SUMMARY
Interleukin-25 (IL-25 or IL-17E), a member of the structurally related IL-17 family, functions as an important mediator of T helper 2 cell-type (type 2) responses. We examined the cell-type specific role of IL-25-induced Act1-mediated signaling in protective immunity against helminth infection. Targeted Act1 deficiency in epithelial cells resulted in a marked delay in worm expulsion and abolished the expansion of the Lin−c-kit+ innate cell population in the mesenteric lymph node, lung and liver. Th2 cell-inducing cytokines (IL-25 and IL-33) expression were reduced in the intestinal epithelial cells from the infected and IL-25-injected epithelial-specific Act1-deficient mice. Adoptive transfer of Lin−c-kit+ cells or combined injection of IL-25 and IL-33 restored the type 2 responses in these mice. Taken together, these results suggest that epithelial-specific Act1 mediates the expansion of the Lin−c-kit+ innate cell population through the positive feedback loop of IL-25, initiating the type 2 immunity against helminth infection.
INTRODUCTION
T helper 2 (Th2) cell-type cytokines play a critical role in protective immunity against parasitic helminth infections and also mediate the pathogenesis of allergy and asthma(Paul and Zhu, 2010). An important question is how the initiation of Th2 cell-type immune responses (type-2 response) takes place in vivo. Emerging studies have begun to reveal the mechanisms by which the epithelium modulates type-2 responses through the production of a group of epithelial-derived Th2 cell-driving cytokines, including IL-25, IL-33 and TSLP(Artis, 2008;Holgate, 2007a;Kato and Schleimer, 2007;Saenz et al., 2008). These epithelial-derived Th2 cell-driving cytokines maintain the balance of host immune homeostasis and defense against various pathogens(Anthony et al., 2007;Gregory et al., 2010;Strickland et al., 2010).
Homology-based cloning has revealed six IL-17 family members, termed IL-17A to IL-17F. IL-17A (IL-17), a prototypic member of IL-17 family, is required for host defense against extracellular microorganisms and involved in the pathogenesis of various human and animal autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and experimental autoimmune encephalomyelitis (EAE)(Chang and Dong, 2011;Iwakura et al., 2011). The most divergent member of the IL-17 family is IL-17E (IL-25); it is induced in airway epithelial cells in response to allergens, expressed in mouse T lymphocytes of the CD4+ subset with a Th2 cell profile and human innate effector eosinophils and basophils(Fort et al., 2001;Zaph et al., 2007). Transgenic expression as well as recombinant IL-25 induce Th2 cell immunity, and increase Th2 cell-type cytokines IL-4, IL-5, IL-13, eosinophilia and IgE(Angkasekwinai et al., 2007;Strickland et al., 2010;Wang et al., 2007;Zaph et al., 2008). Endogenous IL-25 has been shown to be critical in allergen-induced pulmonary inflammation in a mouse asthma model(Pan et al., 2001). Elevated IL-25 and IL-25R expression were detected in asthmatic lung tissues, linking their roles in allergic pulmonary inflammation(Fort et al., 2001). In IL-25-deficient mice, expulsion of helminth parasites is delayed, indicating the essential role of IL-25 signaling in protective immunity against helminth infection(Fallon et al., 2006;Owyang et al., 2006;Zhao et al., 2010).
IL-17 receptor (IL-17RA and IL-17RC) and IL-25R (IL-17RB and IL-17RA) belong to a newly defined SEFIR protein family, due to a conserved sequence segment called SEFIR in their cytoplasmic domain(Novatchkova et al., 2003). Act1 is a key component in IL-17 and IL-25 signaling (Claudio et al., 2009;Novatchkova et al., 2003;Qian et al., 2007;Swaidani et al., 2009). Act1 contains two TRAF binding sites, a helix-loop-helix domain at the N-terminus, and a SEFIR domain at the C-terminus and therefore, Act1 is also a member of the SEFIR protein family(Li, 2008). Upon IL-17 and IL-25 stimulation, Act1 is recruited to IL-17R and IL-25R through its SEFIR domain. We recently found that Act1 also contains a U-box-like region and has E3 ubiquitin ligase activity(Liu et al., 2009), which is critical for its function. Mice deficient in Act1 have reduced allergen-induced pulmonary eosinophilia. Furthermore, Act1 deficiency in epithelial cells resulted in diminished type-2 responses and less lung inflammation.
Previous studies showed that CD4 mAb treatment attenuates expulsion of N. brasiliensis, indicating the importance of CD4+ T cells in protective immunity against parasite infection(Day et al., 1979;Katona et al., 1988). IL-25 alone can polarize naïve CD4+ T cells to Th2 cells(Wang et al., 2007) and augments type-2 immune responses by enhancing the expansion and function of TSLP-DC-activated Th2 memory cells(Angkasekwinai et al., 2007). We found that Act1 deficiency in the T cell compartment results in the abrogation of eosinophilic airway infiltration as well as airway hyperresponsiveness (AHR) in a mouse model of antigen-induced airway inflammation(Swaidani et al., 2011). Our findings suggest that Act1 expression in T cells is required for cellular and humoral Th2 cell-mediated allergic responses through its function in IL-25-induced development of Th2 cells.
Several studies have also reported that IL-25 elicits an innate immune cell population(s) by using helminth models, which is capable of shaping Th2 cell immune responses and required for worm expulsion(Neill and McKenzie, 2011;Saenz et al., 2010a). IL-25-induced innate effector cell populations [termed either natural helper cells (NHCs), multi-potent progenitor type 2 (MPPtype2) cells, nuocytes or innate type 2 helper (Ih2) cells] for type 2 immunity that share similar features, all lacking expression of lineage-defining markers with c-Kit expression (Fallon et al., 2006;Moro et al., 2010;Neill et al., 2010;Price et al., 2010;Saenz et al., 2010b). Following exposure to helminth infections or allergens, epithelial cells located at mucosal surfaces secrete IL-25 and IL-33. IL-25 has been shown to induce the population expansion of MPPtype2 cells, which can be differentiated into macrophages, basophils and mast cell populations. While IL-25 and IL-33 have the capacity to expand IL-13 (and IL-5) producing nuocytes and Ih2 cells in vivo, NHCs have been shown to produce IL-4, IL-5 and IL-13 following treatment with IL-33 and IL-25. The functional commonality of MPPtype2 cells, nuocytes, Ih2 cells and NHCs is to promote CD4+ Th2 cell cytokine responses and type 2 inflammation.
In this study, we used cell-type specific Act1 (a key adaptor for IL-25 signaling)-deficient mice to examine how IL-25-mediated signaling in different cellular compartments participates in protective immunity against helminth infection through its impact on the Lin−c-Kit+ innate immune cell population. Act1 deficiency in macrophages or in T cells did not substantially impact on worm expulsion. However, targeted Act1 deficiency in epithelial cells resulted in diminished Linc-Kit+ innate immune cell population in the mesenteric lymph nodes(MLN), reduced Th2 cell-type cytokine expression and a significant delay in worm expulsion. Furthermore, the expression of IL-33, IL-25 was reduced in the intestinal epithelial cells (IECs) from the infected epithelial-specific Act1-deficient mice compared to that in wild-type control mice. These findings suggest that epithelial-specific Act1 is critical for IL-25-dependent expansion of the Lin−c-Kit+ innate cell population probably through the amplification of Th2 cell-driving cytokines (IL-33 and IL-25) and consequent helminth expulsion. This study provides an important cellular and molecular mechanism for how the initiation of type-2 immune responses takes place in vivo. Specifically, we elucidate how Epithelial-specific Act1 mediates IL-25-dependent helminth expulsion through the positive feedback loop of IL-25.
