Abstract
Rats fed a high fat diet develop increased adiposity and oxidative stress leading to impaired vasodilation. The purpose of the present study was to examine the effects of high fat-induced increases in adiposity and oxidative stress on vasoconstrictor reactivity of isolated mesenteric arteries. We hypothesized that rats with more adiposity would develop oxidative stress-potentiated increases in iNOS-derived nitric oxide leading to diminished vasoconstriction. Male Sprague-Dawley rats were fed either a control (Chow) or high fat diet for 6 weeks. The roles of oxidative stress and iNOS in the impaired vasoconstrictor responses to endothelin-1 were characterized in small mesenteric arteries. Rats fed the HFD developed significantly more adiposity compared to Chow rats. Plasma levels of nitric oxide and the inflammatory factor tumor necrosis factor α were significantly higher in high fat fed rats compared to Chow rats (nitric oxide: 95.36 ± 19.3 vs. 38.96 ± 6.7 μM; tumor necrosis factor α: 598 ± 111.4 vs. 292 ± 71.8 pg/mL, respectively). Despite exhibiting elevated systolic blood pressure compared to Chow rats (153.5 ± 2.4 vs. 137.5 ± 2.7 mmHg), endothelin-1 mediated vasoconstriction was impaired in isolated mesenteric arteries from high fat fed rats but was normalized by individual or combined inhibition of nitric oxide synthase, iNOS, or oxidative stress. Therefore, oxidative stress and iNOS are involved in the attenuation of endothelin-1 mediated vasoconstriction observed in isolated mesenteric arteries from high fat fed rats.
Keywords: Oxidative stress, Overweight, Antioxidant, Inflammation, Mesenteric Artery
Introduction
Increased adiposity is a major risk factor for cardiovascular disease, diabetes, hypertension, and insulin resistance syndrome [1]. Studies have shown that feeding rats a high fat diet (HFD; 60–66.5% kcal from fat) for only 2 days results in impaired glycolysis [2], and after 2–4 weeks, obesity, insulin resistance, hyperglycemia, hypertension, and oxidative stress develop [2–4]. Likewise, prior studies in our laboratory have shown that feeding rats a HFD (60% kcal from fat) for 6 weeks results in fasting hyperglycemia and oxidative stress [5]. These findings are similar to studies showing a positive correlation between increased adiposity and oxidative stress in humans [6]. Increases in waist circumferences in particular are associated with increased mortality [7], although the mechanisms involved are not well-characterized.
Superoxide (O2·−) is a prominent reactive oxygen species (ROS) that can scavenge the endogenous vasodilator nitric oxide (NO) to form peroxynitrite (ONOO−) [5,8]. Reduced bioavailability of NO therefore directly leads to increased blood pressure. Nitric oxide synthase (NOS) is the enzyme responsible for producing NO. There are three distinct isoforms of NOS: endothelial (eNOS), inducible (iNOS) and neuronal (nNOS) [9]. The predominant isoform found in endothelial cells of the cardiovascular system is eNOS, which is therefore an important regulator of local blood pressure and flow [9]. The expression of iNOS is upregulated in response to inflammatory cytokines that are elevated in many diseases states, including obesity, and can produce ten-fold higher levels of NO compared to eNOS [10–12]. The nNOS isoform is found primarily in developing and adult neurons and will therefore not be discussed further [9]. Prior studies in our laboratory have demonstrated that eNOS protein expression is not altered by the HFD feeding protocol [5]. Therefore, the present study will focus on the role of iNOS in vasoconstrictor reactivity following HFD.
In the HFD rat model, impaired vasodilation of mesenteric arteries is mediated by scavenging of NO by O2·−, reduced sensitivity to NO, and inflammatory factors [5]. In other studies, evidence supports a role for O2·− as a co-stimulator of iNOS protein expression and activity in isolated rat mesangial and endothelial cells treated with inflammatory cytokines [13–14]. Elevated activity of iNOS, in turn, has been shown to produce O2·− [15] leading to further increases in NO scavenging to form ONOO−. Therefore, the purpose of the present study was to explore the relationship between oxidative stress and iNOS on vasoconstrictor responses of rats fed a HFD for 6 weeks.
Obesity has long been linked with hypertension (For review: [16]). Similarly, excess visceral fat in particular has a well-associated link with hypertension (For review: [17]). Moreover, simply being overweight puts individuals at a greater risk of developing increased blood pressure [18]. Our research focus, however, was to examine the chronic effects of increased adiposity on vasoconstrictor reactivity of mesenteric arteries in our overweight HFD rat model. We hypothesized that rats with increased adiposity would develop elevated systolic blood pressure and oxidative stress-potentiated increases in iNOS-derived nitric oxide (NO) leading to diminished vasoconstriction of the mesenteric arteries.
Materials and Methods
All protocols and surgical procedures employed in this study were reviewed and approved by the Institutional Animal Care and Use Committees of the University of New Mexico School of Medicine (Albuquerque, NM, USA) and Arizona State University (Tempe, AZ, USA).
