the Coronary Drug Project (CDP), niacin reduced the rates of myocardial infarction during the initial 5-years follow-up compared to placebo; however, there was no significant difference in the primary endpoint of the rate of death from any cause between the niacin and placebo group (The Coronary Drug Project Research Group, 1975). However, after 15 years of follow-up there was a significant reduction in all-cause mortality in the group that was initially randomised to niacin therapy (Canner et al, 1986), but these results have to be interpreted with caution since the randomised treatment duration was limited to 8 years.
In the HDL-Atherosclerosis Treatment Study (HATS), combination therapy with niacin and simvastatin slowed progression of coronary atherosclerosis and the occurrence rate of a first cardiovascular event in patients with CAD and low HDL-cholesterol levels (Brown et al, 2001). However, in this study the number of patients was limited and there was no simvastatin monotherapy group.
The Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) studies 2, 3 and 6 evaluated the effects of niacin on carotid atherosclerosis in patients with CAD (or CAD risk equivalent) on statin therapy. In ARBITER-2, extended release-niacin reduced carotid intima media thickness (cIMT) in these patients after 1 year of follow-up, but there was no significant difference compared to the placebo group (Taylor et al, 2004). The follow-on open label uncontrolled crossover study, ARBITER-3, reported a significant regression of cIMT after further 12 months on extended release-niacin (Taylor et al, 2006). In ARBITER 6-HALTS, addition of niacin, but not addition of ezetimibe, reduced cIMT after 14 months in patients with CAD (or a CAD risk equivalent) and an HDL-cholesterol level under 50 mg (men)/55 mg (women) per deciliter, who were receiving long-term statin therapy and in whom an LDL cholesterol level under 100 mg per deciliter had been achieved (Taylor et al, 2009).
The recently published Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes trial (AIM-HIGH) included 3414 patients and addressed the question of whether addition of 1.5–2.0 g of ER-niacin per day would reduce the risk of major cardiovascular events in patients with established cardiovascular disease and atherogenic dyslipidemia (Boden, 2011). To maintain an LDL cholesterol level of 40–80 mg per deciliter, all patients received simvastatin (40–80 mg per day), plus ezetimibe (10 mg per day) if needed. The AIM HIGH trial was stopped early after a mean follow-up of 3 years because niacin therapy did not show any clinical benefit and there was an unexpected higher number of ischemic strokes in patients randomized to niacin. However, there are a number of limitations in this trial that need to be taken into consideration. The trial was designed with 85% power to show a 25% reduction in the primary endpoint, which is clearly an overestimation in retrospect, largely because of three reasons: first, the absolute difference in HDL cholesterol levels between the niacin and placebo group was small (i.e. 4–5 mg/dl); second, the LDL cholesterol levels were very low in both groups, 75% of patients in the placebo group were on high-dose statin treatment and a further 20% received ezetimibe as an add-on therapy to simvastatin; third, there was a 25% discontinuation rate in the niacin group that may have biased the results.
Furthermore, AIM-HIGH is the first trial to show a potential association between niacin therapy and ischemic strokes. This needs to be taken seriously and should be further examined in future studies. However, there is no prior study or any biological evidence suggesting that niacin might favour the occurrence of ischemic strokes. The ongoing HPS-2-HRIVE trial examines ER-niacin/laropiprant on-top of statin therapy in secondary prevention, has included already more than 25,000 patients and will therefore provide a more definite answer on the effects of ER-niacin/laropiprant therapy on cardiovascular outcome (NCT00461630).
CETP inhibitors
The interest in pharmacological CETP inhibition as novel therapeutic approach to raise HDL cholesterol levels was kindled by the finding of elevated HDL cholesterol levels in Japanese families with genetic CETP deficiency. However, the first compound tested in large clinical trials, torcetrapib, did not to reduce the progression of carotid atheroslerosis in patients with familial hypercholesterolemia receiving statin treatment and was associated with progression of disease in the common carotid segment (Kastelein et al, 2007). Of note, these effects occurred despite a pronounced increase in HDL cholesterol levels and a substantial decrease in LDL cholesterol and trigylcerides (Kastelein et al, 2007).
More importantly, in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) study, a large clinical outcome trial characterizing the effects of torcetrapib on major cardiovascular events in patients at high cardiovascular risk who were receiving statins, torcetrapib therapy was associated with an increased risk of cardiovascular events and death from any cause (Barter et al, 2007b). This was, at least in part, due to an increase in blood pressure, systemic aldosterone levels and alterations in serum electrolytes related to ‘off-target’ effects of the compound on aldosterone and cortisol synthesis in adrenal cortical cells (Barter & Rye, 2011). Moreover, torcetrapib administration to spontaneously hypertensive rats impairs endothelial NO production and increases production of reactive oxygen species as well as endothelin-1 in the endothelium, leading to endothelial dysfunction in these animals (Simic et al, 2011).
Three other CETP inhibitors are currently in clinical trials, i.e. dalcetrapib, anacetrapib and evacetrapib. In contrast to torcetrapib, none of these agents has been shown to alter blood pressure, electrolytes or serum aldosterone.