RESULTS
Act1 deficiency attenuated the initiation of type 2 responses and delayed worm expulsion
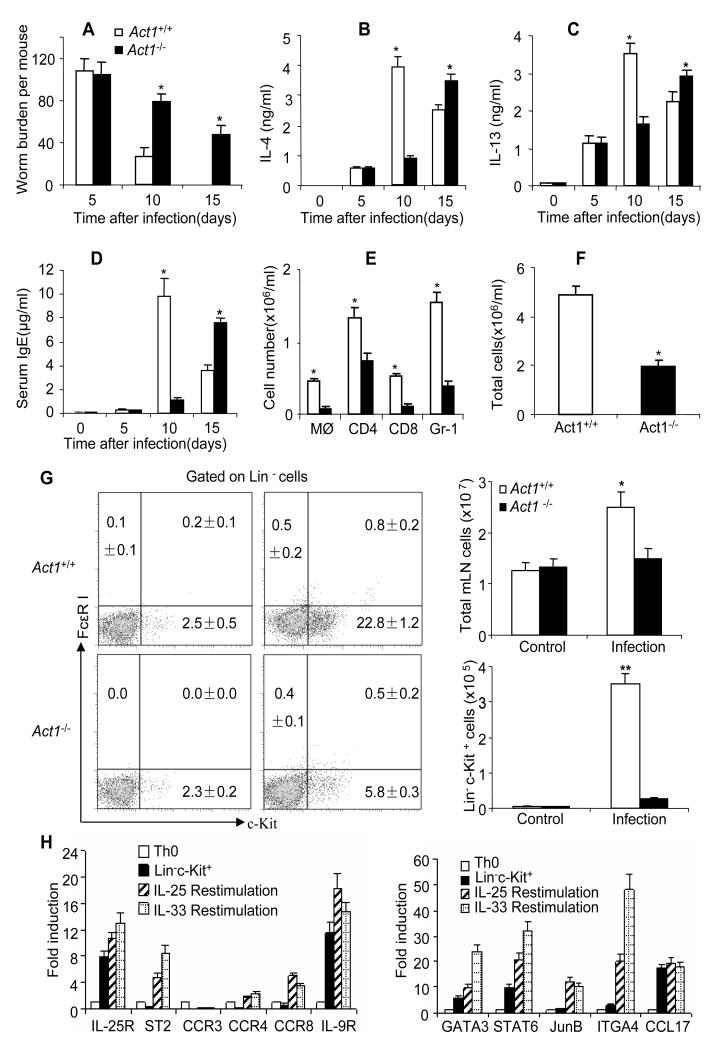
To determine the role of IL-25-induced Act1-mediated signaling in protective immunity against gastrointestinal helminth parasites, wild-type (WT) and Act1-deficient (Act1−/−) mice were infected with N. brasiliensis larvae and sacrificed 5, 10 and 15 days after infection. The infected mice were monitored for worm burden and type-2 responses. As shown in Figure 1A, the WT and Act1−/− mice showed similar worm burdens at day 5 after infection; but the WT mice displayed reduced worm burden compared to the Act1−/− mice by day 10 and day 15 after infection. However, worm burdens eventually went down in Act1−/− mice 20 days after infection, suggesting Act1 deficiency did not completely abolish type-2 immunity, but rather delayed it. We next assessed Th2 cell-type cytokine production from the mesenteric lymph nodes of the infected WT and Act1−/− mice. The WT and Act1−/− mesenteric lymph node cells from the infected mice were re-stimulated with CD3 and CD28 antibodies, followed by measurement for Th2 cell-type cytokines. While similar amounts of IL-4 and IL-13 were detected in the WT and Act1−/− mice at day 5 after infection, the Act1−/− mice produced substantially less IL-4 and IL-13 than the WT mice by day 10 after infection(Figure 1B-C). Interestingly, the production of IL-4 and IL-13 was actually increased in Act1−/− mice by day 15 after infection (Figure 1B-C), probably due to the persistent higher worm burden in the Act1−/− mice compared to the WT mice. These data suggest that Act1 deficiency attenuated the initiation of type-2 responses, resulting in delayed worm expulsion. In support of this, we noted that the IgE concentrations in the serum peaked at a much later time in Act1−/− mice (day 15) as compared to that in WT mice (day 10) in response to infection (Figure 1D). Since Th2 cell-type cytokines are known to mediate lung inflammation during N. brasiliensis infection, we analyzed inflammatory cell infiltration in the bronchoalveolar lavage (BAL) in infected WT and Act1−/− mice. There was more leukocyte infiltration in BAL of the WT mice than that in Act1−/− mice, including macrophages, granulocytes and T cells (Figure 1E-F). Furthermore, the Th2 cell-associated gene expression (Il4, Il5, Il13, Ccl11, Il25 and Tslp) was substantially reduced in the lung tissues of the infected Act1−/− mice compared to that in WT mice (Figure S1A).
Figure 1. Act1 deficiency resulted in reduced type 2 responses and delayed worm expulsion.
(A) Act1−/− and Act1+/+ mice were sacrificed 5, 10 and 15 days after infection, followed by analysis of worm burden in intestine of each mouse. (B-C) Mesenteric lymph nodes were taken from the infected mice at indicated time points. Cells were re-stimulated by anti-CD3 and anti-CD28 for overnight, followed by IL-4 and IL-13 ELISA in the collected supernatants. (D) Serum IgE levels were assayed by ELISA. (E-F) Different cell subsets in BAL of Act1-deficient and wild-type control mice were analyzed by flow cytometry 5 days after infection. Total cell numbers and absolute number of each subset were shown. (G) Act1-deficient and wild-type control mice were sacrificed 5 days after infection and mesenteric lymph nodes were collected. Plots shown are gated on live, lineage negative cells(CD3ε, CD4, CD8, TCRβ, TCRγδ, B220, CD19, NK1.1, Ter119, Gr-1, CD11b and CD11c), The frequency of Lin−c-kit+ cells(left panel) and absolute numbers were shown(right panel). All the data represent mean (n=4)±SEM; *p<0.05, **p<0.01. (H)Lin−c-kit+ cells were sorted by MACS and FACS, then sorted cells were restimulated by IL-25 or IL-33, Th0 helper T cells and unrestimulated Lin−c-kit+ cells were included as controls, specific gene expression was shown as mean(n=4)±SEM. The experiment was repeated three times. See also Figure S1;S2.
Previous studies have shown that infection with N. brasiliensis induces a population of Lin-c-kit+ cells that is required for worm expulsion. We analyzed this population in the mesenteric lymph nodes of WT and Act1−/− mice 5 days after infection. The frequency and absolute number of Lin−c-kit+ cells were dramatically increased in wild-type mice (about 30-fold) but not in Act1−/− mice (Figure 1G). Consistent with the literature, this Lin−c-kit+ innate effector cell population expresses IL-5 and IL-13 (Figure S1B) and a unique set of cell surface markers [including IL-25R, IL-33R (T1/ST2), IL-9R and ITGA4] (Figure 1H). In addition, we also detected expression of certain chemokine receptors such as CCR4 and CCR8 in these Lin−kit+ innate effector cells, which might play an important in the chemotaxis of this cell population during infection (Figure 1H). The expression of the cell surface markers and several important transcription factors (GATA3, STAT6 and JunB) for Th2 cell-type cytokine gene expression were increased upon IL-25 and IL-33 stimulation (Figure 1H). Taken together, Act1 deficiency resulted in reduced expansion of the innate effector cell population, attenuated type-2 responses and delayed worm expulsion.
Act1 is a key component in IL-17 and IL-25 signaling. While Act1 is required for IL-17-dependent signaling and autoimmune inflammatory disease(Qian et al., 2007), Act1 deficiency abolishes IL-25-induced expression of Th2cell-type cytokines and pulmonary eosinophilia(Claudio et al., 2009;Swaidani et al., 2009). While expulsion of helminth parasites is delayed in Il25−/− mice(Fallon et al., 2006;Owyang et al., 2006), it remains unclear whether IL-17 signaling has any impact on the protective immunity against helminth infection. IL-17 signals through a heteromeric receptor complex, consisting of IL-17RA and IL-17RC(Gaffen, 2009;Ho and Gaffen, 2010;Toy et al., 2006;Trajkovic et al., 2001). To determine the impact of IL-17 signaling on worm expulsion, wild-type, Il17a−/− mice were infected with N. brasiliensis larvae and sacrificed 10 and 15 days after infection. IL-17A deficiency did not have substantial impact on worm burden and type-2 responses (Figure S2). These results indicate that IL-17 signaling is dispensable for N. brasiliensis expulsion. The impact of Act1 deficiency on worm expulsion is probably due to the disruption of IL-25 signaling during infection.
Macrophage- and T cell-specific Act1 are dispensable for helminth expulsion
While IL-25 signaling has been shown to play a critical role in worm expulsion, it remains unclear what specific cell types directly participate in the IL-25-Act1-mediated protective immunity. Macrophages are innate immune cells with well-established roles not only in the primary response to pathogens, but also in tissue homeostasis, coordination of the adaptive immune response, inflammation, resolution, and repair (Martinez et al., 2009). Macrophages, known for their association with IFN-γ-dominant Th1 cell-type responses to infections of bacteria and viruses, also play an essential role in the type-2 inflammatory response to helminthes(Kreider et al., 2007). These macrophages have been classified as alternatively activated macrophages (AAMΦs) as they express a specific set of signature genes including RELMα, Ym1 and Arginase1. Rapid increases of AAMΦs are observed in the lung by four days after N. brasiliensis infection and this increase is associated with elevated RELMα, Ym1 and Arginase1(Siracusa et al., 2008).