Experimental Groups
Male Sprague-Dawley rats (140–160 g body weight, Harlan Industries) were divided into two groups and fed either normal chow (Chow) or high fat (HFD) diets. The chow diet contained in kcal: 18.9% protein, 57.33% carbohydrates (% sucrose NA), and 5% fat (2018; Harlan Teklad). The high fat diet contained 20% protein, 20% carbohydrates (6.8% sucrose) and 60% fat (D12492; Research Diets, Inc.). Rats were maintained on the respective diets for 6 weeks and the food was replaced every 3–4 days to prevent spoiling. Animals were housed in identical cages in the same animal facility and exposed to a 12:12h light dark cycle and had free access to food and water.
Measurement of Weight Gain and Plasma Indicators of Adiposity, Oxidative Stress, Inflammation, Nitrates/Nitrites, and Endothelin-1
Animals were weighed at the beginning and end of the 6-week feeding protocol to determine weight gain in response to each diet. Tail length and waist circumference were also measured. At the end of the 6-week feeding protocol, systolic blood pressure was measured by tail vein plethysmography. Tail vein blood samples were collected at the end of the 6-week feeding protocol and used to measure high density lipoprotein (HDL), total cholesterol as well as ketone bodies (as β-hydroxybutyrate, an indicator of fatty acid oxidation), using a portable meter (Cardiocheck PA, Polymer Technology Services, Inc., Indianapolis, IN). Following deep anesthesia (sodium pentobarbital 200 mg/kg, i.p.), epididymal fat pads were extracted and weighed to gauge the degree of adiposity [5]. Cardiac plasma samples were taken to quantify nitrates and nitrites (NOx) as well as tumor necrosis factor alpha (TNFα), an indicator of inflammation. Plasma NOx and TNFα were measured using commercially available kits (NOx: 780001, Cayman Chemical, Ann Arbor, MI, USA; TNFα ELISA: ER3TNFA, Thermo Scientific, Rockford, IL) according to the manufacturer’s protocols. Plasma superoxide dismutase (SOD) activity was measured following the manufacturer’s protocol using a commercially available kit (706002, Cayman Chemical, Ann Arbor, MI, USA). Plasma endothelin-1 was quantified by enzyme immunometric assay (EIA) using a commercially available kit according to the manufacturer’s protocol (ADI-900-020A, Enzo Life Sciences, Plymouth Meeting, PA, USA).
Isolation of Mesenteric Resistance Arteries
The mesenteric arcade was removed by performing a midline laparotomy on anesthetized rats (sodium pentobarbital, 200 mg/kg, i.p.). The arcade was pinned out in a Silastic coated dissection dish containing ice-cold HEPES buffer (in mM: 134.4 NaCl, 6 KCl, 1 MgCl2, 1.8 CaCl2, 10 HEPES, 10 glucose, pH 7.4) and fifth-order mesenteric resistance arterioles (~1mm length; 80–120μm, i.d.) were isolated. Isolated arterioles were transferred to a HEPES filled vessel chamber (Living Systems, CH-1), cannulated with glass pipettes, and secured with silk ligature. The vessels were then pressurized to 60 mmHg with a servo-controlled peristaltic pump (Living Systems Instrumentation, Burlington, VT) and the chamber was placed on a microscope stage for continuous measurement of the inner diameter of the vessels using video microscopy and edge-detection software (IonOptix, Milton, MA). Vessels were superfused with warm aerated physiological salt solution (PSS; 37°C) containing (in mM): 129.8 NaCl, 5.4 KCl, 0.5 NaH2PO4, 0.83 MgSO4, 19 NaHCO3, 1.8 CaCl2, and 5.5 glucose at a rate of 10 mL/min. Vessels were exposed to the vasoconstrictor phenylephrine (PE; 10−6M) followed by the vasodilator acetylcholine (ACh; 10−6M) in the superfusate prior to each experiment to verify vasoconstrictor and vasodilator viability, respectively.
Western Blots for Inducible Nitric Oxide Synthase (iNOS)
Isolated mesenteric arcades were snap-frozen in isopentane cooled by liquid nitrogen and stored at −80°C until analysis. Frozen arcades were homogenized using a ground glass homogenizer containing ice-cold Tris HCl homogenization buffer (10 mM Tris (pH 7.6), 1 mM EDTA, 1% triton X-100, 0.1% Na-deoxycholate, 0.03% protease inhibitor cocktail (Sigma P2714), and 1 mM phenylmethanesulfonyl fluoride). To remove insoluble debris, homogenates were spun at 4000g for 10 min at 4°C and the supernatant removed. The protein concentration of the supernatant was then measured according to the Bradford method (Bio-Rad, Hercules, CA). Tissue sample proteins (50 μg/lane) were resolved by 7.5% Tris-HCl sodium dodecyl sulfate polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA). The separated proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA) and were then incubated in blocking buffer (tween/tris buffered saline (TTBS) containing 3% bovine serum albumin and 5% nonfat dry milk) overnight at 4°C. Following washes in TTBS, membranes were incubated for 4 hours at room temperature with mouse monoclonal antibody specific for iNOS (1:1500; Cat. 610431; BD Transduction Laboratories, San Jose, CA) and rabbit polyclonal antibody to β-actin as a loading control (1:10,000; Cat. Ab13772; AbCam, Cambridge, MA). Membranes were then washed in TTBS followed by incubation with anti-mouse and anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:5000, Cat. PI-1000 and PI-2000, respectively; Vector Laboratories, Burlingame, CA) for 1 hr at room temperature. After washes in tris buffered saline (TBS) and a 1-min exposure to Pierce enhanced chemiluminescence western blotting substrate (Thermo Scientific, Rockford, IL), immunoreactive bands were visualized by exposure to x-ray film (Kodak X-OMAT, Thermo Fisher Scientific, Pittsburgh, PA). The developed films were analyzed using ImageJ software (NIH) and iNOS protein levels were normalized to β-actin protein expression.
