Abstract
Background
While the association between obesity and endometrial cancer (EC) is well established, the underlying mechanisms require further study. We assessed possible links between lipid profiles and EC risk, while also taking into account BMI, parity, and menopausal status at baseline.
Methods
Using the information available from the Swedish Apolipoprotein MOrtality RISk (AMORIS) study we created a cohort of 225,432 women with baseline values for glucose, triglycerides (TG), and total cholesterol (TC). Two subgroups of 31,792 and 26,317 had, in addition, baseline measurements of HDL, LDL, apolipoprotein A-I and apoB and BMI, respectively. We used Multivariate Cox proportional hazards models to analyze quartiles and dichotomized values of these lipid components for a link to EC risk.
Results
During mean follow-up of 12 years (SD: 4.15), 1,144 persons developed endometrial cancer. A statistically significant association was found between TG and EC risk when using both quartiles and a clinical cut-off (Hazard Ratio (HR): 1.10 (95%CI: 0.88-1.37), 1.34 (1.09-1.63), and 1.57 (1.28-1.92)) for the 2nd, 3rd, and 4th quartile, compared to the 1st, with P-value for trend: <0.001). The association remained after exclusion of the first three years of follow-up. Also total cholesterol and TG/HDL ratio were positively associated with EC risk, but no link was found for the other lipid components studied.
Conclusion
This detailed analysis of lipid components showed a consistent relation between TG levels and EC risk. Future research should continue to analyze the metabolic pathway and its relation to EC risk, as a pathway to further understand the relation of obesity and disease.
Keywords: Lipid profiles, risk factor, endometrial cancer, Swedish AMORIS study
Introduction
Increasing body mass index (BMI) is strongly associated with endometrial cancer (EC) incidence and death [1]. A meta-analysis including 19 reviews and prospective studies showed that per 5kg/m2 increase in BMI a woman’s risk of development of EC increased with 59% (RR: 1.59 (95%CI: 1.50-1.68) [2]. The molecular mechanisms underlying how adipose tissue and obesity contribute to the pathogenesis of EC are becoming better understood and have revealed a number of rational strategies, both behavioral and pharmaceutical, for the prevention of both primary and recurrent disease.
A greater amount of adipose tissue is thought to improve efficiency by which androstenedione converts into estrone, resulting in higher estrogen levels, which are positively associated with EC risk [3,4]. Additionally, estrogen is thought to inhibit actions of peroxisome proliferator-activated receptor alpha (PPAR-alpha), a ligand-activated transcription factor that is heavily involved in catabolism of fatty acids and lipoproteins [5]. However, few epidemiological studies have investigated whether commonly measured markers of the lipid metabolism as well as glucose, which may have changed following overweight or obesity, are associated with the risk of EC.
A prospective cohort study of 31, 473 women found no association between low-density lipoprotein (LDL) or high-density lipoprotein (HDL) cholesterol and EC risk, but showed a positive relation between levels of triglycerides (TG) and EC after adjusting for age and BMI (HR: 1.79 (95% CI: 1.04-5.28)) [6]. In contrast, low serum cholesterol and low LDL cholesterol were associated with greater EC risk for women above 55 years of age in a case-control study with 256 cases (OR: 4.15 (95% CI: 1.8-9.7) and 3.06 (95% CI: 1.3-7.0), respectively) [7,8]. To improve the understanding of mechanisms linking obesity and EC risk, we investigated associations between serum lipids and EC risk in a large prospective cohort.
Methods
Study population and data collection
The Swedish AMORIS database has been described in detail elsewhere [9-11]. Briefly, this database is based on linkage of the Central Automation Laboratory (CALAB) database (1985-1996) to several Swedish national registries such as the National Cancer Register, Cause of Death Register, consecutive Swedish Censuses during 1970-1990, Patient Register, MultiGeneration Register, and National Register of Emigration by using the Swedish 10-digit personal identity number to provide information on cancer diagnosis, vital status, socio-economic status (SES), hospitalization, parity, and emigration [12]. The CALAB database includes data from 351,487 male and 338,101 female healthy individuals having clinical laboratory testing as part of a general health check-up or outpatients referred for laboratory testing. This study complied with the Declaration of Helsinki and was approved by the ethics review board of the Karolinska Institute.