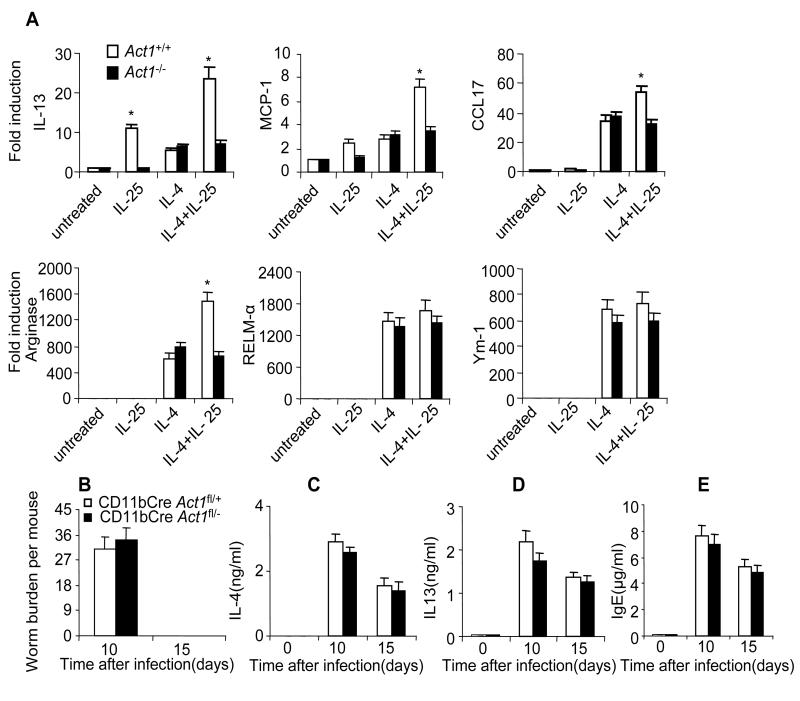
One important question is whether IL-25 can augment alternative macrophage activation. To test this, we treated bone-marrow-derived macrophages (BMDMs) with IL-25, IL-4 or IL-25+IL-4 for 24 hours, followed by analysis of the signature genes associated with alternatively activated macrophages (AAMΦs). While IL-25 stimulation alone did not induce the expression of Arginase 1, Ym1 and RELMα, IL-25 had a synergistic effect on IL-4-induced Arginase 1 expression. Interestingly, IL-25 alone up-regulated the expression of IL-13 and MCP-1 in BMDMs and also synergized with IL-4 to further induce the expression of IL-13, MCP-1 and CCL-17 (Figure 2A). Importantly, the IL-25-mediated impact on gene expression in BMDMs was Act1-dependent.
Figure 2. Macrophage Act1 is dispensable for worm expulsion.
(A) Bone marrow macrophages from Act1−/− and Act1+/+ mice were untreated or treated with IL-25 (100ng/ml), IL-4 (10ng/ml) or IL-25+IL-4 for 24 hours, followed by analysis of gene expression by real-time PCR. (B) CD11bCre Act1fl/− mice and CD11bCre Act1fl/+ were sacrificed 10 and 15 days after infection, followed by analysis of worm burden in intestine of each mouse. (C-D) Mesenteric lymph nodes were taken from the infected mice at indicated time points. Cells were re-stimulated by anti-CD3 and anti-CD28 for overnight, followed by IL-4 and IL-13 ELISA in the collected supernatants. (E) Serum IgE levels were measured by ELISA. Data represent mean(n=4)±SEM; *p<0.05. The experiment was repeated three times.
We then tested if deletion of Act1 in macrophages would have an effect on worm expulsion in vivo. CD11bCre transgenic mice (generated using the promoter for CD11b, an integrin expressed exclusively in the myeloid lineage) express Cre in macrophages and also in other myeloid cells such as neutrophils. CD11bCre Act1+/− mice were bred onto Act1fl/fl to generate control mice (CD11bCreAct1fl/+) and myeloid-specific Act1-deficient mice (CD11bCre Act1fl/−). The deletion of Act1 in macrophages has been confirmed (Kang et al., 2010). CD11bCre Act1fl/− mice and littermate control CD11bCreAct1fl/+ mice were infected by N. brasiliensis and sacrificed 10 and 15 days after infection. Myeloid-specific Act1 deficiency did not have substantial impact on worm burden and type-2 responses (Figure 2B-E). Furthermore, we also analyzed the Lin−c-kit+ cell population in the mesenteric lymph nodes (MLN) of control mice (CD11bCreAct1fl/+) and macrophage-specific Act1-deficient mice (CD11bCre Act1fl/−) 5 days after infection. The frequency and absolute number of Lin−c-kit+ cells were induced to similar levels in these two groups of mice (data not shown). Taken together, these results clearly indicate that that IL-25-induced Act1-mediated signaling in macrophages is dispensable for N. brasiliensis expulsion.
Expulsion of N. brasiliensis is a T-cell-dependent process(Voehringer et al., 2006). We recently found that Act1 deficiency in the T cell compartment results in the abrogation of eosinophilic airway infiltration as well as airway hyperresponsiveness (AHR) in a mouse model of antigen induced airway inflammation(Swaidani et al., 2011) Our findings suggested that Act1 expression in T cells is required for cellular and humoral Th2 cell-mediated allergic responses through its function in IL-25-induced development of Th2 cells. Therefore, it is important to determine whether T cell-specific Act1 is also critical for helminth expulsion. LckCre transgenic mice express Cre in T cell progenitor cells. LckCreAct1+/− mice were bred onto Act1fl/fl to generate control mice (LckCreAct1fl/+) and T cell-specific Act1-deficient mice (LckCreAct1fl/−). LckCreAct1fl/− mice and littermate control LckCreAct1fl/+ mice were infected by N. brasiliensis. T cell-specific Act1 deficiency did not have substantial impact on worm burden and type 2 responses (Supplemental Figure 3 and data not shown). Consistently, the Lin−c-kit+ cell population in the mesenteric lymph nodes (MLN) were induced to similar numbers 5 days after infection in control mice (LckCreAct1fl/+) and T cell-specific Act1-deficient mice (LckCreAct1fl/−) (data not shown). These results clearly indicate that IL-25-induced Act1-mediated signaling in T cells is dispensable for N. brasiliensis expulsion.