In a 24 h ambulatory blood pressure study, anacetrapib has been shown to effectively raise HDL cholesterol levels in patients with dyslipidemia after 4 weeks of therapy without affecting blood pressure levels (Krishna et al, 2007). Comparable effects were seen in 589 patients with hypercholesterolemia or mixed hyperlipidemia and in this study, 8 weeks of anacetrapib treatment led to an increase in HDL cholesterol by up to 139% (Bloomfield et al, 2009). Of note, in this trial co-administration of anacetrapib with atorvastatin resulted in significant incremental LDL cholesterol reductions and similar HDL cholesterol increases compared to atorvastatin therapy alone. In the recent DEFINE trial, 1623 patients with coronary heart disease or at high risk for coronary heart disease who were taking a statin and who had a baseline LDL cholesterol level of 81 mg/dl were assigned to receive 100 mg of anacetrapib or placebo daily for 18 months. As compared to placebo, anacetrapib reduced LDL cholesterol levels by 39.8% and resulted in 138% increase in HDL cholesterol levels (Cannon et al, 2010). No changes were observed in blood pressure, electrolyte or aldosterone levels and no increase in adverse cardiovascular events was reported. The effects of anacetrapib treatment on cardiovascular outcome will be examined in the large global REVEAL HPS-3 TIMI-55 trial, which will test whether anacetrapib reduces death and cardiovascular morbidity in a population of approximately 30,000 patients with cardiovascular disease.
Dalcetrapib has been shown to confer a more modest inhibition of CETP activity and is currently evaluated in a large clinical trial program of phase II and III studies. The recently completed dal-VESSEL study has demonstrated no adverse effects of dalcetrapib on arterial blood pressure or endothelial function. The results of the dal-PLAQUE imaging trial that characterized the effects of dalcetrapib or placebo treatment on vessel wall structure (by MRI) and inflammation (by 18FDG-PET/CT) for 24 months in patients with or without high risk of CAD were recently published (Fayad et al, 2011). Importantly, there was no evidence for an adverse effect of dalcetrapib on structural parameters and inflammatory indices of the arterial wall. At present, the dal-OUTCOMES trial is ongoing (and has included approximately 15,600 patients) and will examine the effects of dalcetrapib on cardiovascular outcome in patients after a recent acute coronary syndrome.
Potential mechanisms underlying impaired HDL functionality in patients with cardiovascular disease
As described in more detail above, the capacity of HDL particles of different size or structure to exert anti-atherogenic effects differs. Changes in HDL functionality may have several causes, including changes of the HDL proteome (protein composition or modifications) and lipidome. Moreover, physicochemical properties of the HDL particle can influence its anti-inflammatory capacity, as the smaller and denser HDL3 subfraction has been shown to be superior to the larger and less dense HDL2 subfraction in inhibiting TNF-α-induced VCAM-1 expression in endothelial cells (Ashby et al, 1998).
Heterogeneity of the HDL proteome
Recent proteomics studies have identified between 28 and 67 HDL-associated proteins using liquid chromatography–electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) (Davidson et al, 2009; Vaisar et al, 2007). Interestingly, these proteomics studies of HDL have found not only proteins with well-characterized roles in lipid metabolism and antioxidant properties of HDL, but also a number of proteins involved in the acute phase response, complement regulation and proteinase inhibition. Of note, the protein composition of HDL may differ between healthy subjects and patients with CAD (Vaisar et al, 2007), suggesting a potential remodelling of the HDL particle in these patients. A combined statin and niacin therapy partially reversed changes in the HDL proteome observed in patients with CAD, yielding a protein composition that resembled that in apparently healthy age- and sex-matched control subjects (Green et al, 2008). However, whether changes in the HDL proteome are of relevance for the altered vascular effects of HDL in patients with CAD needs to be examined in future studies.
In addition, the protein composition of HDL changes during acute inflammation as observed in mice after endotoxin treatment or in humans after cardiac surgery (Chiba et al, 2011; Van Lenten et al, 1995). This has led to the term ‘acute phase HDL’ that is characterized by an increase in serum amyloid A (SAA), group IIa secretory phospholipase A2 (sPLA2-IIa), and ceruloplasmin content, and a reduced apoA-I content. Controversial data have been obtained for the cholesterol efflux capacity of HDL during an acute phase response. While McGillicuddy and coworkers observed an impaired macrophage-to-feces RCT during subacute endotoxemia in mice (McGillicuddy et al, 2009), others observed a rather increased ABCG-1 dependent cholesterol efflux capacity of HDL isolated from mice during endotoxin challenge (de Beer et al, 2011), and a preserved ABCA-1- and ABCG-1-dependent cholesterol efflux capacity of HDL isolated from patients after cardiac surgery (Jahangiri et al, 2009). However, the interpretation of these data is complicated by the fact that SAA can itself act as an acceptor of cellular cholesterol.
Posttranslational modifications of apoA-I
Oxidative modifications of apoA-I have been shown to impair the cholesterol efflux capacity of HDL (Fig 4). In particular, myeloperoxidase (MPO), a hypochlorous acid (HOCl)-generating enzyme that is enriched in human atheroma, can modify apoA-I and induce chloro- and nitrotyrosine formation (Zheng et al, 2004) as well as methionine oxidation (Shao et al, 2008). Modification of HDL by MPO leads to a profound impairment of the cholesterol efflux capacity of HDL (Bergt et al, 2004; Zheng et al, 2004) and an impaired capacity of HDL to stimulate endothelial NO production (Sorrentino et al, 2010). Interestingly, nitro-and chloro-tyrosine levels of apoA-I are higher in patients with CAD as compared to healthy subjects (Zheng et al, 2004). However, there is an ongoing controversy about the mechanisms whereby MPO modification renders apoA-I dysfunctional.
Figure 4. Alterations of the atheroprotective effects of HDL in patients with CAD.