Agonist-induced Vasoreactivity
Phenylephrine (PE), acetylcholine (ACh) and human/porcine endothelin-1 (ET1) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and were prepared in deionized water, aliquoted, and frozen (−20°C) until use.
Following equilibration of isolated mesenteric arteries in heated PSS for 30-min (37°C; 10 mL/min), arteries were superfused for 1 hour with either a control PSS solution or PSS with the addition of inhibitors of NOS, iNOS or oxidative stress alone or in combination as described below. Vasoconstrictor responses were determined by exposing vessels to increasing concentrations of PE (10−9 to 10−5 M, 3 min each step) or ET1 (10−12 to 10−8 M, 3 min each step) in the superfusate. Vasodilatory responses to ACh (10−8 to 10−5 M, 3 min each step) were measured in arteries pre-constricted to 50% of resting inner diameter using increasing concentrations of PE in the superfusate. The inner diameter was continuously monitored from bright field images using video microscopy and edge-detection software (IonOptix, Milton, MA). Following the concentration response curves, arteries were superfused for 30-min with calcium-free PSS containing (in mM): 129.8 NaCl, 5.4 KCl, 0.5 NaH2PO4, 0.83 MgSO4, 19 NaHCO3, 5.5 glucose, and 3 EGTA to obtain the passive inner diameter from which percent vasoconstriction or vasodilation was calculated.
The synthetic SOD and catalase mimetic, chloro[[2,2′-[1,2-ethanediylbis[(nitrilo-κN)methylidyne]]bis[6-methoxyphenolato-κO]]]-manganese (EUK-134; 10 μM; Cayman Chemical Company, Ann Arbor, MI) [19] was used to determine the role of oxidative stress in the impaired vasoconstriction to ET1. In prior studies, acteylcholine-mediated vasodilation was shown to be dependent on eNOS in arteries from Chow rats [5]. Pre-exposure of arteries to the non-specific inhibitor of NOS Nω-nitro-L-arginine (LNNA; 100 μM; Sigma) or the iNOS selective inhibitor N6-(1-iminoethyl)-L-lysine dihydrochloride (LNIL; 10 μM; Cayman Chemical) was used to determine the role of NOS- and iNOS-derived NO in the vasoconstrictor and vasodilator responses of arteries from Chow and HFD rats. Specificity of the iNOS inhibitor, LNIL, was verified in preliminary experiments showing no effect of iNOS inhibition on acetylcholine-mediated vasodilation of mesenteric arteries from Chow rats (data not shown). Additional experiments to assess the combined effects of LNNA or LNIL and EUK-134 on vasoconstrictor responses were performed.
Statistics
Data are expressed as means ± SEM. Analysis of Variance (ANOVA) was used to compare changes in blood pressure, weight gain and the plasma indicators of adiposity, oxidative stress, inflammation, nitrates/nitrites, and endothelin-1 within and between groups (Tables 1–2). Percent vasoconstriction to PE and ET1 were calculated as the percent difference of inner diameter observed at each concentration vs. calcium free values. Percent vasodilation to ACh was calculated as the percent reversal of tone elicited by PE as well as inherent myogenicity. This was determined by measuring the difference in intraluminal diameter observed at each concentration of ACh vs. the inner diameter observed in a calcium free solution. Data for all dose response curves were arcsine transformed to approximate a normal distribution and analyzed using two-way repeated measures analysis of variance (ANOVA). Where significant effects occurred, individual groups were compared using Student-Newman-Keuls post hoc analysis. A probability of ≤0.05 was accepted as statistically significant for all comparisons.
Table 1.
Various Diet-induced Changes
| Parameter | Chow | HFD |
|---|---|---|
| Change in body mass (g), n=13–16 | 172.9 ± 4.6 | 212.6 ± 7.0** |
| Waist circumference (cm), n=10–12 | 16.8 ± 0.28 | 18.0 ± 0.21** |
| Tail length (cm), n=10–12 | 20.3 ± 0.29 | 20.7 ± 0.22 |
| Epididymal fat pad mass (g), n=11–14 | 3.46 ± 0.15 | 5.66 ± 0.31** |
| Plasma ketone bodies (mg/dL), n=5 | 4.72 ± 0.31 | 7.18 ± 0.77* |
| Plasma total cholesterol (mg/dL), n=9–10 | 147.8 ± 6.9 | 121.8 ± 3.5** |
| Plasma HDL cholesterol (mg/dL), n=9–10 | 83.3 ± 4.0 | 62.3 ± 1.9** |
| Systolic blood pressure (mmHg), n=6–9 | 137.5 ± 2.7 | 153.5 ± 2.4** |
P<0.05 from Chow,
P≤0.005 from Chow.
Table 2.