Epithelial cell-specific Act1 is critical for IL-25-dependent helminth expulsion
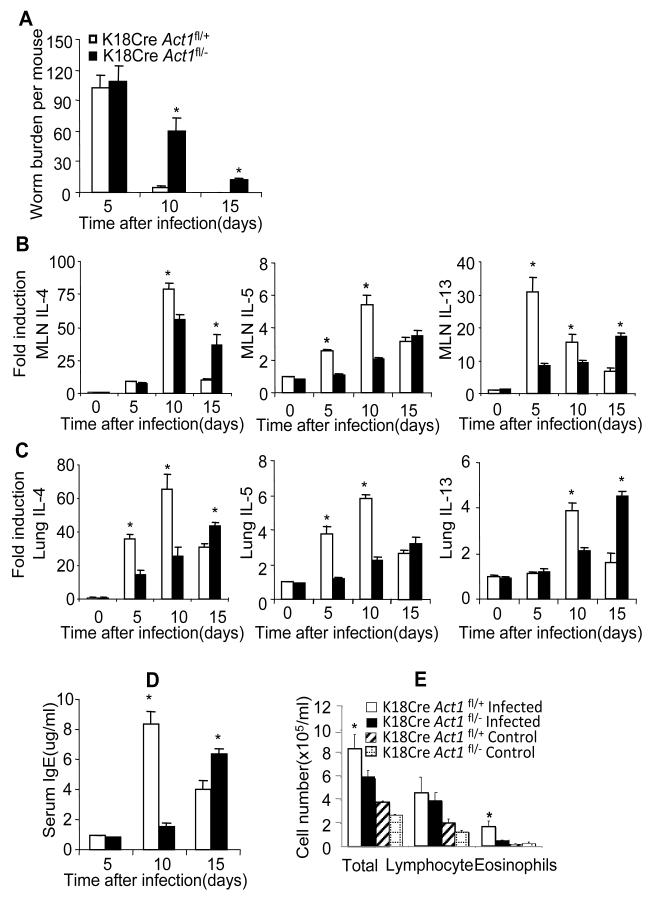
Previous studies suggest that epithelial cells play an important role in protective immunity against gastrointestinal helminth parasites (Herbert et al., 2009;Zaph et al., 2007). Epithelial cells are a major source of IL-25, promoting Th2 cytokine responses at mucosal sites. One important observation from our previous study was that Act1 deletion in the epithelial compartment resulted in attenuation of pulmonary inflammation following the induction of an antigen-induced murine asthma model. These results suggested that epithelial cells produce IL-25 and also respond to IL-25. Therefore, we examined whether the epithelial-specific Act1 is also critical for worm expulsion. K18Cre transgenic mice express Cre in epithelial cells. K18CreAct1+/− mice were bred onto Act1fl/fl to generate control mice (K18CreAct1fl/+) and epithelial-specific Act1-deficient mice (K18CreAct1fl/−). K18CreAct1fl/− mice and littermate control K18CreAct1fl/+ mice were infected by N. brasiliensis and sacrificed 5, 10 and 15 days after infection. Importantly, we observed that epithelial-specific Act1-deficient mice displayed very similar phenotype as the complete Act1-deficient mice in response to infection. Worm expulsion was substantially delayed in K18CreAct1fl/− mice compared to the K18CreAct1fl/+ control mice (Figure 3A). It should be noted that while worm burdens were greatly reduced in control mice 10 days after infection, worms were expelled in epithelial-specific Act1-deficient mice 20 days after infection (Figure 3A and data not shown). Consistent with the worm burden results, the serum IgE levels and the Th2 cytokines (IL-4, IL-5 and IL-13) in MLN and in the lung tissues, peaked at much later time in the epithelial-specific Act1-deficient mice (day 15) as compared to that in the K18CreAct1fl/+ mice (day 10) (Figure 3B-D). Leukocyte infiltration (especially eosinophils) in BAL was significantly decreased in the Act1-deficient mice 5 days after infection compared to that in wild-type mice (Figure 3E). Taken together, these data demonstrated that epithelial-specific Act1 signaling is critical for type 2 responses and worm expulsion.
Figure 3. Epithelial cell-specific Act1 is critical for type-2 dependent worm expulsion.
(A) Epithelial-specific Act1-deficient and littermate control mice were sacrificed 10 and 15 days after infection, followed by analysis of worm burden in intestine of each mouse. (B-C) The expression of IL-4, IL-5 and IL-13 in the lung and mesenteric lymph nodes after infection were analyzed by real-time PCR. (D) Serum IgE concentrations were measured by ELISA. (E) BAL infiltration. Data represent mean(n=4)±SEM; *p<0.05. The experiment was repeated three times.
Act1-mediated signaling in epithelium is required for the IL-25-induced expansion of Lin−c-kit+ cells
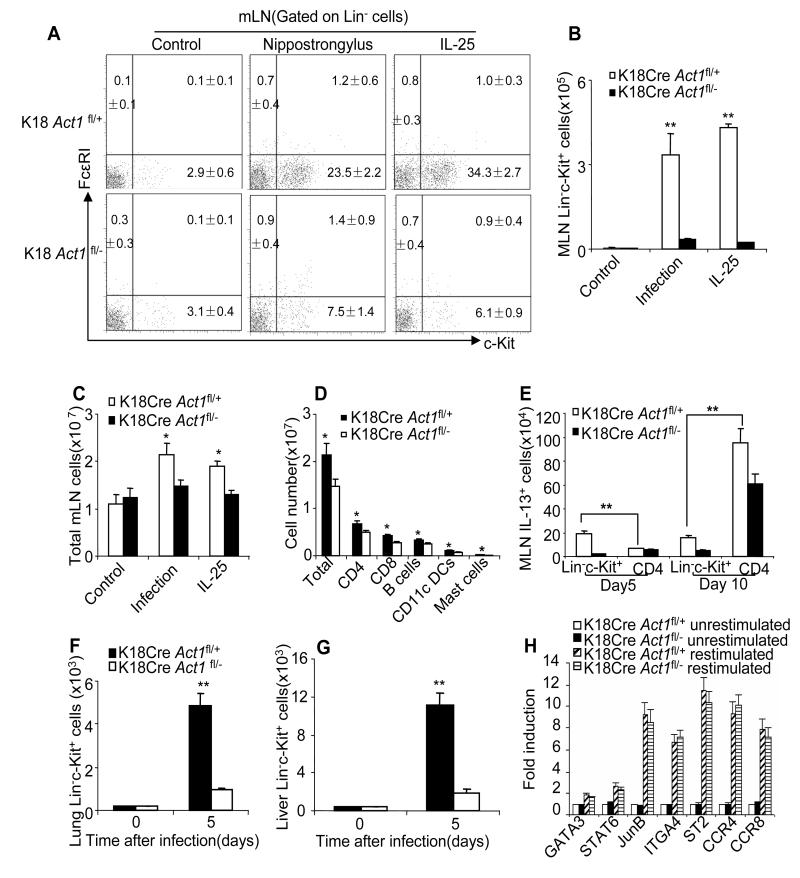
Because our results indicate that epithelial-specific Act1 is necessary for worm expulsion and type 2 responses, we hypothesized that IL-25 signaling in epithelium mediates the expansion of the Lin−c-Kit+ cell population that promotes Th2 responses. To test this hypothesis, we examined the Lin−c-Kit+ cell population in MLN of the IL-25-injected or worm infected epithelial-specific Act1-deficient (K18CreAct1fl/−) and control wild-type (K18CreAct1fl/+) mice (Figure 4A-B). IL-25-induced expansion of the Lin−c-kit+ cell population in MLN was much reduced in the epithelial-specific Act1-deficient (K18CreAct1fl/−) mice as compared to that in wild-type (K18CreAct1fl/+) mice. Similar results were obtained with worm-infected K18CreAct1fl/− and K18CreAct1fl/+ mice (Figure 4A-B). In addition, the total cell number of MLN cells and the absolute number of T cells, B cells, macrophages and mast cells were also moderately increased in both IL-25-treated and infected wild-type mice compared with that in epithelial Act1-deficient mice (Figure 4C-E and data not shown). However, the Lin−c-kit+ cell population expanded the most following IL-25 treatment and worm infection. While about 70% of the Lin−c-Kit+ cells were IL-13 positive, less than 5% of the CD4+ T cells were IL-13 producing cells in MLN 5 days after infection (data not shown). Act1 deficiency in epithelium greatly diminished the Lin−c-Kit+ cells but not CD4+ Th2 cell population 5 days after infection (Figure 4E). The IL-13-producing MLN CD4+ Th2 cell population was expanded 10 days after infection, which was only partially dependent on the epithelial-specific Act1.
Figure 4. Act1-mediated signaling in epithelium is required for the IL-25-induced expansion of Lin−c-kit+ cells.
(A) Frequencies of Lin−c-kit+ cells in the mesenteric lymph nodes of K18Cre Act1fl/− and K18Cre Act1fl/+ mice after 4 days of PBS or IL-25 treatment by i.p. or 5 days after worm infection. Plots shown are gated on lineage negative cells. (B-C) Total cell numbers (B) or Lin-c-kit+ cell numbers (C) in mesenteric lymph nodes from K18Cre Act1fl/− and K18Cre Act1fl/+ mice after 4 days of PBS or IL-25 treatment by i.p. or 5 days after worm infection. (D) Number of total MLN cells or of T cells(CD4+ or CD8+), B cells (CD19+), Dendritic cells(CD11c+) and mast cells from K18Cre Act1fl/− and K18Cre Act1fl/+ mice 5 days after worm infection. (E) Absolute number of MLN IL-13+ cells 5 or 10 days after infection. (F) Absolute number of Lin−c-kit+ cells in the lung. (G) Absolute number of Lin-c-kit+ cells in the liver. (H) Gene expression of MLN Lin−c-kit+ cells from K18Cre Act1fl/− and K18Cre Act1fl/+ mice 5 days after worm infection. Isolated cells were restimulated with IL-25, and cells without restimulation were included as controls. Data represent mean(n=4)±SEM; *p<0.05, **p<0.01. The experiment was repeated two times. See also Figure S3.