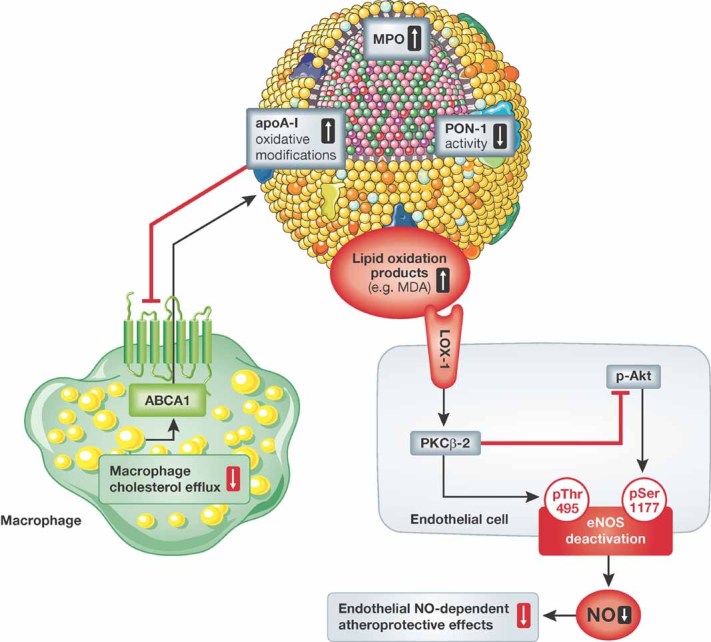
Modification of apoA-I by MPO has been shown to impair the macrophage cholesterol efflux capacity of HDL. More recently, accumulation of MDA in HDL from patients with CAD due to an impaired HDL-associated PON1 activity has been observed to stimulate activation of endothelial PKCbeta-II via the LOX-1 receptor. PKCbeta-II activation by HDL from patients with CAD inhibited Akt-dependent phosphorylation of eNOS at serine residue 1177 and increased the inhibitory eNOS phosphorylation at threonine 495, leading to reduced endothelial NO production.
Besides MPO-mediated oxidation, modification of HDL by malondialdehyde, an advanced lipid oxidation product, has been shown to impair the cholesterol efflux capacity of HDL in vitro (Shao et al, 2010), suggesting that lipid oxidation itself can induce alterations of HDL-associated proteins.
Characterization of the HDL lipidome
Considerably less is known about the HDL lipidome. It has been demonstrated that HDL is enriched in phosphatidylcholine, lysophosphatidylcholine, phosphatidylethanolamine and phosphatidylethanolamine-based plasmalogens, when compared to other lipoprotein fractions in plasma (Wiesner et al, 2009). However, a comprehensive and detailed profiling of the molecular lipid species in HDL including information on the type of fatty acids and the position where they are attached to the glycerol backbone is not yet available. Furthermore, it remains unknown whether the HDL-associated lipid profile differs in patients with stable CAD or acute coronary syndrome. Interestingly, it has been demonstrated that the phosphatidylcholine species composition of HDL influences its anti-inflammatory activity (Baker et al, 2000), suggesting that the lipid composition might be an important determinant of HDL functionality. However, this needs to be tested in more detail in future studies.
Role of PON1 for lipid oxidation and impaired endothelial-protective effects of HDL in patients with CAD
PON1 is an HDL-associated esterase/lactonase that has been shown to protect against lipid peroxide formation in LDL and HDL. Notably, we have recently shown that the activity of HDL-associated PON1 was profoundly impaired in HDL from patients with CAD as compared to HDL from age- and gender-matched healthy subjects (Besler et al, 2011). Inhibition of HDL-associated PON1, as observed in patients with CAD, led to an increase of MDA-lysine adducts in HDL that subsequently activated endothelial protein kinase C beta II (PKCβII) via the endothelial LOX-1 receptor (Fig 4). In turn, stimulation of endothelial PKCβII by HDL from patients with CAD led to an inhibition of signalling pathways that are necessary for activation of eNOS, i.e. phosphorylation of eNOS at serine residue 1177 and dephosphorylation of eNOS at threonine residue 495. Compatible with the concept that activation of endothelial PKCβII limits eNOS stimulation by HDL from patients with CAD, inhibition of endothelial PKCβII partially restored the impaired capacity of HDL from patients with CAD to increase endothelial NO production (Besler et al, 2011).
Pending issues
The molecular basis of potential anti-atherogenic effects of HDL needs to be further investigated. For example, it remains at present unclear, what the relative contribution of HDL-mediated macrophage RCT versus direct endothelial-protective effects of HDL is.
It has become clear that HDL cholesterol serum levels alone are insufficient as a therapeutic target. Moreover, HDL becomes ‘dysfunctional’ in patients with cardiovascular disease. HDL functional analysis may therefore aid in the evaluation of HDL-targeted therapies, since it is likely that only increasing HDL with vasoprotective properties will lead to beneficial vascular effects. Such HDL function assays need to be standardized and validated in larger patient cohorts.
The molecular mechanisms leading to ‘dysfunctional’ HDL need to be further characterized, including a better understanding of the role of alterations of the protein and lipid composition of HDL in patients with cardiovascular disease and their relevance for impaired HDL functionality. HDL ‘dysfunction’ may represent a potential therapeutic target.
Conclusion
Low plasma levels of HDL cholesterol are associated with an increased risk of coronary disease and myocardial infarction. HDL is considered an anti-atherogenic lipoprotein, largely based on experimental studies suggesting several potential anti-atherogenic properties of HDL. At the same time it has become clear that the biological functions of HDL, i.e. endothelial-atheroprotective effects, are highly heterogenous and are altered in patients with coronary disease or diabetes. These observations may have profound implications for the understanding of the vascular effects of HDL-targeted or HDL-raising therapies. Indeed, the simple notion that raising plasma HDL cholesterol levels by itself will always translate into a reduction of atherosclerotic vascular disease can no longer be sustained, because HDL cholesterol levels alone represent an insufficient measure of the antiatherogenic capacity of HDL.