We selected all females aged 25+, whose levels of triglycerides (TG) (mmol/L), total cholesterol (TC) (mmol/L), and glucose (mmol/L) were measured at baseline and who did not have hysterectomy, as registered in the Patient Register, prior to t h is index examination (n=225,432). Of those, 26,317 had baseline measurement of body-mass index (BMI, kg/m2) (Subgroup A) and 32,659 persons had baseline information of LDL (mmol/L), HDL (mmol/L), apolipoprotein (apo) B (g/L), and apoA-I (g/L) (Subgroup B) (Figure 1). Follow-up started six months after baseline examination and subjects were followed until date of EC diagnosis, hysterectomy, death, emigration out of Sweden or study closing date (31 December 2002), whichever occurred first.
Figure 1.
Overview of study population.
Endometrial cancer diagnosis was taken from the National Cancer Registry (ICD-7: 172), which has an underreporting of <4%. All incident cases of cancer in Sweden must be separately reported to the cancer register by the responsible clinician as well as the respective pathologist/cytologist. All cancers are thus histologically or cytologically confirmed and record linkage by means of the personal identity number ensures that each tumour is only registered once [13,14]. SES is based on the occupational group recorded in the census information, which classifies gainfully employed subjects into manual and non-manual workers, designated here as blue-collar and white-collar workers [15]. Number of children and mother’s age at birth of first child, as measures of parity, were obtained from the Swedish Multi-Generation Register. The concentrations of LDL and HDL were calculated and validation procedures have been reported [11]. The balance of cholesterol can be assessed either by ratios of TC to HDL (TC/HDL) or LDL to HDL cholesterol (LDL/HDL) or by using a ratio of apoB/apoA-I, which are component particles of LDL and HDL that bind and transport cholesterol [16].
To measure total cholesterol and TG we applied enzymatic methods whereas apoB and apoA-I were measured by immunoturbidimetric methods [9,10]. Glucose was measured enzymatically with a glucose-oxidase/peroxidase method. All methods were fully automated with automatic calibration and performed at one accredited laboratory [9] (Table 1).
Table 1.
Laboratory methods used to assess the biomarkers used in AMORIS.
| Instrument | Method | Total imprecision | |
|---|---|---|---|
| Triglycerides | Technicon DAXTM 96 Multichannel Analyzer (Bayer Diagnostics, Tarrytown, USA) | GPO-PAP: Enzymatic determination of glycerol with glycerol-phosphate-oxidase (GPO) after hydrolysis with lipoprotein lipase | ≤5.0% CV |
| Total cholesterol | Technicon DAXTM 96 Multichannel Analyzer (Bayer Diagnostics, Tarrytown, USA) | CHOD-PAP: Enzymatic cholesterol assay based on cholesterol esterase and cholesterol oxidase conversion followed by a Trinder-type sequence of reactions | ≤2.7% CV |
| Glucose | Technicon DAXTM 96 Multichannel Analyzer (Bayer Diagnostics, Tarrytown,USA) | GOD-PAP method: Enzymatic colorimetric test in which glucose in serum reacts with oxygen to give gluconate and hydrogen peroxide in the presence of glucose oxidase | <2.2% CV |
| Apolipoprotein A-I | Technicon DAXTM 96 Multichannel Analyzer (Bayer Diagnostics, Tarrytown, USA) | Immunoturbidimetry using polyclonal antisera from Orion (Helsinki, Finland) | <4.0% CV |
| Apolipoprotein B | Technicon DAXTM 96 Multichannel Analyzer (Bayer Diagnostics, Tarrytown, USA) | mmunoturbidimetry using polyclonal antisera from Orion (Helsinki, Finland) | <4.0% CV |
Data analysis
Associations between quartiles of lipid components and EC risk were studied by using multivariate Cox proportional hazards models. All models were adjusted for continuous levels of glucose, TG and TC, as well as age, parity, fasting status, and SES. The association between glucose and EC risk has been studied in detail previously in AMORIS [12]. An analysis of lipid components in relation to EC risk was also carried out using dichotomized values based on cut-offs used in cardiovascular disease prevention (cut-offs: 1.03 mmol/L, 4.10 mmol/L, 5.00, 3.50, 1.50, 1.05, 1.00, and 0.50 for HDL, LDL, TC/HDL, LDL/HDL, apoB, apoA-I, apoB/apoA-I, and log(TG/HDL), respectively) [10,17,18]. Levels of TG and TC were also dichotomized based on the National Cholesterol Education Program (NCEP) (cut-offs: 1.71 and 6.50mmol/L) [18].