Previous studies have shown that Lin−c-kit+ cells can be induced systemically after worm infection (Price et al., 2010). Since K18 promoter allows expression of Cre in epithelium of all internal organs (including lung and liver), the K18CreAct1fl/− mice provide an important tool to examine the impact of Act1 deficiency in epithelium on the expansion of Lin−c-kit+ cell population in other organs in addition to the gut. Importantly, we indeed found that the Lin-c-Kit+ cells were dramatically expanded in the lung and liver of wild-type control mice, but not in that of epithelial-specific Act1-deficient mice (Figure 4F-G; Figure S3). These findings suggest that IL-25 signaling in epithelium plays a critical role in the expansion of the Lin−c-Kit+ cell population in several organs during worm infection, promoting type 2 responses and worm expulsion. In support of this, we found that the Lin−c-Kit+ cells sorted from the infected epithelial-specific Act1-deficient (K18CreAct1fl/−) mice were actually as responsive to IL-25 as those from wild-type (K18CreAct1fl/+) mice (Figure 4H), indicating the necessity of IL-25 signaling in epithelium for the initial expansion of the Lin−c-Kit+ cell population during worm infection.
Epithelial cells produce IL-25 and IL-33 to induce the expansion of the Lin−c-kit+ cell population
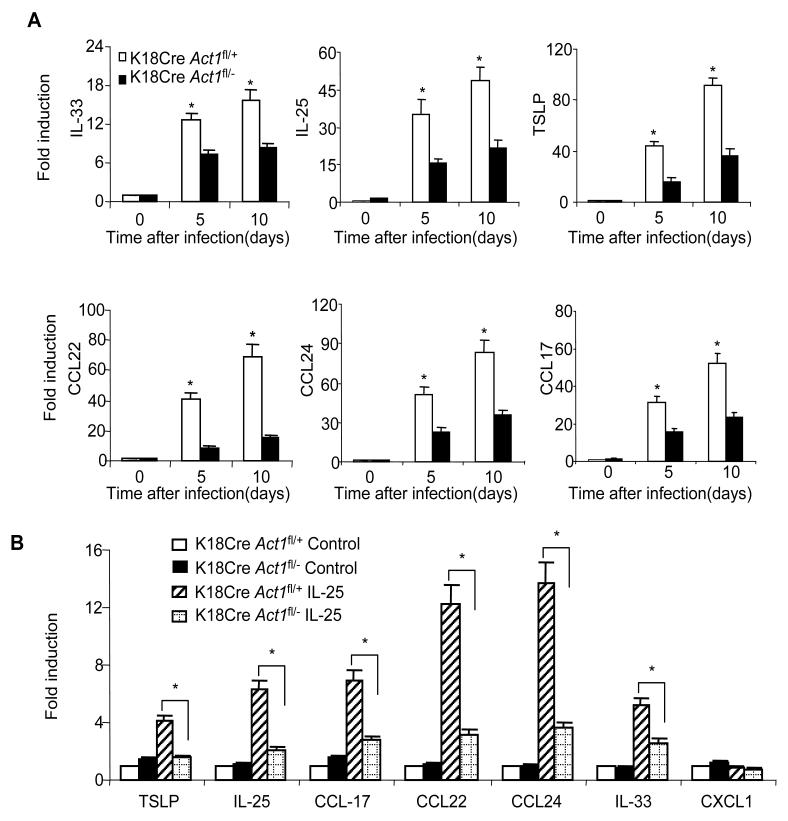
It has been shown that Th2-driving cytokines and Th2-attracting chemokines (including IL-33, IL-25, TSLP, CCL17, CCL22 and CCL24) can be produced by epithelial cells, playing an important role in worm expulsion. We measured their expression in intestinal epithelial cells from the infected K18CreAct1fl/− and K18CreAct1fl/+ control mice. As shown in Figure 5A, the intestinal epithelial cells from the infected K18CreAct1fl/− mice had reduced IL-33, IL-25, TSLP, CCL-17, CCL-22, and CCL-24 expression compared to that from the infected K18CreAct1fl/+ mice. To examine whether IL-25 injection has similar effects on intestinal epithelial cells, the K18CreAct1fl/− and K18CreAct1fl/+ mice were injected intraperitoneally with IL-25 at day 0 and day 1. The IECs were isolated from the injected mice at day 2, and analyzed for gene expression by real-time PCR. We found that the expression of same cytokines and chemokines (IL-33, IL-25, TSLP, CCL17, CCL22, and CCL24) were reduced in the IECs from the K18CreAct1fl/− mice compared to that in the K18CreAct1fl/+ mice (Figure 5B). Taken together, these data implicate that epithelial-specific Act1 likely contributes to the IL-25-dependent helminth expulsion through the induction of Th2-driving cytokines and Th2-attracting chemokines, some of which (such as IL-33 and IL-25) may directly participate in the expansion of the Lin−c-kit+ cell population.
Figure 5. IL-25 induced type 2 cytokines and chemokines in epithelial cells.
(A) Infected K18Cre Act1fl/− and K18Cre Act1fl/+ mice were sacrificed 10 days after infection. Gene expressions in intestinal epithelial cells after infection were analyzed by real-time PCR. (B) Epithelial-specific Act1-deficient and littermate control mice were subjected to intra-peritoneal injection of IL-25 (2μg/mouse, every 24 hours for two days), followed by analysis of gene expression in intestinal epithelial cells by real-time PCR. Data represent mean(n=4)±SEM; *p<0.05. The experiment was repeated two times.
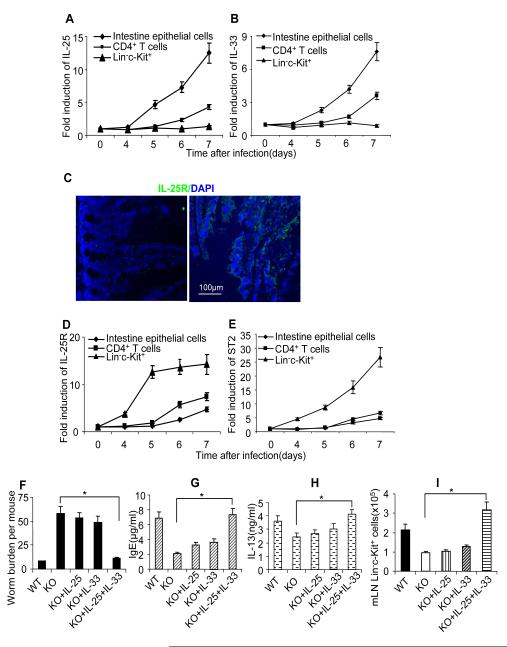
To further determine the sources of IL-33 and IL-25 during worm infection, a kinetic experiment was performed to measure the induction IL-33 and IL-25 expression in different cells types after worm infection. We found that the induction of IL-33 and IL-25 were much earlier and higher in intestinal epithelial cells as compared to that in CD4+ T cells from MLN, supporting the concept that epithelial cells are the initial and main source of IL-33 and IL-25 after worm infection(Figure 6A-B). Up-regulation of IL-33 and IL-25 expression was not observed in Lin−c-Kit+ cells. In contrast, the expression kinetics of IL-25R and IL-33R (ST2) were distinct from the ligands. While IL-25R protein was readily detectable in intestinal epithelial cells, the induction of IL-33R (ST2) and IL-25R mRNA were much earlier and higher in Lin−c-Kit+ cells than that in intestinal epithelial cells and CD4+ T cells from MLN (Figure 6C-E). Taken together, these results suggest that while epithelial cells are probably the major sources of IL-25 and IL-33, Lin−c-Kit+ cells are the key target cell population of IL-33 and IL-25 during worm infection.
Figure 6. Combined injection of IL-25 and IL-33 restored type 2 immunity in epithelial-specific Act1-deficient mice.
Wild type mice were infected with N. brasiliensis, mice were sacrificed at different time points as indicated and CD4+ T cells, Lin−c-kit+ cells and intestinal epithelial cells were isolated and gene expression were analyzed by real-time PCR. (A) IL-25 expression. (B) IL-33 expression. (C) cross sections of small intestine from naïve C57BL/6 mice were stained with anti-IL-25R antibodies(Right), isotype antibody staining was included as control(left). Green fluorescence suggests IL-25R positive. Blue shows DAPI staining. (D) IL-25R expression (samples from A-B). (E) ST2 expression (samples from A-B). K18Cre Act1fl/−(KO) and K18Cre Act1fl/+ (WT) mice were infected(F-I), three groups of KO mice received IL-25, IL-33 and IL-25+IL-33 injection (i.p, 500ng/mouse for 5 days starting from the first day of infection), respectively. All the mice were sacrificed 10 days after infection. (F) worm burden in small intestine was analyzed. (G) serum IgE levels were measured by ELISA. H. MLN Cells were re-stimulated by anti-CD3 and anti-CD28 for overnight, followed by IL-13 ELISA in the collected supernatants. I. Absolute numbers of Lin−c-kit+ cells in MLN are shown. Data represent mean(n=4)±SEM; *p<0.05. The experiment was repeated two times.