Therefore reliable and validated assays of HDL functionality are needed to better assess the therapeutic effects of HDL-targeted therapeutic interventions on vasoprotective effects of HDL. In this respect, an important open question in the field remains which alterations of the atheroprotective functions of HDL are related to adverse clinical cardiovascular outcome in patients with CAD. It is at present unknown which potential atheroprotective function of HDL, or which particular protein or lipid marker in HDL, may be suited best to assess the antiatherogenic capacity of HDL. It seems likely that only raising HDL with vasoprotective properties can be expected to exert beneficial cardiovascular effects. In addition, the molecular target leading to increased HDL cholesterol levels will represent a critical determinant for the vascular effects of HDL-raising therapeutic interventions, and should likely not involve mechanisms that hinder vasoprotective functions of HDL (such as blockade of the SR-BI receptor).
Acknowledgments
This work was supported by the Leducq Foundation, the Swiss Heart Foundation and the Swiss National Science Foundation (SNF-138486).
Conflict of interest statement: U.L. has received speaker fees and a research grant from Roche and Merck. T.F.L. has received speaker fees and a research grant from Roche and MSD. C.B. has no conflict of interest.
References
- Aiello RJ, Brees D, Bourassa PA, Royer L, Lindsey S, Coskran T, Haghpassand M, Francone OL. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler Thromb Vasc Biol. 2002;22:630–637. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- Ameli S, Hultgardh-Nilsson A, Cercek B, Shah PK, Forrester JS, Ageland H, Nilsson J. Recombinant apolipoprotein A-I Milano reduces intimal thickening after balloon injury in hypercholesterolemic rabbits. Circulation. 1994;90:1935–1941. doi: 10.1161/01.cir.90.4.1935. [DOI] [PubMed] [Google Scholar]
- Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST, et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–2756. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- Ashby DT, Rye KA, Clay MA, Vadas MA, Gamble JR, Barter PJ. Factors influencing the ability of HDL to inhibit expression of vascular cell adhesion molecule-1 in endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:1450–1455. doi: 10.1161/01.atv.18.9.1450. [DOI] [PubMed] [Google Scholar]
- Badimon JJ, Badimon L, Galvez A, Dische R, Fuster V. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab Invest. 1989;60:455–461. [PubMed] [Google Scholar]
- Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PW, Rye KA, Gamble JR, Vadas MA, Barter PJ. Phospholipid composition of reconstituted high density lipoproteins influences their ability to inhibit endothelial cell adhesion molecule expression. J Lipid Res. 2000;41:1261–1267. [PubMed] [Google Scholar]
- Barter P, Rye KA. Cholesteryl ester transfer protein inhibition to reduce cardiovascular risk: where are we now. Trends Pharmacol Sci. 2011;32:694–699. doi: 10.1016/j.tips.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- Bellanger N, Orsoni A, Julia Z, Fournier N, Frisdal E, Duchene E, Bruckert E, Carrie A, Bonnefont-Rousselot D, Pirault J, et al. Atheroprotective reverse cholesterol transport pathway is defective in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2011;31:1675–1681. doi: 10.1161/ATVBAHA.111.227181. [DOI] [PubMed] [Google Scholar]
- Bergt C, Pennathur S, Fu X, Byun J, O'Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121:2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisoendial RJ, Hovingh GK, Levels JH, Lerch PG, Andresen I, Hayden MR, Kastelein JJ, Stroes ES. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003;107:2944–2948. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
- Bloomfield D, Carlson GL, Sapre A, Tribble D, McKenney JM, Littlejohn TW, III, Sisk CM, Mitchel Y, Pasternak RC. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am Heart J. 2009;157:352–360. doi: 10.1016/j.ahj.2008.09.022. e 352. [DOI] [PubMed] [Google Scholar]
- Boden WE. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- Bortnick AE, Rothblat GH, Stoudt G, Hoppe KL, Royer LJ, McNeish J, Francone OL. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J Biol Chem. 2000;275:28634–28640. doi: 10.1074/jbc.M003407200. [DOI] [PubMed] [Google Scholar]
- Bowry VW, Stanley KK, Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci USA. 1992;89:10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi L, Baldassarre D, Castelnuovo S, Conca P, Bocchi L, Candini C, Frigerio B, Amato M, Sirtori CR, Alessandrini P, et al. Functional lecithin/cholesterol acyltransferase is not required for efficient atheroprotection in humans. Circulation. 2009;120:628–635. doi: 10.1161/CIRCULATIONAHA.108.818143. [DOI] [PubMed] [Google Scholar]
- Calabresi L, Favari E, Moleri E, Adorni MP, Pedrelli M, Costa S, Jessup W, Gelissen IC, Kovanen PT, Bernini F, et al. Functional LCAT is not required for macrophage cholesterol efflux to human serum. Atherosclerosis. 2009;204:141–146. doi: 10.1016/j.atherosclerosis.2008.08.038. [DOI] [PubMed] [Google Scholar]
- Calabresi L, Baldassarre D, Simonelli S, Gomaraschi M, Amato M, Castelnuovo S, Frigerio B, Ravani A, Sansaro D, Kauhanen J, et al. Plasma lecithin/cholesterol acyltransferase and carotid intima-media thickness in European individuals at high cardiovascular risk. J Lipid Res. 2011;52:1569–1574. doi: 10.1194/jlr.P014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, Gotto AM, Stepanavage M, Liu SX, Gibbons P, Ashraf TB, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–2415. doi: 10.1056/NEJMoa1009744. [DOI] [PubMed] [Google Scholar]
- Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256:2835–2838. [PubMed] [Google Scholar]
- Chiba T, Chang MY, Wang S, Wight TN, McMillen TS, Oram JF, Vaisar T, Heinecke JW, De Beer FC, De Beer MC, et al. Serum amyloid A facilitates the binding of high-density lipoprotein from mice injected with lipopolysaccharide to vascular proteoglycans. Arterioscler Thromb Vasc Biol. 2011;31:1326–1332. doi: 10.1161/ATVBAHA.111.226159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis. Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler Thromb Vasc Biol. 2009;29:870–876. doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer MC, Ji A, Jahangiri A, Vaughan AM, de Beer FC, van der Westhuyzen DR, Webb NR. ATP binding cassette G1-dependent cholesterol efflux during inflammation. J Lipid Res. 2011;52:345–353. doi: 10.1194/jlr.M012328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries-van der Weij J, Zadelaar S, Toet K, Havekes LM, Kooistra T, Rensen PC. Human CETP aggravates atherosclerosis by increasing VLDL-cholesterol rather than by decreasing HDL-cholesterol in APOE*3-Leiden mice. Atherosclerosis. 2009;206:153–158. doi: 10.1016/j.atherosclerosis.2009.02.038. [DOI] [PubMed] [Google Scholar]
- Degoma EM, Rader DJ. Novel HDL-directed pharmacotherapeutic strategies. Nat Rev Cardiol. 2011;8:266–277. doi: 10.1038/nrcardio.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Duivenvoorden R, Holleboom AG, van den Bogaard B, Nederveen AJ, de Groot E, Hutten BA, Schimmel AW, Hovingh GK, Kastelein JJ, Kuivenhoven JA, et al. Cholesterol acyltransferase gene mutations have accelerated atherogenesis as assessed by carotid 3.0-T magnetic resonance imaging: carriers of lecithin. J Am Coll Cardiol. 2011;58:2481–2487. doi: 10.1016/j.jacc.2010.11.092. [DOI] [PubMed] [Google Scholar]
- Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–1559. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Lievens J, Jacobs F, Hoekstra M, Van Craeyveld E, Gordts SC, Snoeys J, De Geest B. Hepatocyte-specific ABCA1 transfer increases HDL cholesterol but impairs HDL function and accelerates atherosclerosis. Cardiovasc Res. 2010;88:376–385. doi: 10.1093/cvr/cvq204. [DOI] [PubMed] [Google Scholar]
- Foger B, Chase M, Amar MJ, Vaisman BL, Shamburek RD, Paigen B, Fruchart-Najib J, Paiz JA, Koch CA, Hoyt RF, et al. Cholesteryl ester transfer protein corrects dysfunctional high density lipoproteins and reduces aortic atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. J Biol Chem. 1999;274:36912–36920. doi: 10.1074/jbc.274.52.36912. [DOI] [PubMed] [Google Scholar]
- Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjaerg-Hansen A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299:2524–2532. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- Genest JJ, McNamara JR, Salem DN, Schaefer EJ. Prevalence of risk factors in men with premature coronary artery disease. Am J Cardiol. 1991;67:1185–1189. doi: 10.1016/0002-9149(91)90924-a. [DOI] [PubMed] [Google Scholar]
- Gille A, Bodor ET, Ahmed K, Offermanns S. Nicotinic acid: pharmacological effects and mechanisms of action. Annu Rev Pharmacol Toxicol. 2008;48:79–106. doi: 10.1146/annurev.pharmtox.48.113006.094746. [DOI] [PubMed] [Google Scholar]
- Glomset JA. The plasma lecithins/cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- Green PS, Vaisar T, Pennathur S, Kulstad JJ, Moore AB, Marcovina S, Brunzell J, Knopp RH, Zhao XQ, Heinecke JW. Combined statin and niacin therapy remodels the high-density lipoprotein proteome. Circulation. 2008;118:1259–1267. doi: 10.1161/CIRCULATIONAHA.108.770669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghpassand M, Bourassa PA, Francone OL, Aiello RJ. Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels. J Clin Invest. 2001;108:1315–1320. doi: 10.1172/JCI12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek T, Masucci-Magoulas L, Jiang X, Walsh A, Rubin E, Breslow JL, Tall AR. Decreased early atherosclerotic lesions in hypertriglyceridemic mice expressing cholesteryl ester transfer protein transgene. J Clin Invest. 1995;96:2071–2074. doi: 10.1172/JCI118255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovingh GK, Brownlie A, Bisoendial RJ, Dube MP, Levels JH, Petersen W, Dullaart RP, Stroes ES, Zwinderman AH, de Groot E, et al. A novel apoA-I mutation (L178P) leads to endothelial dysfunction, increased arterial wall thickness, and premature coronary artery disease. J Am Coll Cardiol. 2004;44:1429–1435. doi: 10.1016/j.jacc.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Hovingh GK, Hutten BA, Holleboom AG, Petersen W, Rol P, Stalenhoef A, Zwinderman AH, de Groot E, Kastelein JJ, Kuivenhoven JA. Compromised LCAT function is associated with increased atherosclerosis. Circulation. 2005;112:879–884. doi: 10.1161/CIRCULATIONAHA.105.540427. [DOI] [PubMed] [Google Scholar]