Following our study on the association between glucose and EC risk, we also conducted an analysis stratified by glucose levels with 6.11 mmol/L as the cut-off [12]. In subgroup A, the association between TG, TC, and EC risk was also stratified by BMI. We chose BMI stratification rather than BMI adjustment because we wanted to test whether overweight (BMI≥25kg/m2) was associated with increased EC risk via changes in lipid components. Overweight rather than obesity (BMI≥30kg/m2) was chosen because there were too few obese persons in the cohort. The number of EC cases in subgroup B was too small to allow for stratification by BMI. Wald Tests were used to check for statistical significance of the interaction terms tested above.
Since parity at baseline is thought to be inversely associated with EC risk [19], we conducted a stratified analysis by parity status (0 versus ≥1 children) in which an additional adjustment for age at first birth was made. To account for the unfavorable effects of menopause on the lipid profile [20], we also did an age-stratified analysis as a proxy for menopausal status at time of baseline [21]. In the analysis of premenopausal women, individuals were followed to age 50 after which they were censored [12]. In the assessment of postmenopausal risk, individuals with a baseline measurement taken before age 50 entered the study at age 50 by means of delayed entry. We evaluated reverse causation by excluding all women with follow-up < 3 years [22]. All Cox proportional hazard models were tested for proportionality by visual inspection of the log(-log(survival) versus log(time) plot and there were no signs of model violations. All statistical methods are consistent with our studies on lipid profiles and risk of prostate and kidney cancer [23-25] and were conducted with Statistical Analysis Systems (SAS) release 9.1.3 (SAS Institute, Cary, NC) and R version 2.7.2 (R Foundation for Statistical Computing, Vienna, Austria). This study complied with the Declaration of Helsinki and was approved by the ethics review board of the Karolinska Institute.
Results
A total of 1,144 women developed EC during mean follow-up of 12.16 years (SD: 4.15) . Descriptive statistics of the study population are shown in Table 2. These baseline characteristics were similar for both subgroups A and B (results not shown).
Table 2.
Descriptive statistics by endometrial cancer status.
| Endometrial Cancer N=1144 | No Endometrial Cancer N=224288 | |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 56.09 (10.06) | 46.52 (13.78) |
| Parity, n (%) | ||
| 0 children | 316 (31.98) | 62139 (32.08) |
| 1 child | 247 (25) | 43991 (22.71) |