Since both IL-33 and IL-25 expression were much reduced in the IECs from the infected and IL-25-injected K18CreAct1fl/− mice compared to that in the K18CreAct1fl/+ mice, we tested the ability of these two cytokines to rescue the type 2 protective immunity against worm infection in the epithelial-specific Act1-deficient mice. Importantly, combined injection of IL-25 and IL-33 (but not the individual cytokines) rescued the type 2 protective immunity against worm infection in the epithelial-specific Act1-deficient mice, including reduced worm burdens, enhanced IL-13 production and serum IgE levels (Figure 6F-H). Furthermore, injection of IL-33 and IL-25 also restored the Lin−c-Kit+ cell population in the epithelial-specific Act1-deficient recipient mice in response to worm infection (Figure 6I). These results suggest that the impaired type 2 responses in epithelial-specific Act1-deficient mice could be, at least partly, due to the reduced expression of IL-33 and IL-25, which was rescued by the injection of the exogenous cytokines. It is intriguing that ex vivo studies showed that IL-25 stimulation can indeed directly induce the expression of IL-25 and IL-33 in airway and kidney epithelial cells (unpublished data by Zepp and Li). One exciting possibility is that epithelial-specific Act1 might mediate IL-25-dependent helminth expulsion through the positive feedback loop of IL-25, which mediates the expansion of the Lin−c-Kit+ cell population in conjunction with the production and action of IL-33.
IL-13-producing cells can rescue type 2 protective immunity in epithelial-specific Act1-deficient mice
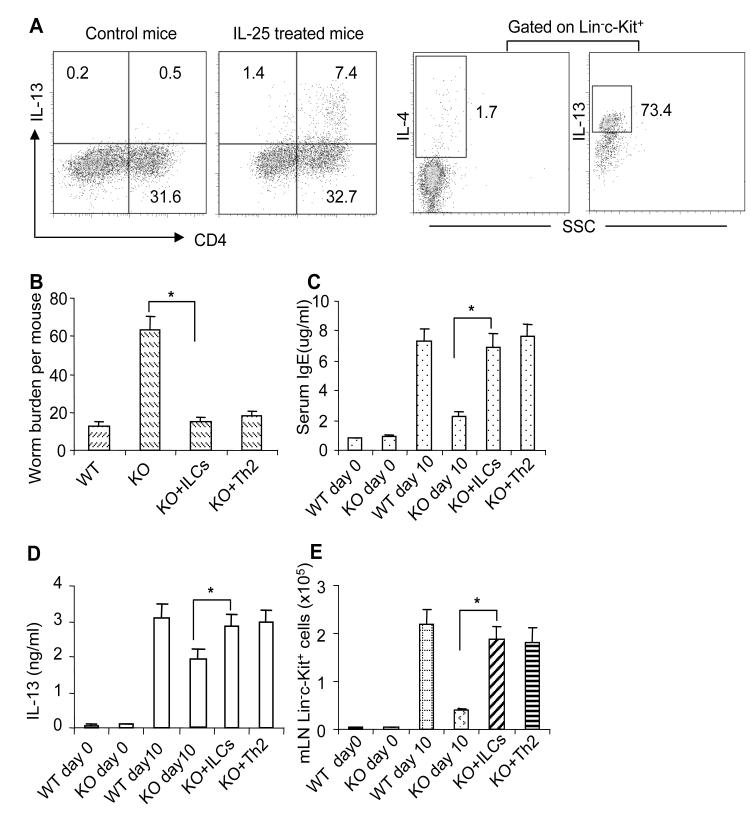
Previous studies reported that the innate Lin−c-kit+ cell population is critical for initiation of type-2 responses and that adoptive transfer of nuocytes to Il17rb−/− mice restored protective immunity against worm infection (Neill et al., 2010). We therefore hypothesized that the reduced expansion of Lin−c-kit+ cells in epithelial-specific Act1-deficient mice might contribute to the delayed type 2 immunity in these mice. To test this hypothesis, we isolated Lin−c-kit+ cells from MLN of IL-25-injected mice and adoptively transferred them to the epithelial-specific Act1-deficient mice on the same day of worm infection. It is important to note that about 70% of the Lin−c-kit+ cells were IL-13 positive (Figure 7A). Mice were sacrificed 10 days after infection followed by analyses for type 2 responses. As shown in Figure 7B-E, the transferred Lin−c-Kit+ cells indeed rescued type 2 protective immunity in epithelial-specific Act1-deficient mice, including reduced worm burdens and increased production of IL-13 and serum IgE.
Figure 7. IL-13-producing cells rescued type 2 protective immunity in epithelial-specific Act1-deficient mice.
(A) Th2 cells were induced by i.p injection of IL-25 consecutively for 7 days at a dose of 1000 ng/mouse each day. Lin−c-Kit+ cells were induced by i.p injection of IL-25 consecutively for 5 days at a dose of 500 ng/mouse each day. The frequencies of IL-13-producing CD4+ T cells and Lin−c-Kit+ cells are shown. K18Cre Act1fl/−(KO) and K18Cre Act1fl/+ (WT) mice were infected(B-E), one group of KO mice received 1×106 Lin−c-kit+ cells/mouse and another group of KO mice received 107 CD4+ T cells/mouse at the same day of infection. All the mice were sacrificed 10 days after infection. (B)worm burden in small intestine were analyzed. (C) Serum IgE levels were measured by ELISA. (D) MLN Cells were re-stimulated by anti-CD3 and anti-CD28 for overnight, followed by IL-13 ELISA in the collected supernatants. (E) Absolute numbers of Lin−c-kit+ cells in MLN are shown. Data represent mean(n=4)±SEM; *p<0.05. The experiment was repeated two times. See also Figure S4.
Although IL-25-induced Act1-mediated signaling in T cells is dispensable for N. brasiliensis expulsion (Figure S4), CD4+ T cells from MLN of infected mice were reduced in epithelial-specific Act1-deficient mice compared to that in wild-type mice. Therefore, we were interested to examine whether adoptive transfer of CD4+ Th2 cells can restore the protective immunity in the epithelial-specific Act1-deficient mice. Th2 cells were induced by i.p injection of IL-25 in wild-type mice and characterized by intracellular staining. CD4+ T cells from MLN of IL-25-injected mice (containing about 20% IL-13 positive cells, Figure 7A) were isolated and adoptively transferred to the epithelial-specific Act1-deficient mice on the same day of worm infection. Type 2 responses were assessed 10 days after infection. As shown in Figure 7B-D, adoptive transfer of Th2 cells restored the type 2 responses in epithelial-specific Act1-deficient recipient mice, including reduced worm burdens and increased production of IL-13 and serum IgE. It is interesting to note that adoptive transfer of Th2 cells restored the expansion of Lin−c-kit+ cell population. As shown in Figure 6A-B, IL-33 and IL-25 expression was induced in CD4+ T cells from MLN of infected mice, which might help to explain the ability of IL-25-induced CD4+ Th2 cells in promoting the expansion of Lin−c-Kit+ cell population in this adoptive transfer experiment.
DISCUSSION
In this study, we report that Act1 deficiency in epithelial cells leads to a significant delay in worm expulsion and decreased IL-25-induced expansion of the Lin−c-kit+ cell population in the MLN, Lung and liver. IL-25-induced expression of Th2 cell-driving cytokines (including IL-25, IL-33 and TSLP) and Th2 cell-attracting chemokines (including CCL17, CCL22 and CCL24) were reduced in the IECs from the IL-25-treated or infected K18CreAct1fl/− mice compared to that in the K18CreAct1fl/+ mice, suggesting that epithelial-specific Act1 is critical for IL-25-dependent helminth expulsion..
The effort to delineate how IL-25 mediates the induction of type 2 cytokine production during helminth infection has led to the discovery of a unique cell type mediating anti-helminth responses(Neill et al., 2010;Price et al., 2010;Saenz et al., 2010a). . In our study, we focused on the Lin−c-kit+ cell population in the MLN during helminth infection, confirming the absence of defining markers for T cells, B cells, NKT cells, dendritic cells, macrophages, neutrophils, eosinophils, mast cells, and basophils. Similar to Ih2, NHC and nuocytes, we also detected the expression of the IL-25 receptor (IL-17RA and IL-17RB), the IL-33 receptor (T1/ST2) and as well as type 2 cytokines (IL-13 and IL-5) in the sorted Lin−c-kit+ cell population from the MLN of infected or IL-25-injected wild-type mice.