Diet-Induced Vascular Changes
| Parameter | Chow | HFD |
|---|---|---|
| Plasma nitrates/nitrites (NOx) (μM), n=4 | 39.0 ± 6.7 | 95.4 ± 19.3* |
| Plasma tumor necrosis factor alpha (pg/mL), n=4–6 | 292 ± 72 | 598 ± 111* |
| Plasma superoxide dismutase activity (U/mL), n=6 | 6.71 ± 0.53 | 4.70 ± 0.74* |
| Plasma endothelin-1 (pg/mL), n=9 | 3.41 ± 0.2 | 2.92 ± 0.2 |
p≤0.05 from Chow.
Results
Diet-induced Alterations in Blood Pressure, Weight Gain and Plasma Indicators of Adiposity, Oxidative Stress, Inflammation, Nitrates/Nitrites, and Endothelin-1
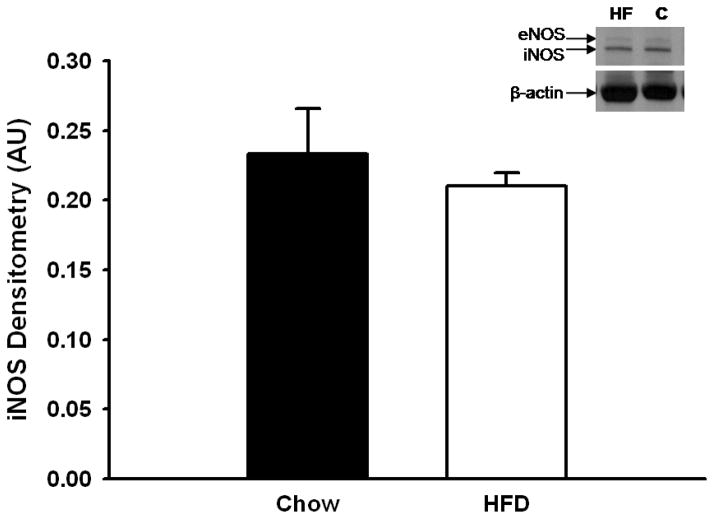
Rats fed the HFD for 6 weeks gained significantly more weight attributed to increased adiposity (Table 1). This is evidenced by larger waist circumferences and epididymal fat pad masses (Table 1). These increases were not attributed to overall growth since the tail lengths were similar between HFD and Chow-fed rats (Table 1). Consistent with ingesting a HFD, rats in this group had elevated plasma ketone levels concomitant with decreased high density lipoprotein (HDL) and total cholesterol concentrations compared to their Chow-fed counterparts (Table 1). Systolic blood pressure was also significantly higher following the HFD (Table 1). Plasma concentrations of nitrates/nitrites (NOx) and TNFα were significantly greater, whereas plasma superoxide dismutase (SOD) activity was significantly less in the HFD rats compared to the Chow rats (Table 2). Plasma levels of endothelin-1 were not significantly different between Chow and HFD rats (Table 2). Protein expression of iNOS in mesenteric arcade homogenates was not significantly different between groups (Figure 1).
Figure 1.
Immunostaining of inducible nitric oxide synthase (iNOS) in mesenteric artery homogenates from Chow and HFD-fed rats. Densitized values were normalized to β-actin protein concentrations and are expressed as a ratio (mean ± SEM). n=6 per group. Data are not significantly different.
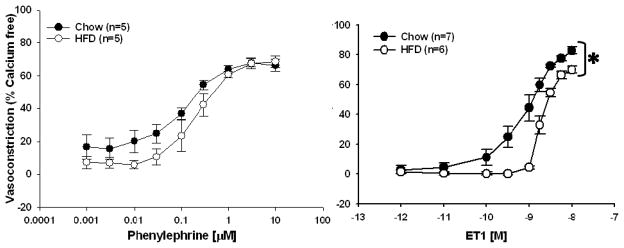
Agonist-induced Vasoreactivity
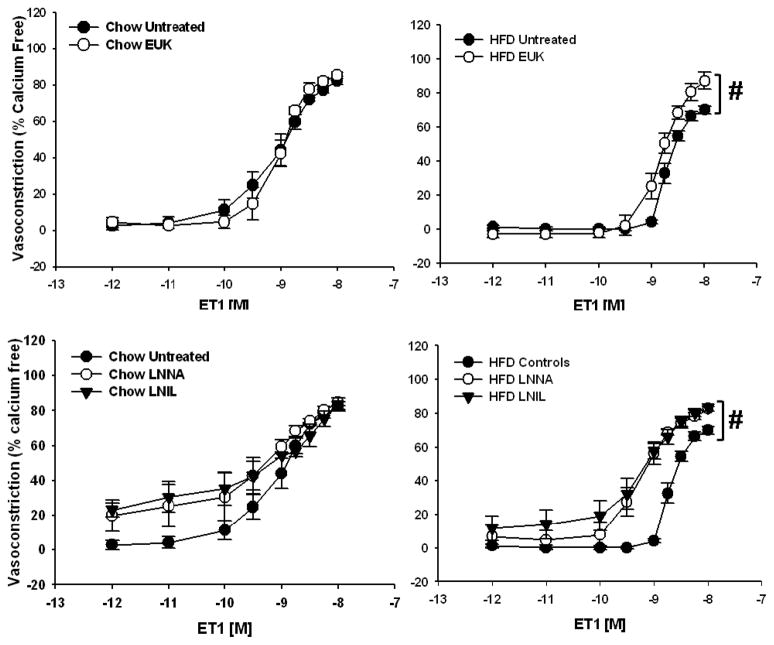
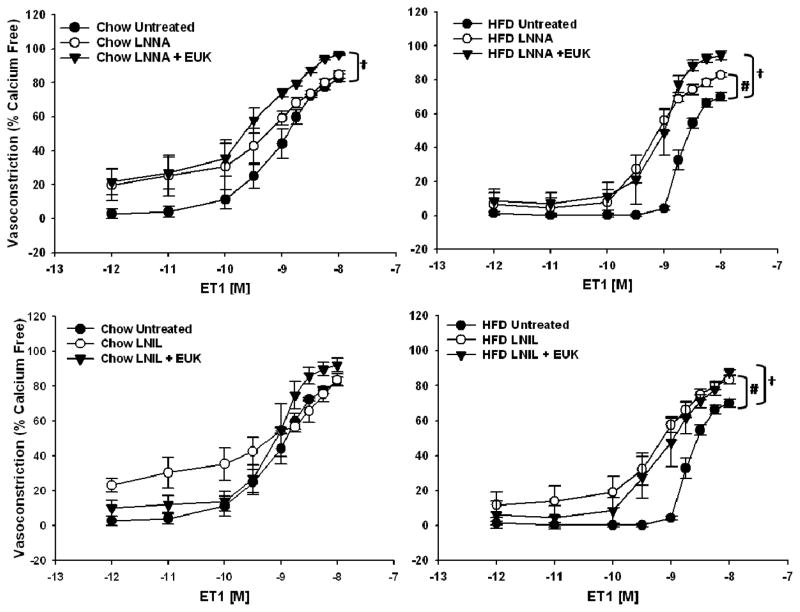
Responsiveness to PE in isolated mesenteric arteries was not affected by the HFD (Figure 2, left panel). In contrast, responsiveness to ET1 was significantly attenuated in the HFD rats compared to the Chow-fed controls (Figure 2, right panel). In fact, arteries from HFD rats required a significantly larger dose of ET1 to reach EC50 compared to those from Chow-fed rats, 10−8.71±0.04 vs. 10−9.09±0.15 M (p<0.05, n=5–6), respectively. ET1-mediated vasoconstriction was normalized in arteries from HFD rats by inhibition of ROS using the SOD and catalase mimetic, EUK-134 (Figure 3, top right panel). The