| 2 children | 372 (37.65) | 77083 (39.79) |
| 3+ children | 53 (5.36) | 10516 (5.43) |
| Age at Birth of First Child | ||
| <21 | 24.86 (4.63) | 24.69 (4.84) |
| BMI (kg/m2)^ | ||
| <18.5 | 1 (0.09) | 822 (0.37) |
| 18.5- 24.99 | 61 (5.33) | 17661 (7.87) |
| 25-29.99 | 33 (2.88) | 5831 (2.60) |
| >30 | 20 (1.75) | 1888 (0.84) |
| Missing | 1029 (89.95) | 198086 (88.32) |
| SES | ||
| White Collar | 325 (28.41) | 66216 (29.52) |
| Blue Collar | 643 (56.21) | 120583 (53.76) |
| Not gainfully employed or Missing | 176 (15.38) | 37489 (16.71) |
| Follow-up time (years) | ||
| Mean (SD) | 8.23 (4.46) | 12.18 (4.14) |
| Fasting status | ||
| Fasting | 724 (63.29) | 124650 (55.58) |
| Non-fasting | 309 (27.01) | 74232 (33.10) |
| Missing | 111 (9.70) | 25406 (11.33) |
| Glucose (mmol/l) | ||
| Mean (SD) | 5.15 (1.47) | 4.86 (1.13) |
| Triglycerides (mmol/l) | ||
| Mean (SD) | 1.36 (0.835) | 1.11(0.72) |
| Total cholesterol (mmol/l) | ||
| Mean (SD) | 6.12 (1.13) | 5.59 (1.17) |
| Apolipoprotein A-I (mmol/l)* | ||
| Mean (SD) | 1.54 (0.25) | 1.51 (0.24) |
| Apolipoprotein B (mmol/l)* | ||
| Mean (SD) | 1.32 (0.36) | 1.16 (0.34) |
| HDL-cholesterol (mmol/l)* | ||
| Mean (SD) | 1.72 (0.46) | 1.72 (0.43) |
| LDL-cholesterol (mmol/l)* | ||
| Mean (SD) | 3.84 (1.13) | 3.48 (4.14) |
| ApoB/apoA-I ratio* | ||
| Mean (SD) | 0.87(0.30) | 0.79 (0.27) |
| LDL/HDL ratio* | ||
| Mean (SD) | 2.47 (1.26) | 2.21 (1.22) |
| Total Cholesterol/HDL ratio* | ||
| Mean (SD) | 3.89 (1.60) | 3.56 (1.61) |
| Triglycerides/HDL ratio* | ||
| Mean (SD) | 0.93 (0.91) | 0.77 (1.08) |
Measured in subgroup A;
Measured in subgroup B
Hazard ratios (HR) for the associations between lipid components and EC risk are presented in Table 3. A statistically significant positive association was noted for quartiles of TG and TC (e.g. HR for TG: 1.10 (95%CI: 0.88-1.37), 1.34 (95%CI: 1.09-1.63), 1.57 (95%CI: 1.28-1.92) for the 2nd, 3rd, and 4th quartile compared to the 1st, P-value for trend: <0.001). The TG/HDL ratio was the only lipid ratio found to be statistically significantly positively associated with EC risk (HR: 1.18 (95%CI: 0.71-1.95), 1.38 (95%CI: 0.86-2.24), 1.67 (95%CI: 1.05-2.66) for the 2nd, 3rd, and 4th quartile compared to the 1st, P-value for trend: 0.021). In Table 4 we show how lipid components are associated with EC risk in relation to clinical cut-off points. We found a positive association between elevated levels of TG, TG/HDL ratio, and EC risk (Table 4).
Table 3.
Hazard ratios of endometrial cancer in quartiles of lipoprotein components and ratios, adjusted for age, parity, glucose (continuous), triglycerides (continuous), total cholesterol (continuous), fasting status, and SES.