In addition to IL-25, it has been shown that IL-33 can also induce this Lin−c-kit+ cell population. Previous studies have shown that adoptive transfer of Lin−c-kit+ IL-13 producing cells into N. brasiliensis-infected Il17rb−/− Il1rl1−/− mice (which are deficient in IL-25 and IL-33 receptor, respectively, and severely impaired in their ability to expel worms) enabled these animals to expel the worms (Neill et al., 2010). The fact that Act1 deficiency in epithelial cells diminished the IL-25-induced expansion of the Lin−c-kit+ cell population in the MLN indicates that the responsiveness of the epithelium to IL-25 is required for the expansion of this cell population. Since the expansion of the Lin−c-kit+ cell population has been shown to play a critical role in promoting type 2 responses, the reduced Lin−c-kit+ cell population in epithelial-specific Act1-deficient mice might account for the delayed worm expulsion in these mice. Through adoptive transfer experiment, IL-13-producing Lin−c-Kit+ cells were indeed able to rescue type 2 protective immunity in epithelial-specific Act1-deficient mice, including reduced worm burdens and increased production of IL-13 and serum IgE, demonstrating the Linc-Kit+ cells are the missing link between IL-25-Act1 axis in the epithelium and type 2 responses in epithelial-specific Act1-deficient mice after worm infection.
IL-33 expression was reduced in the IECs from the IL-25-treated or infected K18CreAct1fl/− mice compared to that in the K18CreAct1fl/+ mice, suggesting that epithelial-specific Act1 might mediate IL-25-dependent helminth expulsion through the induction of IL-33. In support of this, it was reported that although IL-25 and IL-33 can lead to the expansion of the Lin−c-kit+ cell population in vivo and render anti-helminth immunity through their ability to induce type 2 cytokines, receptor ligation by IL-33, but not IL-25, facilitates in vitro IL-7-dependent expansion of the Lin−c-kit+ cell population(Neill et al., 2010). Paradoxically, whereas IL-25 signaling is essential for the expansion of the Lin−c-kit+ cell population during infection and indispensable for worm expulsion, IL-33 signaling is only partially required. We noted that IL-25 expression was also induced in the IECs from the IL-25-treated or infected mice, implicating a possible role of IL-25R signaling in epithelial compartment to amplify IL-25. Indeed, the induction of IL-25 expression was diminished in the IECs from K18CreAct1fl/− mice compared to that in the K18CreAct1fl/+ mice, suggesting that epithelial-specific Act1 might also mediate IL-25-dependent helminth expulsion through the positive feedback loop of IL-25. Of note, combined treatment of IL-25 and IL-33 (but not individual cytokine) rescued the type 2 protective immunity against worm infection in the epithelial-specific Act1-deficient mice, including reduced worm burdens and restored the Lin−c-Kit+ cell population. These results suggest that epithelial-specific Act1 might mediate IL-25-dependent helminth expulsion through the positive feedback loop of IL-25, which mediates the expansion of Lin−c-Kit+ cell population in conjunction with the production and action of IL-33.
These findings are consistent with the previous reports that the mucosal epithelium plays a critical role in the mounting of type 2 responses and is central to the pathogenesis of allergic inflammation through the production of a wide range of cytokines, chemokines and growth factors (Holgate, 2008;Holgate, 2007a;Holgate, 2007b;Kato and Schleimer, 2007;Schleimer et al., 2007). IL-25 expression is induced in lung epithelial cells in response to allergens. IL-25 stimulation of airway epithelial cells induced the expression of type 2 cytokines (IL-33, IL-25 and TSLP) and Th2 cell-attracting chemokines(CCL17, CCL22 and CCL24). In this study, we confirm that while epithelial cells are the major source of IL-25 and IL-33, Lin−c-Kit+ cells are the key target cell population of IL-33 and IL-25 during worm infection. Moreover, the Lin−c-Kit+ cells were also dramatically expanded in the lung and liver of wild-type control mice, but not in that of epithelial-specific Act1-deficient mice. These findings suggest that IL-25 signaling in epithelium plays a critical role in the expansion of the Lin−c-Kit+ cell population in several organs during worm infection, promoting type 2 responses and worm expulsion. These findings indicate that epithelium initiates type-2 responses by producing IL-25 and responding to it.
In addition to IL-25 and IL-33, IL-25-induced expression of TSLP and Th2 cell-attracting chemokines were also reduced in the IECs from the K18CreAct1fl/− mice compared to that in the K18CreAct1fl/+ mice. Consistently, the expression of TSLP, CCL17, CCL22 and CCL24 were reduced in the IECs from the infected K18CreAct1fl/− mice compared to that in the K18CreAct1fl/+ mice. It is important to note that whereas Act1 deficiency in epithelium greatly diminished the Lin−c-Kit+ cells but not the CD4+ Th2 cell population 5 days after infection, the IL-13-producing MLN CD4+ Th2 cell population was expanded 10 days after infection, which was partially dependent on the epithelial-specific Act1. Future studies are required to determine whether IL-25-induced expression of TSLP and chemokines in IECs might contribute to the expansion of the CD4+ Th2 cell population during worm infection. The IL-4- and IL-13-reporter mice has allowed more detailed studies of the dynamic and kinetic localization of IL-13-producing the Lin−c-Kit+ and CD4+ Th2 cells in vivo during worm infection(Liang et al., 2011;Neill et al., 2010;Price et al., 2010;Saenz et al., 2010b). It will be important to further investigate how the Lin−c-Kit+ and CD4+ Th2 cells coordinately initiate, amplify and sustain Th2 cell protective immunity.
EXPERIMENTAL PROCEDURES
Mice and Helminth infection
Act1-deficient C57BL/6 mice and K18 Cre transgenic mice were generated as described previously (Qian et al., 2004;Wen et al., 2003). C57BL/6J and B6.Cg-Tg (Lck-cre) mice were purchased from Jackson laboratory. CD11bCre transgenic mice were provided by Dr. George Kollias (Biomedical Sciences Research Centre “Alexander Fleming”) (Boillee et al., 2006). CD11bCre-mediated and K18Cre-mediated specific deletion of Act1 in vivo were confirmed as described previously (Kang et al., 2010b;Swaidani et al., 2009). IL-17-deficient mice were provided by by Dr. Yoichiro Iwakura(Nakae et al., 2002). Individual mice were inoculated subcutaneously with 500 viable third-stage N. brasiliensis larvae. Animals were sacrificed 5, 10, and 15 days after infection. The intestinal worm burdens were determined, and serum was taken. All mice used in the experiment were housed under specific pathogen-free conditions. Experimental protocols were approved by the Institutional Animal Care and Use Committee of Cleveland Clinic.
Culture of Bone-marrow-derived macrophages
Bone marrow cell suspensions were prepared from one femur and then ACK lysed. A total number of 5×106 cells/ml was cultured with 30ng/ml M-CSF for 5 days. Then cells were harvested and incubated in fresh medium with 100ng/ml IL-25, 10ng/ml IL-4 or a combination of IL-25+IL-4 for 24 hours. Cells incubated without cytokines were included as control. Then RNA was isolated and assayed by real-time PCR.
Isolation of Intestinal epithelial cells (IECs)
Intesinal epithelial cells were isolated by using a modification of the methods as described previously(Whitehead et al., 1999;Evans et al., 1994;Evans et al., 1992). Intestines were cut into 2-3mm fragments and washed by PBS for 5 times with vigorous shaking. Then intestines were incubated with 3mM EDTA and 0.5mM dithiothreitol at room temperature for 30 min with shaking. Crypts released from the intestine by shaking were washed with PBS and were solubilized by cell lysis buffer. To test the contamination of leukocytes, crypts were digested by collagenase I and dispase I; and single cells were stained by anti-CD45 antibodies. Less than 2% of CD45 positive cells were detected suggesting high purity of collected IECs.
Isolation lung and liver mononuclear cells for flow cytometry
Lung and liver tissues were cut into small pieces and digested with collagenase D(Sigma). Then the tissues were filtered through a cell strainer. For lung MNCs, red cells were lysed and ready for flow cytometry. For liver, 30% percol were used to isolate the MNCs and red cells were lysed before flow cytometry. CD45 antibodies were used for gating on leukocytes.