impaired vasoconstriction was also normalized to the same degree using either the nonspecific inhibitor of NOS, LNNA, or the specific inhibitor of iNOS, LNIL (Figure 3, bottom right panel). The combined inhibition of ROS and NOS, or iNOS specifically, significantly improved vasoconstriction in HFD arteries (Figure 4, right panels) whereas vasoconstriction in Chow arteries was only improved with the combined inhibition of ROS and nonspecific inhibition of NOS (Figure 4, top left panel).
Figure 2.
Agonist-induced vasoconstriction of mesenteric arteries from Chow and HFD rats. Left Panel: Responses to phenylephrine (PE) were not significantly different between Chow and HFD rats. Right Panel: Endothelin-1 (ET1)-mediated vasoconstriction was significantly impaired in arteries from HFD rats compared to Chow fed controls. Data expressed as mean ± SEM. *p <0.005 from Chow curves.
Figure 3.
Vasoconstrictor responses to ET1 in mesenteric resistance arterioles from Chow (n=5–7) and HFD-fed rats (n=5–6). Top Panels: Arteries were exposed to the superoxide dismutase and catalase mimetic EUK-134 (EUK) in the superfusate prior to the concentration response curve. Responses from Chow and HFD untreated arteries repeated from Figure 2 for comparison. Data are expressed as means ± SEM. #p<0.05 from Chow curves. Bottom Panels: Vasoconstrictor responses to ET1 in mesenteric resistance arterioles from Chow and HFD-fed rats (n= 4–6 per group) in the presence of the non-specific NOS inhibitor LNNA (100μM) or iNOS specific inhibitor LNIL (10μM). Arteries were exposed to LNNA or LNIL in the lumen and superfusate prior to the concentration response curve. Responses from Chow and HFD untreated arteries repeated from Figure 2 for comparison. Data are expressed as means ± SEM. #p<0.005 HFD LNNA and LNIL curves vs. HFD Untreated curve.
Figure 4.
Top Panels Vasoconstrictor responses in mesenteric resistance arterioles from Chow (n=4–7) and HFD-fed rats (n=5–6) to increasing concentrations of ET1. Responses from untreated Chow and HFD arteries repeated from Figure 3 and Chow and HFD LNNA data repeated from Figure 5 for comparison. Data are expressed as means ± SEM. #p<0.05 LNNA treated vs untreated vessels; †p<0.05 LNNA + EUK treated vs untreated vessels. Bottom Panels: Vasoconstrictor responses in mesenteric resistance arterioles from Chow (n=5–7) and HFD-fed rats (n=5–6) to increasing concentrations of endothelin-1 (ET1). Responses from untreated Chow and HFD arteries repeated from Figure 3 and Chow and HFD LNIL data repeated from Figure 5 for comparison. Data are expressed as means ± SEM. #p<0.05 HFD LNIL curve vs. HFD untreated curve; †p<0.05 HFD LNIL + EUK curve vs. HFD untreated curve.