- Huby T, Doucet C, Dachet C, Ouzilleau B, Ueda Y, Afzal V, Rubin E, Chapman MJ, Lesnik P. Knockdown expression and hepatic deficiency reveal an atheroprotective role for SR-BI in liver and peripheral tissues. J Clin Invest. 2006;116:2767–2776. doi: 10.1172/JCI26893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangiri A, de Beer MC, Noffsinger V, Tannock LR, Ramaiah C, Webb NR, van der Westhuyzen DR, de Beer FC. HDL remodeling during the acute phase response. Arterioscler Thromb Vasc Biol. 2009;29:261–267. doi: 10.1161/ATVBAHA.108.178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik GP, Rajagopalan R, Rosenthal EA, Wolfbauer G, McKinstry L, Vaze A, Brunzell J, Motulsky AG, Nickerson DA, Heagerty PJ, et al. Genetic and nongenetic sources of variation in phospholipid transfer protein activity. J Lipid Res. 2010;51:983–990. doi: 10.1194/jlr.M000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Wang N, Ramakrishnan R, Sehayek E, Huszar D, Breslow JL, Tall AR. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J Biol Chem. 1999;274:33398–33402. doi: 10.1074/jbc.274.47.33398. [DOI] [PubMed] [Google Scholar]
- Jiang XC, Qin S, Qiao C, Kawano K, Lin M, Skold A, Xiao X, Tall AR. Apolipoprotein B secretion and atherosclerosis are decreased in mice with phospholipid-transfer protein deficiency. Nat Med. 2001;7:847–852. doi: 10.1038/89977. [DOI] [PubMed] [Google Scholar]
- Joyce CW, Wagner EM, Basso F, Amar MJ, Freeman LA, Shamburek RD, Knapper CL, Syed J, Wu J, Vaisman BL, et al. ABCA1 overexpression in the liver of LDLr-KO mice leads to accumulation of pro-atherogenic lipoproteins and enhanced atherosclerosis. J Biol Chem. 2006;281:33053–33065. doi: 10.1074/jbc.M604526200. [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, van Leuven SI, Burgess L, Evans GW, Kuivenhoven JA, Barter PJ, Revkin JH, Grobbee DE, Riley WA, Shear CL, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356:1620–1630. doi: 10.1056/NEJMoa071359. [DOI] [PubMed] [Google Scholar]
- Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna R, Anderson MS, Bergman AJ, Jin B, Fallon M, Cote J, Rosko K, Chavez-Eng C, Lutz R, Bloomfield DM, et al. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet. 2007;370:1907–1914. doi: 10.1016/S0140-6736(07)61813-3. [DOI] [PubMed] [Google Scholar]
- Kuvin JT, Ramet ME, Patel AR, Pandian NG, Mendelsohn ME, Karas RH. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am Heart J. 2002;144:165–172. doi: 10.1067/mhj.2002.123145. [DOI] [PubMed] [Google Scholar]
- Lagor WR, Rader DJ. Phospholipidation of HDL—how much is too much. Clin Chem. 2011;57:4–6. doi: 10.1373/clinchem.2010.156224. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis. Circulation. 2004;109:II27–II33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- Li H, Reddick RL, Maeda N. Lack of apoA-I is not associated with increased susceptibility to atherosclerosis in mice. Arterioscler Thromb. 1993;13:1814–1821. doi: 10.1161/01.atv.13.12.1814. [DOI] [PubMed] [Google Scholar]
- Lie J, de Crom R, van Gent T, van Haperen R, Scheek L, Lankhuizen I, van Tol A. Elevation of plasma phospholipid transfer protein in transgenic mice increases VLDL secretion. J Lipid Res. 2002;43:1875–1880. doi: 10.1194/jlr.m200166-jlr200. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest. 2011;121:1163–1173. doi: 10.1172/JCI41651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D, Jiang XC, Lagrost L, Tall AR. The role of plasma lipid transfer proteins in lipoprotein metabolism and atherogenesis. J Lipid Res. 2009;50:S201–S206. doi: 10.1194/jlr.R800061-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D, Deckert V, Gautier T, Klein A, Desrumaux C, Viglietta C, Pais de Barros JP, Le Guern N, Grober J, Labbe J, et al. Worsening of diet-induced atherosclerosis in a new model of transgenic rabbit expressing the human plasma phospholipid transfer protein. Arterioscler Thromb Vasc Biol. 2011;31:766–774. doi: 10.1161/ATVBAHA.110.215756. [DOI] [PubMed] [Google Scholar]
- Matsuura F, Wang N, Chen W, Jiang XC, Tall AR. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J Clin Invest. 2006;116:1435–1442. doi: 10.1172/JCI27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo C, Shaul PW. Role of high-density lipoprotein and scavenger receptor B type I in the promotion of endothelial repair. Trends Cardiovasc Med. 2007;17:156–161. doi: 10.1016/j.tcm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J Biol Chem. 2003;278:9142–9149. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- Modesto KM, Dispenzieri A, Gertz M, Cauduro SA, Khandheria BK, Seward JB, Kyle R, Wood CM, Bailey KR, Tajik AJ, et al. Vascular abnormalities in primary amyloidosis. Eur Heart J. 2007;28:1019–1024. doi: 10.1093/eurheartj/ehm066. [DOI] [PubMed] [Google Scholar]
- Moore RE, Kawashiri MA, Kitajima K, Secreto A, Millar JS, Pratico D, Rader DJ. Apolipoprotein A-I deficiency results in markedly increased atherosclerosis in mice lacking the LDL receptor. Arterioscler Thromb Vasc Biol. 2003;23:1914–1920. doi: 10.1161/01.ATV.0000092328.66882.F5. [DOI] [PubMed] [Google Scholar]
- Moore RE, Navab M, Millar JS, Zimetti F, Hama S, Rothblat GH, Rader DJ. Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation. Circ Res. 2005;97:763–771. doi: 10.1161/01.RES.0000185320.82962.F7. [DOI] [PubMed] [Google Scholar]
- Navab M, Hama SY, Cooke CJ, Anantharamaiah GM, Chaddha M, Jin L, Subbanagounder G, Faull KF, Reddy ST, Miller NE, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res. 2000;41:1481–1494. [PubMed] [Google Scholar]
- Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res. 2001;42:1308–1317. [PubMed] [Google Scholar]
- Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Dusting GJ, Cutri B, Bao S, Drummond GR, Rye KA, Barter PJ. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–1550. doi: 10.1161/01.CIR.0000159351.95399.50. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Yonemori F, Wakitani K, Minowa T, Maeda K, Shinkai H. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 2000;406:203–207. doi: 10.1038/35018119. [DOI] [PubMed] [Google Scholar]