| N(%) | Hazard Ratio (95% CI) | ||
|---|---|---|---|
| Endometrial Cancer | No Endometrial Cancer | ||
| Triglycerides (mmol/L)1 | |||
| <0.70 | 148 (12.94) | 53155 (23.70) | 1.00 (Ref) |
| 0.70-0.90 | 183 (16.00) | 47176 (21.03) | 1.10 (0.88-1.37) |
| 0.90-1.30 | 339 (29.63) | 61508 (27.42) | 1.34 (1.09-1.63) |
| ≥1.30 | 474 (41.43) | 62449 (27.84) | 1.57 (1.28-1.92) |
| P-value for trend | <0.001 | ||
| Total cholesterol (mmol/L)2 | |||
| <4.80 | 122 (10.66) | 56221(25.07) | 1.00 (Ref) |
| 4.80-5.50 | 223 (19.49) | 54931 (24.49) | 1.28 (1.02-1.60) |
| 5.50-6.30 | 324 (28.32) | 55272 (24.64) | 1.28 (1.03-1.59) |
| ≥6.30 | 475 (41.52) | 57864 (25.80) | 1.15 (0.93-1.43) |
| P-value for trend | 0.731 | ||
| LDL-cholesterol (mmol/L)b,2 | |||
| <2.70 | 19 (11.45) | 7726 (23.78) | 1.00 (Ref) |
| 2.70-3.36 | 41 (24.70) | 8152 (25.09) | 1.48 (0.85-2.56) |
| 3.36-4.13 | 42 (25.30) | 8204 (25.25) | 1.11 (0.64-1.94) |
| ≥4.13 | 64 (38.55) | 8411 (25.89) | 1.16 (0.67-2.00) |
| P-value for trend | 0.857 | ||
| HDL-cholesterol (mmol/L) b,2 | |||
| <1.45 | 49 (29.52) | 8345 (25.68) | 1.00 (Ref) |
| 1.45-1.70 | 42 (25.30) | 8240 (25.23) | 1.03 (0.66-1.60) |
| 1.70-1.98 | 29 (17.47) | 7983 (24.57) | 0.72 (0.44-1.19) |
| ≥1.98 | 46 (27.71) | 7925 (24.39) | 0.97 (0.62-1.54) |
| P-value for trend | 0.645 | ||
| Apolipoprotein B (g/L)b | |||
| <0.93 | 19 (11.46) | 8071 (24.84) | 1.00 (Ref) |
| 0.93-1.11 | 34 (20.48) | 8063 (24.81) | 1.06 (0.60-1.89) |
| 1.11-1.34 | 41 (24.70) | 7841 (24.13) | 0.90 (0.49-1.65) |
| ≥1.34 | 72 (43.37) | 8518 (26.21) | 1.01 (0.50-2.03) |
| P-value for trend | 0.885 | ||
| Apolipoprotein A-I (g/L)b | |||
| <1.35 | 37 (22.29) | 8434 (25.96) | 1.00 (Ref) |
| 1.35-1.49 | 46 (27.71) | 8128 (25.01) | 1.14 (0.74-1.77) |
| 1.49-1.65 | 36 (21.69) | 7706 (26.72) | 0.89 (0.56-1.42) |
| ≥1.65 | 47 (28.31) | 8225 (25.31) | 0.96 (0.61-1.51) |
| P-value for trend | 0.606 | ||
| Total Cholesterol/HDL ratio b,2 | |||
| <2.69 | 30 (18.07) | 7758 (23.88) | 1.00 (Ref) |
| 2.69-3.22 | 34 (20.48) | 7951 (24.47) | 0.93 (0.56-1.52) |
| 3.22-3.98 | 40 (24.10) | 8276 (25.34) | 0.85 (0.52-1.39) |
| ≥3.98 | 62 (37.35) | 8508 (26.18) | 0.88 (0.53-1.47) |
| P-value for trend | 0.605 | ||
| LDL/HDL ratiob,2 | |||
| <1.52 | 33 (19.88) | 8210 (25.27) | 1.00 (Ref) |
| 1.52-2.00 | 31 (18.67) | 8053 (24.78) | 0.80 (0.49-1.31) |
| 2.00-2.66 | 47 (28.31) | 8161 (25.12) | 0.97 (0.61-1.53) |
| ≥2.66 | 55 (33.13) | 8069 (24.88) | 0.81 (0.50-1.31) |
| P-value for trend | 0.564 | ||
| ApoB/apoA-I ratiob | |||
| <0.60 | 26 (15.66) | 7988 (24.58) | 1.00 (Ref) |
| 0.60-0.75 | 38 (22.89) | 8431 (25.95) | 1.02 (0.62-1.68) |
| 0.75-0.93 | 40 (24.10) | 7830 (24.10) | 0.86 (0.52-1.43) |
| ≥0.93 | 62 (37.35) | 8244 (25.37) | 0.93 (0.56-1.54) |
| P-value for trend | 0.657 | ||
| Triglycerides/HDL ratiob,2 | |||
| <0.37 | 28 (16.87) | 8359 (25.73) | 1.00 (Ref) |
| 0.37-0.54 | 34 (20.48) | 7941 (24.44) | 1.18 (0.71-1.95) |
| 0.54-0.87 | 44 (26.51) | 8124 (25.00) | 1.38 (0.86-2.24) |
| ≥0.87 | 60 (36.14) | 8069 (24.83) | 1.67 (1.05-2.66) |
| P-value for trend | 0.021 | ||
Not adjusted for TG;
Not adjusted for TC;
Measured in subgroup B
Table 4.