Discussion
Feeding rats a HFD for 6 weeks results in symptoms associated with metabolic syndrome: weight gain, increased adiposity, fasting hyperglycemia, oxidative stress, and impaired endothelium-mediated vasodilation [5]. The impaired vasodilation was attributed to inflammatory factors, O2·−-mediated scavenging of NO, and decreased sensitivity to NO [5]. In obesity, iNOS expression and activity are upregulated in response to inflammatory cytokines [10–12] a process that can be co-stimulated by O2·− [13–14]. As a result of these observations, we hypothesized that rats with increased adiposity would develop oxidative stress-potentiated increases in iNOS-derived nitric oxide (NO) leading to diminished vasoconstriction. The major conclusions from the present study are: 1) Plasma NOx and TNFα are higher, whereas plasma superoxide dismutase activity is lower, in HFD rats and 2) Oxidative stress and iNOS impair ET1-mediated vasoconstriction in isolated mesenteric arteries from HFD rats.
Rats fed a HFD for 6 weeks develop larger waist circumferences (Table 1). In humans, increases in waist circumference are associated with increased mortality [7]. Moreover, indices of altered lipid metabolism are evident in the HFD rats: elevated ketone bodies with reductions in total and HDL cholesterol (Table 1). Adipose tissue is a source of many factors that could alter the vasoreactivity of nearby arteries and may therefore regulate local blood pressure as well as systemic blood pressure. The release of inflammatory cytokines rises in individuals with increased central adiposity leading to a state of chronic inflammation [20,21]. Consistent with these findings in humans, HFD rats likewise have increased circulating levels of the inflammatory cytokine TNFα (Table 2). Oxidative stress is also elevated in humans with increased adiposity [6]. In studies of mice, higher quantities of perivascular adipose tissue in particular results in impaired vasodilation of the abdominal aorta that was attributed to O2·− [22]. Similarly, small mesenteric arteries from HFD rats with increased perivascular adipose tissue [personal observation] also exhibit impaired vasodilation resulting from O2·−-mediated scavenging of NO [5]. These findings support an important regulatory role for adipokines in the regulation of vascular reactivity.
Consistent with the impaired vasodilation observed in prior studies [5], HFD rats in the present study were found to have elevated systolic blood pressure (Table 1). However, plasma nitrates/nitrites (NOx) were elevated (Table 2), which if indicative of bioavailable NO, would act to oppose vasoconstriction and protect against elevations in blood pressure. In prior studies, isolated mesenteric arteries from these HFD rats were shown to have elevated oxidative stress as measured by DCF fluorescence, a dye sensitive to peroxynitrite (ONOO−), hydrogen peroxide, and hydroxyl radicals [5]. Therefore, it is possible that the increased plasma NOx also represents elevated peroxynitrite (ONOO−), a product formed by O2·−-mediated scavenging of NO. Similar elevations in blood pressure and NOx have been observed in mice fed a HFD for 4–8 weeks. In these studies, the increased NOx production was attributed to iNOS [12]. As mentioned, iNOS expression and activity are upregulated in response to inflammatory cytokines [10–12] and can be co-stimulated by O2·− [13–14]. The diminished superoxide dismutase (SOD) activity and increased TNFα observed in HFD rats support a similar mechanism following HFD although iNOS protein expression was not significantly different from Chow rats (Figure 1).
In contrast to PE, which showed no difference in reactivity between groups (Figure 1, left panel), vasoconstriction to ET1 was significantly attenuated in mesenteric arteries from HFD rats (Figure 1, right panel) resulting in a higher EC50 for arteries from HFD rats compared to Chow rats. The difference in sensitivity to these vasoconstrictors may be related to the pathways through which they elicit vasoconstriction. Phenylephrine elicits vasoconstriction through activation of the adrenergic receptor on vascular smooth muscle cells to activate inositol 1,4,5-triphosphate resulting in the release of calcium from intracellular stores [23]. Similarly, endothelin-1 binding to the endothelin A or B (ETA or ETB) receptors on vascular smooth muscle cells elicits vasoconstriction through activation of 1,4,5-triphosphate and the release of calcium from intracellular stores [24,25]. Unlike phenylephrine, however, endothelin-1 may also bind to ETB receptors on the vascular endothelium resulting in vasodilation through the production of NO [26]. Therefore, with increased adiposity, alterations in the expression pattern or sensitivity of the ETA and ETB receptors may lead to reduced sensitivity to this vasoconstrictor, but normal sensitivity to PE. Since responses to ET1 were normalized following inhibition of either ROS or NOS, differences in the expression patterns of the ETA or ETB receptors are unlikely. Plasma levels of ET1 were not significantly different between Chow and HFD rats (Table 2) suggesting that at similar levels of ET1, vasoconstrictor responses to ET1 would be impaired in either intact or isolated mesenteric arteries from HFD rats. At first glance, this diminished vasoconstrictor reactivity to ET1 appears to contradict the overall increase in blood pressure observed in the HFD rats. However, it is important to note that in vivo the combined effect of multiple endogenous vasoconstrictors and vasodilators, along with potential regional heterogeneity in sensitivity to these vasoactive agents, help to explain the increased systemic blood pressure in animals with impaired mesenteric vasoconstriction.