- Out R, Jessup W, Le Goff W, Hoekstra M, Gelissen IC, Zhao Y, Kritharides L, Chimini G, Kuiper J, Chapman MJ, et al. Coexistence of foam cells and hypocholesterolemia in mice lacking the ABC transporters A1 and G1. Circ Res. 2008;102:113–120. doi: 10.1161/CIRCRESAHA.107.161711. [DOI] [PubMed] [Google Scholar]
- Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump AS, Masucci-Magoulas L, Bruce C, Bisgaier CL, Breslow JL, Tall AR. Increased atherosclerosis in ApoE and LDL receptor gene knock-out mice as a result of human cholesteryl ester transfer protein transgene expression. Arterioscler Thromb Vasc Biol. 1999;19:1105–1110. doi: 10.1161/01.atv.19.4.1105. [DOI] [PubMed] [Google Scholar]
- Proudfoot JM, Barden AE, Loke WM, Croft KD, Puddey IB, Mori TA. HDL is the major lipoprotein carrier of plasma F2-isoprostanes. J Lipid Res. 2009;50:716–722. doi: 10.1194/jlr.M800607-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader DJ. Lecithin/cholesterol acyltransferase and atherosclerosis: another high-density lipoprotein story that doesn't quite follow the script. Circulation. 2009;120:549–552. doi: 10.1161/CIRCULATIONAHA.109.881979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramet ME, Ramet M, Lu Q, Nickerson M, Savolainen MJ, Malzone A, Karas RH. High-density lipoprotein increases the abundance of eNOS protein in human vascular endothelial cells by increasing its half-life. J Am Coll Cardiol. 2003;41:2288–2297. doi: 10.1016/s0735-1097(03)00481-9. [DOI] [PubMed] [Google Scholar]
- Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong JX, Li J, Reis ED, Choudhury RP, Dansky HM, Elmalem VI, Fallon JT, Breslow JL, Fisher EA. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–2452. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180:1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- Schaefer EJ. Clinical, biochemical, and genetic features in familial disorders of high density lipoprotein deficiency. Arteriosclerosis. 1984;4:303–322. doi: 10.1161/01.atv.4.4.303. [DOI] [PubMed] [Google Scholar]
- Schaefer EJ, Zech LA, Schwartz DE, Brewer HB., Jr Coronary heart disease prevalence and other clinical features in familial high-density lipoprotein deficiency (Tangier disease) Ann Intern Med. 1980;93:261–266. doi: 10.7326/0003-4819-93-2-261. [DOI] [PubMed] [Google Scholar]
- Schaefer EJ, Santos RD, Asztalos BF. Marked HDL deficiency and premature coronary heart disease. Curr Opin Lipidol. 2010;21:289–297. doi: 10.1097/MOL.0b013e32833c1ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CC, Halloran LG, Vlahcevic ZR, Gregory DH, Swell L. Preferential utilization of free cholesterol from high-density lipoproteins for biliary cholesterol secretion in man. Science. 1978;200:62–64. doi: 10.1126/science.204996. [DOI] [PubMed] [Google Scholar]
- Schwartz CC, Berman M, Vlahcevic ZR, Swell L. Multicompartmental analysis of cholesterol metabolism in man. Quantitative kinetic evaluation of precursor sources and turnover of high density lipoprotein cholesterol esters. J Clin Invest. 1982;70:863–876. doi: 10.1172/JCI110683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PK, Nilsson J, Kaul S, Fishbein MC, Ageland H, Hamsten A, Johansson J, Karpe F, Cercek B. Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation. 1998;97:780–785. doi: 10.1161/01.cir.97.8.780. [DOI] [PubMed] [Google Scholar]
- Shao B, Heinecke JW. HDL, lipid peroxidation, and atherosclerosis. J Lipid Res. 2009;50:599–601. doi: 10.1194/jlr.E900001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao B, Cavigiolio G, Brot N, Oda MN, Heinecke JW. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc Natl Acad Sci USA. 2008;105:12224–12229. doi: 10.1073/pnas.0802025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao B, Pennathur S, Pagani I, Oda MN, Witztum JL, Oram JF, Heinecke JW. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J Biol Chem. 2010;285:18473–18484. doi: 10.1074/jbc.M110.118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- Simic B, Hermann M, Shaw SG, Bigler L, Stalder U, Dorries C, Besler C, Luscher TF, Ruschitzka F. Torcetrapib impairs endothelial function in hypertension. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr348. DOI: 10.1093/eurheartj/ehr348. [DOI] [PubMed] [Google Scholar]
- Singaraja RR, Fievet C, Castro G, James ER, Hennuyer N, Clee SM, Bissada N, Choy JC, Fruchart JC, McManus BM, et al. Increased ABCA1 activity protects against atherosclerosis. J Clin Invest. 2002;110:35–42. doi: 10.1172/JCI15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH, Mueller M, Horvath T, Doerries C, Heinemann M, et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121:110–122. doi: 10.1161/CIRCULATIONAHA.108.836346. [DOI] [PubMed] [Google Scholar]
- Spieker LE, Sudano I, Hurlimann D, Lerch PG, Lang MG, Binggeli C, Corti R, Ruschitzka F, Luscher TF, Noll G. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002;105:1399–1402. doi: 10.1161/01.cir.0000013424.28206.8f. [DOI] [PubMed] [Google Scholar]
- Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- Tailleux A, Duriez P, Fruchart JC, Clavey V. Apolipoprotein A-II, HDL metabolism and atherosclerosis. Atherosclerosis. 2002;164:1–13. doi: 10.1016/s0021-9150(01)00751-1. [DOI] [PubMed] [Google Scholar]
- Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- Tanigawa H, Billheimer JT, Tohyama J, Zhang Y, Rothblat G, Rader DJ. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation. 2007;116:1267–1273. doi: 10.1161/CIRCULATIONAHA.107.704254. [DOI] [PubMed] [Google Scholar]