T cell re-stimulation
Mice were sacrificed at different time points after infection. Mesenteric lymph nodes were collected. T cells were re-stimulated by coated CD3+CD28 antibodies (eBioscience) for overnight. Supernatants were harvested for ELISA assays.
Bronchoalveolar lavage(BAL) and tissue collection
Mice were sacrificed at the times indicated. A total of 0.7ml of HL-1 medium (BioWhittaker) was used to obtain BAL fluid through trachea using a blunt needle and 1ml syringe. Total cells were counted manually and cell subsets were analyzed by flow cytometry. Macrophages were characterized by F4/80 antibody (Serotech) and granulocytes by Gr-1 antibody (BD Biosciences). Flow cytometry of stained cells was performed with a FACSCalibur (BD Biosciences). And data were analyzed Using FlowJo software (Tree Star). Lungs and mesenteric lymph nodes were collected and snap frozen immediately in liquid nitrogen container for RNA extraction.
Flow cytometry and cell sorting
Fluorescence-conjugated CD3, CD4, CD8, TCRβ, TCRγδ, B220, CD19, NK1.1, Ter119, Gr-1, CD11b, CD11c, FcεRI, ckit antibodies and isotype controls were all purchased from BD Biosciences. For cell surface staining, cells were blocked with 1% BSA plus 2μg/ml CD16/32 FcR blocker(BD Biosciences) for 30min on ice. Then cells were incubated with antibodies for 30min on ice. Cells were then washed three times and fixed by 1% paraformaldehyde. Data were analyzed by FlowJo software(Tree Star). For sorting of Lin−c-kit+ cells, MLN cells from wild-type mice 5 days after worm infection were collected, and Lin−c-kit+ cells were enriched by negative deletion of lineage-positive cells(CD3, CD4, CD8, TCRβ, TCRγδ, B220, CD19, NK1.1, Ter119, Gr-1, CD11b, CD11c and FcεRI) by MACS, Then Lin−c-Kit+ cells were sorted by FACS. Portion of isolated cells were stained with Lineage markers to check the purity, the cell purity was higher than 99%.
ELISA
IgE, IL-4 and IL-13 level were assayed by Mouse IgE ELISA Set (BD Biosciences) and murine IL-4 and IL-13 Quantikine ELISA Kit (R&D systems). All procedures followed the manufacture’s instruction.
Real-time PCR
Total RNA was extracted from lungs and lymph nodes with TRIzol (Invitrogen) according to the manufacture’s instructions. All gene expression results are expressed as arbitrary units relative to expression of the gene encoding β-actin. The specific primer sequences used in reaction are listed in Supplemental table 1.
Statistical analysis
The significance of differences between two groups was determined by Student’s t test (Two-tailed). Unless otherwise specified, all results are shown as mean and the standard error of the mean (mean±SEM). A p value of <0.05 was considered significant.
Supplementary Material
Highlights.
Epithelial cell-specific Act1 is critical for IL-25-dependent helminth expulsion.
Act1 in epithelium is required for IL-25-induced expansion of Lin−c-kit+ cells.
Epithelial cells produce IL-25 etc to induce the expansion of the Lin−c-kit+ cells. Lin−c-kit+ cells rescued type 2 immunity in epithelial-specific Act1-deficient mice.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. George Kollias for provision of CD11bCre transgenic mice. This investigation was supported by a grant from the National Institutes of Health (RO1HL098935-01A1) and Sandler award for asthma.
Footnotes
SUPPLEMENTAL DATA Supplemental Data include four figures and one table.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23:1069–1075. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio E, Sonder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, Chariot A, Garcia-Perganeda A, Leonardi A, Paun A, Chen A, Ren NY, Wang H, Siebenlist U. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol. 2009;182:1617–1630. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day KP, Howard RJ, Prowse SJ, Chapman CB, Mitchell GF. Studies on chronic versus transient intestinal nematode infections in mice. I. A. comparison of responses to excretory/secretory (ES) products of Nippostrongylus brasiliensis and Nematospiroides dubius worms. Parasite Immunol. 1979;1:217–239. doi: 10.1111/j.1365-3024.1979.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Evans GS, Flint N, Potten CS. Primary cultures for studies of cell regulation and physiology in intestinal epithelium. Annu Rev Physiol. 1994;56:399–417. doi: 10.1146/annurev.ph.56.030194.002151. [DOI] [PubMed] [Google Scholar]
- Evans GS, Flint N, Somers AS, Eyden B, Potten CS. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci. 1992;101(Pt 1):219–231. doi: 10.1242/jcs.101.1.219. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory LG, Mathie SA, Walker SA, Pegorier S, Jones CP, Lloyd CM. Overexpression of Smad2 drives house dust mite-mediated airway remodeling and airway hyperresponsiveness via activin and IL-25. Am J Respir Crit Care Med. 2010;182:143–154. doi: 10.1164/rccm.200905-0725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, Urban JF, Jr., Rothenberg ME, Finkelman FD. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AW, Gaffen SL. IL-17RC: a partner in IL-17 signaling and beyond. Semin Immunopathol. 2010;32:33–42. doi: 10.1007/s00281-009-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007a;120:1233–1244. doi: 10.1016/j.jaci.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Holgate ST. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol. 2007b;28:248–251. doi: 10.1016/j.it.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergol Int. 2008;57:1–10. doi: 10.2332/allergolint.R-07-154. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, Stohlman S, Tuohy VK, Li X. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona IM, Urban JF, Jr., Finkelman FD. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol. 1988;140:3206–3211. [PubMed] [Google Scholar]
- Kreider T, Anthony RM, Urban JF, Jr., Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Act1 modulates autoimmunity through its dual functions in CD40L/BAFF and IL-17 signaling. Cytokine. 2008;41:105–113. doi: 10.1016/j.cyto.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2011;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, Misra S, Deng L, Chen ZJ, Li X. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal. 2009;2:ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Neill DR, McKenzie AN. Nuocytes and beyond: new insights into helminth expulsion. Trends Parasitol. 2011 doi: 10.1016/j.pt.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, French D, Mao W, Maruoka M, Risser P, Lee J, Foster J, Aggarwal S, Nicholes K, Guillet S, Schow P, Gurney AL. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J Immunol. 2001;167:6559–6567. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF, Fairchild RL, Omori SA, Rickert RC, Scott M, Kotzin BL, Li X. Act1, a negative regulator in. Immunity. 2004;21:575–587. doi: 10.1016/j.immuni.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010a;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr., Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010b;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa MC, Reece JJ, Urban JF, Jr., Scott AL. Dynamics of lung macrophage activation in response to helminth infection. J Leukoc Biol. 2008;84:1422–1433. doi: 10.1189/jlb.0308199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland DH, Upham JW, Holt PG. Epithelial-dendritic cell interactions in allergic disorders. Curr Opin Immunol. 2010;22:789–794. doi: 10.1016/j.coi.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Swaidani S, Bulek K, Kang Z, Gulen MF, Liu C, Yin W, Abbadi A, Aronica M, Li X. T cell-derived Act1 is necessary for IL-25-mediated Th2 responses and allergic airway inflammation. J Immunol. 2011;187:3155–3164. doi: 10.4049/jimmunol.1002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, Aronica M, Li X. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol. 2009;182:1631–1640. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy D, Kugler D, Wolfson M, Vanden BT, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- Trajkovic V, Stosic-Grujicic S, Samardzic T, Markovic M, Miljkovic D, Ramic Z, Mostarica SM. Interleukin-17 stimulates inducible nitric oxide synthase activation in rodent astrocytes. J Neuroimmunol. 2001;119:183–191. doi: 10.1016/s0165-5728(01)00391-5. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen F, Cecena G, Munoz-Ritchie V, Fuchs E, Chambon P, Oshima RG. Expression of conditional cre recombinase in epithelial tissues of transgenic mice. Genesis. 2003;35:100–106. doi: 10.1002/gene.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead RH, Demmler K, Rockman SP, Watson NK. Clonogenic growth of epithelial cells from normal colonic mucosa from both mice and humans. Gastroenterology. 1999;117:858–865. doi: 10.1016/s0016-5085(99)70344-6. [DOI] [PubMed] [Google Scholar]
- Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, Yu Y, Artis D. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- Zhao A, Urban JF, Jr., Sun R, Stiltz J, Morimoto M, Notari L, Madden KB, Yang Z, Grinchuk V, Ramalingam TR, Wynn TA, Shea-Donohue T. Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J Immunol. 2010;185:6921–6929. doi: 10.4049/jimmunol.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.