Prior studies indicated that the mesenteric vascular bed develops increased oxidative stress following HFD [5]. Rats from the current study likewise show diminished SOD activity (Table 2), which would lead to increased O2·− levels. As mentioned, O2·− can increase iNOS expression and activity [13,14], which may be a cause of the impaired ET-1 mediated vasoconstriction seen in this vascular bed. Likewise, increases in the activity of iNOS have been shown to result in excess production of O2·− [15]. Inhibition of O2·− using the SOD and catalase mimetic EUK-134 normalized vasoconstriction to ET1 in arteries from HFD rats (Figure 3, top right panel) supporting a role for O2·− in the impaired response. Moreover, incubation of arteries with either the non-specific NOS inhibitor LNNA or the specific iNOS inhibitor LNIL restored vasoconstrictor responsiveness to ET1 in arteries from this group (Figure 3, bottom right panel). Further support for the interaction between O2·− and iNOS in the impaired vasoconstriction is shown in figure 4 wherein the combined inhibition of O2·− and NOS, or iNOS specifically, normalized vasoconstriction in arteries from HFD rats. In contrast, vasoconstriction in Chow arteries was only enhanced with the combined inhibition of O2·− and nonspecific inhibition of NOS (Figure 4). Since inhibition of O2·− alone had no effect on vasoconstrictor responses in arteries from Chow rats (Figure 3, top left panel) whereas non-specific NOS inhibition had a tendency to augment vasoconstriction (Figure 3, bottom left panel), this effect can be attributed to inhibition of NOS.
Taken together, the data suggest a central role of iNOS in altered vasoreactivity following HFD, however we were not able to detect a difference in iNOS protein levels in pooled mesenteric arteries (Figure 1). Although activity of iNOS is typically linked to its level of expression, there are reports of acute regulation of the catalytic activity of the enzyme (e.g.: [27]). Thus it is possible that iNOS activity rather than expression may be enhanced following HFD in this model. A link between O2·− and increased iNOS activity is supported by both the vasoreactivity studies (Figures 3–4) and by increased circulating NOx (Table 2) measured in the HFD rats. As mentioned, the elevated NOx levels observed in the HFD rats reflects both biologically active NO as well as levels of ONOO− produced from the interaction of O2·− and NO. Since vasoconstrictor responses were normalized by inhibition of ROS or iNOS either alone or in combination (Figures 3 & 4), these findings support a role for uncoupled iNOS-mediated production of O2·− in the impaired vasoconstrictor responses to ET1 observed in the overweight rats. Alternatively, our method of collecting arteries for western blot determinations could have led to a false negative result. To obtain sufficient protein for a western blot, entire mesenteric arcades containing vessels of varying sizes were used. This lack of discrimination between smaller arterioles, like the ones used for the vasoactive studies, and larger arteries may have reduced the sensitivity of the assay to detect iNOS expressed in the small arterioles.
In conclusion, the major findings of this study are that despite developing higher blood pressure compared to Chow-fed rats, those fed a HFD for 6 weeks showed impaired mesenteric artery vasoconstriction as a result of oxidative stress and iNOS. Adipokines may act locally or systemically to contribute to the regulation of blood pressure in vivo. Since HFD rats develop more adipose tissue than Chow rats, this increase in available adipokines may exert a paracrine effect on mesenteric arteries to affect local blood pressure through increased ROS and iNOS while maintaining elevated systemic blood pressure.