Hazard ratios of endometrial cancer for lipid components and lipid ratios using cut-off points used in cardiovascular disease prevention, adjusted for age, glucose (continuous), triglycerides (continuous), fasting status, and SES.
| N(%) | Hazard Ratio (95% CI) | ||
|---|---|---|---|
| Endometrial cancer | No Endometrial cancer | ||
| Triglycerides (mmol/L)1 | |||
| <1.71 | 875 (76.49) | 196146 (87.45) | 1.00 (Ref) |
| ≥1.71 | 269 (23.51) | 28142 (12.55) | 1.54 (1.33-1.79) |
| Total cholesterol (mmol/L)2 | |||
| <6.50 | 724 (63.29) | 176582 (78.33) | 1.00 (Ref) |
| ≥6.50 | 420 (36.71) | 47706 (21.16) | 0.99 (0.87-1.12) |
| LDL-cholesterol (mmol/L)b2 | |||
| <4.10 | 100 (60.24) | 23812 (73.28) | 1.00 (Ref) |
| ≥4.10 | 66 (39.76) | 8681 (26.72) | 0.96 (0.69-1.34) |
| HDL-cholesterol (mmol/L) b2 | |||
| ≥1.03 | 156 (93.98) | 31061 (95.59) | 1.00 (Ref) |
| <1.03 | 10 (6.02) | 1432 (4.41) | 0.86 (0.41-1.81) |
| Apolipoprotein B (mmol/L) b | |||
| <1.50 | 116 (69.88) | 27570 (84.85) | 1.00 (Ref) |
| ≥1.50 | 50 (30.12) | 4923 (15.15) | 1.36 (0.86-2.14) |
| Apolipoprotein A-I (mmol/L) b | |||
| ≥1.05 | 166 (100.0) | 32103 (98.80) | 1.00 (Ref) |
| <1.05 | 0 (0) | 390 (1.20) | NA |
| TC/HDL b2 | |||
| <5.00 | 133 (80.12) | 29019 (89.31) | 1.00 (Ref) |
| ≥5.00 | 33 (19.88) | 3474 (10.69) | 1.23 (0.77-1.98) |
| LDL/HDL b2 | |||
| <3.5 | 138 (83.13) | 29281 (90.11) | 1.00 (Ref) |
| ≥3.50 | 28 (16.87) | 3212 (9.89) | 1.08 (0.68-1.73) |
| ApoB/apoA-I b | |||
| <1.00 | 111 (66.87) | 26382 (81.19) | 1.00 (Ref) |
| ≥1.00 | 55 (33.13) | 6111 (18.81) | 1.31 (0.89-1.94) |
| Log(TG/HDL) b2 | |||
| <0.50 | 143 (86.14) | 30017 (92.38) | 1.00 (Ref) |
| ≥0.50 | 23 (13.86) | 2476 (7.62) | 1.62 (1.03-2.54) |
Not adjusted for TG;
Not adjusted for TC;
Measured in subgroup B.
No effect modification by levels of glucose was seen for the association between TG, TC, and EC risk (Table 5). In subgroup A, we found no effect modification by BMI levels (</≥ 25kg/m2) (Table 5). Figure 2 provides further insight into the associations between quartiles of TG and TC, and EC risk by strata of BMI (</≥25.) For TG it can be seen that those with BMI ≥ 25 had a higher cumulative incidence for all quartiles while the difference between quartiles is smaller, particularly among the first and fourth quartiles, explaining why relative risk estimates are slightly larger for those with BMI<25.
Table 5.