- Tanigawa H, Billheimer JT, Tohyama J, Fuki IV, Ng DS, Rothblat GH, Rader DJ. Lecithin/cholesterol acyltransferase expression has minimal effects on macrophage reverse cholesterol transport in vivo. Circulation. 2009;120:160–169. doi: 10.1161/CIRCULATIONAHA.108.825109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif JC, Gregoire J, L'Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin. 2006;22:2243–2250. doi: 10.1185/030079906x148508. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Villines TC, Stanek EJ, Devine PJ, Griffen L, Miller M, Weissman NJ, Turco M. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361:2113–2122. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T, Li R, Welch CL, Goldberg IJ, et al. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest. 2008;118:3701–3713. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka N, Westerterp M, Koetsveld J, Fernandez-Hernando C, Yvan-Charvet L, Wang N, Sessa WC, Tall AR. ATP-binding cassette transporter G1 and high-density lipoprotein promote endothelial NO synthesis through a decrease in the interaction of caveolin-1 and endothelial NO synthase. Arterioscler Thromb Vasc Biol. 2010;30:2219–2225. doi: 10.1161/ATVBAHA.110.213215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360–381. [PubMed] [Google Scholar]
- Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uittenbogaard A, Shaul PW, Yuhanna IS, Blair A, Smart EJ. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem. 2000;275:11278–11283. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Craeyveld E, Lievens J, Jacobs F, Feng Y, Snoeys J, De Geest B. Apolipoprotein A-I and lecithin/cholesterol acyltransferase transfer induce cholesterol unloading in complex atherosclerotic lesions. Gene Ther. 2009;16:757–765. doi: 10.1038/gt.2009.8. [DOI] [PubMed] [Google Scholar]
- Van Craeyveld E, Gordts SC, Nefyodova E, Jacobs F, De Geest B. Regression and stabilization of advanced murine atherosclerotic lesions: a comparison of LDL lowering and HDL raising gene transfer strategies. J Mol Med (Berl) 2011;89:555–567. doi: 10.1007/s00109-011-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eck M, Bos IS, Kaminski WE, Orso E, Rothe G, Twisk J, Bottcher A, Van Amersfoort ES, Christiansen-Weber TA, Fung-Leung WP, et al. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc Natl Acad Sci USA. 2002;99:6298–6303. doi: 10.1073/pnas.092327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eck M, Singaraja RR, Ye D, Hildebrand RB, James ER, Hayden MR, Van Berkel TJ. Macrophage ATP-binding cassette transporter A1 overexpression inhibits atherosclerotic lesion progression in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:929–934. doi: 10.1161/01.ATV.0000208364.22732.16. [DOI] [PubMed] [Google Scholar]
- van Haperen R, van Tol A, van Gent T, Scheek L, Visser P, van der Kamp A, Grosveld F, de Crom R. Increased risk of atherosclerosis by elevated plasma levels of phospholipid transfer protein. J Biol Chem. 2002;277:48938–48943. doi: 10.1074/jbc.M209128200. [DOI] [PubMed] [Google Scholar]
- Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–2767. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001;103:2283–2288. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- Vergeer M, Boekholdt SM, Sandhu MS, Ricketts SL, Wareham NJ, Brown MJ, de Faire U, Leander K, Gigante B, Kavousi M, et al. Genetic variation at the phospholipid transfer protein locus affects its activity and high-density lipoprotein size and is a novel marker of cardiovascular disease susceptibility. Circulation. 2010;122:470–477. doi: 10.1161/CIRCULATIONAHA.109.912519. [DOI] [PubMed] [Google Scholar]
- Vergeer M, Korporaal SJ, Franssen R, Meurs I, Out R, Hovingh GK, Hoekstra M, Sierts JA, Dallinga-Thie GM, Motazacker MM, et al. Genetic variant of the scavenger receptor BI in humans. N Engl J Med. 2011;364:136–145. doi: 10.1056/NEJMoa0907687. [DOI] [PubMed] [Google Scholar]
- Voyiaziakis E, Goldberg IJ, Plump AS, Rubin EM, Breslow JL, Huang LS. ApoA-I deficiency causes both hypertriglyceridemia and increased atherosclerosis in human apoB transgenic mice. J Lipid Res. 1998;39:313–321. [PubMed] [Google Scholar]
- Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res. 2009;50:574–585. doi: 10.1194/jlr.D800028-JLR200. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BJ, Yan L, Charlton F, Witting P, Barter PJ, Rye KA. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler Thromb Vasc Biol. 2010;30:968–975. doi: 10.1161/ATVBAHA.109.201129. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Tanigawa H, Li X, Komaru Y, Billheimer JT, Rader DJ. Pharmacologic suppression of hepatic ATP-binding cassette transporter 1 activity in mice reduces high-density lipoprotein cholesterol levels but promotes reverse cholesterol transport. Circulation. 2011;124:1382–1390. doi: 10.1161/CIRCULATIONAHA.110.009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XP, Yan D, Qiao C, Liu RJ, Chen JG, Li J, Schneider M, Lagrost L, Xiao X, Jiang XC. Increased atherosclerotic lesions in apoE mice with plasma phospholipid transfer protein overexpression. Arterioscler Thromb Vasc Biol. 2003;23:1601–1607. doi: 10.1161/01.ATV.0000085841.55248.13. [DOI] [PubMed] [Google Scholar]
- Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler TA, Wang N, Senokuchi T, Brundert M, Li H, Rinninger F, Tall AR. SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL. J Lipid Res. 2008;49:107–114. doi: 10.1194/jlr.M700200-JLR200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]