References
- 1.Montani JP, Antic V, Yang Z, Dulloo A. Pathways from obesity to hypertension: from the perspective of a vicious triangle. Int J Obes Relat Metab Disord. 2002;26 (Suppl 2):S28–38. doi: 10.1038/sj.ijo.0802125. [DOI] [PubMed] [Google Scholar]
- 2.Kim JK, Wi JK, Youn JH. Metabolic impairment precedes insulin resistance in skeletal muscle during high-fat feeding in rats. Diabetes. 1996;45:651–658. doi: 10.2337/diab.45.5.651. [DOI] [PubMed] [Google Scholar]
- 3.Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW. Fat feeding causes widespread in vivo insulin resistance, decreased energy expenditure, and obesity in rats. Am J Physiol. 1986;251:E576–583. doi: 10.1152/ajpendo.1986.251.5.E576. [DOI] [PubMed] [Google Scholar]
- 4.Viswanad B, Srinivasan K, Kaul CL, Ramarao P. Effect of tempol on altered angiotensin II and acetylcholine-mediated vascular responses in thoracic aorta isolated from rats with insulin resistance. Pharmacol Res. 2006;53:209–215. doi: 10.1016/j.phrs.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Sweazea KL, Lekic M, Walker BR. Comparison of mechanisms involved in impaired vascular reactivity between high sucrose and high fat diets in rats. Nutr Metab (Lond) 2010;7:48. doi: 10.1186/1743-7075-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason C, Craig CL, Katzmarzyk PT. Influence of central and extremity circumferences on all-cause mortality in men and women. Obesity (Silver Spring) 2008;16:2690–2695. doi: 10.1038/oby.2008.438. [DOI] [PubMed] [Google Scholar]
- 8.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 9.Mungrue IN, Bredt DS, Stewart DJ, Husain M. From molecules to mammals: what’s NOS got to do with it? Acta Physiol Scand. 2003;179:123–135. doi: 10.1046/j.1365-201X.2003.01182.x. [DOI] [PubMed] [Google Scholar]
- 10.Haque MM, Panda K, Tejero J, Aulak KS, Fadlalla MA, Mustovich AT, Stuehr DJ. A connecting hinge represses the activity of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:9254–9259. doi: 10.1073/pnas.0700332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mastronardi CA, Yu WH, McCann SM. Resting and circadian release of nitric oxide is controlled by leptin in male rats. Proc Natl Acad Sci U S A. 2002;99:5721–5726. doi: 10.1073/pnas.082098499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noronha BT, Li JM, Wheatcroft SB, Shah AM, Kearney MT. Inducible nitric oxide synthase has divergent effects on vascular and metabolic function in obesity. Diabetes. 2005;54:1082–1089. doi: 10.2337/diabetes.54.4.1082. [DOI] [PubMed] [Google Scholar]
- 13.Beck KF, Eberhardt W, Walpen S, Apel M, Pfeilschifter J. Potentiation of nitric oxide synthase expression by superoxide in interleukin 1 beta-stimulated rat mesangial cells. FEBS Lett. 1998;435:35–38. doi: 10.1016/s0014-5793(98)01035-7. [DOI] [PubMed] [Google Scholar]
- 14.Wu F, Cepinskas G, Wilson JX, Tyml K. Nitric oxide attenuates but superoxide enhances iNOS expression in endotoxin- and IFNgamma-stimulated skeletal muscle endothelial cells. Microcirculation. 2001;8:415–425. doi: 10.1038/sj/mn/7800113. [DOI] [PubMed] [Google Scholar]
- 15.Heusch P, Aker S, Boengler K, Deindl E, van de Sand A, Klein K, Rassaf T, Konietzka I, Sewell A, Menazza S, Canton M, Heusch G, Di Lisa F, Schulz R. Increased inducible nitric oxide synthase and arginase II expression in heart failure: no net nitrite/nitrate production and protein S-nitrosylation. Am J Physiol Heart Circ Physiol. 2010;299:H446–453. doi: 10.1152/ajpheart.01034.2009. [DOI] [PubMed] [Google Scholar]
- 16.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386–393. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- 17.Despres JP. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol. 2007;6:51–59. doi: 10.1097/HPC.0b013e318057d4c9. [DOI] [PubMed] [Google Scholar]
- 18.Feldstein C, Julius S. The complex interaction between overweight, hypertension, and sympathetic overactivity. J Am Soc Hypertens. 2009;3:353–365. doi: 10.1016/j.jash.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998;284:215–221. [PubMed] [Google Scholar]
- 20.Korner J, Woods SC, Woodworth KA. Regulation of energy homeostasis and health consequences in obesity. Am J Med. 2009;122:S12–18. doi: 10.1016/j.amjmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Fischer-Posovszky P, Wabitsch M, Hochberg Z. Endocrinology of adipose tissue - an update. Horm Metab Res. 2007;39:314–321. doi: 10.1055/s-2007-976539. [DOI] [PubMed] [Google Scholar]
- 22.Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial Adipose Tissue Promotes Endothelial Dysfunction via Oxidative Stress in Diet-Induced Obese C57Bl/6 Mice. Circ J. 2010;74:1479–1487. doi: 10.1253/circj.cj-09-0661. [DOI] [PubMed] [Google Scholar]
- 23.Lee CH, Poburko D, Sahota P, Sandhu J, Ruehlmann DO, van Breemen C. The mechanism of phenylephrine-mediated [Ca(2+)](i) oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol. 2001;534:641–650. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo B, Oemar BS, Siebenmann R, von Segesser L, Luscher TF. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation. 1994;89:1203–1208. doi: 10.1161/01.cir.89.3.1203. [DOI] [PubMed] [Google Scholar]
- 25.Simonson MS, Wann S, Mene P, Dubyak GR, Kester M, Dunn MJ. Endothelin-1 activates the phosphoinositide cascade in cultured glomerular mesangial cells. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S80–84. doi: 10.1097/00005344-198900135-00019. [DOI] [PubMed] [Google Scholar]
- 26.Tsukahara H, Ende H, Magazine HI, Bahou WF, Goligorsky MS. Molecular and functional characterization of the non-isopeptide-selective ETB receptor in endothelial cells. Receptor coupling to nitric oxide synthase. J Biol Chem. 1994;269:21778–21785. [PubMed] [Google Scholar]
- 27.Zhang Y, Brovkovych V, Brovkovych S, Tan F, Lee BS, Sharma T, Skidgel RA. Dynamic receptor-dependent activation of inducible nitric-oxide synthase by ERK-mediated phosphorylation of Ser745. J Biol Chem. 2007;282:32453–32461. doi: 10.1074/jbc.M706242200. [DOI] [PubMed] [Google Scholar]