Hazard ratios of endometrial cancer in quartiles of lipoprotein components and ratios, adjusted for age, parity, glucose (continuous), triglycerides (continuous), total cholesterol (continuous), fasting status, and SES, stratified by parity (nulliparous vs. parous) or menopausal stage.
| N(%) | Hazard Ratio (95% CI) | N(%) | Hazard Ratio (95% CI) | P-values for Interaction | |||
|---|---|---|---|---|---|---|---|
| Endometrial Cancer | No Endometrial Cancer | Endometrial Cancer | No Endometrial Cancer | ||||
| GLUCOSE <6.11 MMOL/L | GLUCOSE ≥6.11 MMOL/L | ||||||
| Triglycerides (mmol/L)1 | |||||||
| <0.70 | 142 (13.63) | 52368 (24.51) | 1.00 (Ref) | 6 (5.88) | 787 (7.42) | 1.00 (Ref) | 0.922 |
| 0.70-0.90 | 178 (17.08) | 46142 (21.59) | 1.12 (0.90-1.40) | 5 (4.90) | 1034 (9.75) | 0.46 (0.13-1.64) | |
| 0.90-1.30 | 322 (30.90) | 59102 (27.66) | 1.35 (1.10-1.66) | 17 (16.67) | 2406 (22.69) | 0.66 (0.25-1.74) | |
| ≥1.30 | 400 (38.39) | 56070 (26.24) | 1.56 (1.27-1.92) | 74 (72.55) | 6279 (60.15) | 0.91 (0.37-2.20) | |
| P-value for trend | <0.001 | 0.482 | |||||
| Total cholesterol (mmol/L)2 | |||||||
| <4.80 | 113 (10.84) | 54673 (25.59) | 1.00 (Ref) | 9 (8.82) | 1548 (14.60) | 1.00 (Ref) | 0.084 |
| 4.80-5.50 | 206 (19.77) | 52916 (24.76) | 1.27 (1.01-1.60) | 17 (16.67) | 2015 (19.00) | 1.23 (0.49-3.09) | |
| 5.50-6.30 | 293 (28.12) | 52505 (24.57) | 1.24 (0.99-1.55) | 31 (30.39) | 2767 (26.09) | 1.57 (0.67-3.67) | |
| ≥6.30 | 430 (41.27) | 52588 (25.08) | 1.12 (0.89-1.40) | 45 (44.12) | 4276 (40.32) | 1.11 (0.47-2.61) | |
| P-value for trend | 0.974 | 0.997 | |||||
| BMI <25 | BMI ≥25 | ||||||
| Triglycerides (mmol/L)1,a | 1.00 (Ref) | 1.00 (Ref) | |||||
| <0.70 | 12 (19.35) | 5445 (29.46) | 1.16 (0.53-2.56) | 4 (7.55) | 915 (11.85) | 0.85 (0.23-3.19) | 0.438 |
| 0.70-0.90 | 13 (20.97) | 4388 (23.74) | 1.09 (0.50-2.36) | 5 (9.43) | 1146 (14.85) | 0.91 (0.28-2.88) | |
| 0.90-1.30 | 16 (25.81) | 5053 (27.34) | 1.73 (0.79-3.78) | 11 (20.75) | 2140 (27.72) | 1.51 (0.51-4.44) | |
| ≥1.30 | 21 (33.87) | 3597 (19.46) | 0.198 | 33 (62.26) | 3518 (45.58) | 0.190 | |
| P-value for trend | |||||||
| Total cholesterol (mmol/L)2,a | |||||||
| <4.80 | 8 (12.90) | 5299 (28.67) | 1.00 (Ref) | 6 (11.32) | 1260 (16.32) | 1.00 (Ref) | 0.547 |
| 4.80-5.50 | 20 (32.26) | 5014 (27.13) | 1.92 (0.84-4.40) | 7 (13.21) | 1680 (21.76) | 0.63 (0.21-1.90) | |
| 5.50-6.30 | 19 (30.65) | 4575 (24.75) | 1.35 (0.57-3.20) | 18 (33.96) | 2141 (27.75) | 0.92 (0.36-2.39) | |
| ≥6.30 | 15 (24.19) | 3595 (19.45) | 0.84 (0.33-2.16) | 22 (41.51) | 2637 (34/16) | 0.65 (0.25-1.72) | |
| P-value for trend | 0.309 | 0.524 | |||||
| NULLIPAROUS | PAROUS | ||||||
| Triglycerides (mmol/L)1 | |||||||
| <0.70 | 45 (14.24) | 16586 (26.69) | 1.00 (Ref) | 77 (13.25) | 26651 (22.56) | 1.00 (Ref) | 0.667 |
| 0.70-0.90 | 45 (14.24) | 13489 (21.71) | 0.94 (0.62-1.43) | 99 (17.04) | 24639 (20.85) | 1.13 (0.83-1.52) | |
| 0.90-1.30 | 87 (27.53) | 16573 (26.67) | 1.23 (0.85-1.79) | 173 (29.78) | 32695 (27.67) | 1.29 (0.98-1.70) | |
| ≥1.30 | 139 (43.99) | 15491 (24.93) | 1.73 (1.19-2.50) | 232 (39.93) | 34173 (28.92) | 1.45 (1.09-1.92) | |
| P-value for trend | <0.001 | 0.005 | |||||
| Total cholesterol (mmol/L)2 | |||||||
| <4.80 | 37 (11.71) | 19013 (30.60) | 1.00 (Ref) | 65 (11.19) | 26713 (22.61) | 1.00 (Ref) | 0.405 |
| 4.80-5.50 | 58 (18.35) | 15783 (25.40) | 1.18 (0.78-1.79) | 120 (20.65) | 28692 (24.28) | 1.24 (0.92-1.68) | |
| 5.50-6.30 | 82 (25.95) | 13764 (22.15) | 1.18 (0.79-1.78) | 169 (29.09) | 30608 (25.90) | 1.18 (0.88-1.58) | |
| ≥6.30 | 139 (43.99) | 13579 (21.85) | 1.15 (0.77-1.73) | 227 (39.07) | 32145 (27.21) | 1.01 (0.75-1.36) | |
| P-value for trend | 0.656 | 0.455 | |||||
| PRE-MENOPAUSAL | POST-MENOPAUSAL | ||||||
| Triglycerides (mmol/L)1 | |||||||
| <0.70 | 66 (23.32) | 43160 (30.72) | 1.00 (Ref) | 188 (16.43) | 61695 (27.51) | 1.00 (Ref) | |
| 0.70-0.90 | 64 (22.61) | 33019 (23.51) | 1.12 (0.79-1.58) | 183 (16.00) | 47176 (21.03) | 1.07 (0.86-1.32) | |
| 0.90-1.30 | 75 (26.50) | 36393 (25.91) | 1.15 (0.82-1.62) | 339 (29.63) | 61508 (27.42) | 1.31 (1.09-1.58) | |
| ≥1.30 | 78 (27.56) | 27904 (19.86) | 1.41 (0.99-2.01) | 434 (37.94) | 53909 (24.04) | 1.57 (1.30-1.90) | |
| P-value for trend | 0.066 | <0.0001 | |||||
| Total cholesterol (mmol/L)2 | |||||||
| <4.80 | 63 (22.26) | 49716 (35.39) | 1.00 (Ref) | 142 (12.41) | 61022 (27.21) | 1.00 (Ref) | |
| 4.80-5.50 | 80 (28.27) | 41710 (29.69) | 1.06 (0.76-1.48) | 223 (19.49) | 54931 (24.49) | 1.26 (1.01-1.58) | |
| 5.50-6.30 | 81 (28.62) | 31644 (22.53) | 1.12 (0.80-1.57) | 324 (28.32) | 55275 (24.64) | 1.23 (1.00-1.52) | |
| ≥6.30 | 59 (20.85) | 17406 (12.39) | 1.17 (0.81-1.71) | 455 (39.77) | 53060 (23.66) | 1.13 (0.92-1.39) | |
| P-value for trend | 0.368 | 0.671 | |||||
Not adjusted for TG;
Not adjusted for TC;
Measured in subgroup A.
Figure 2.
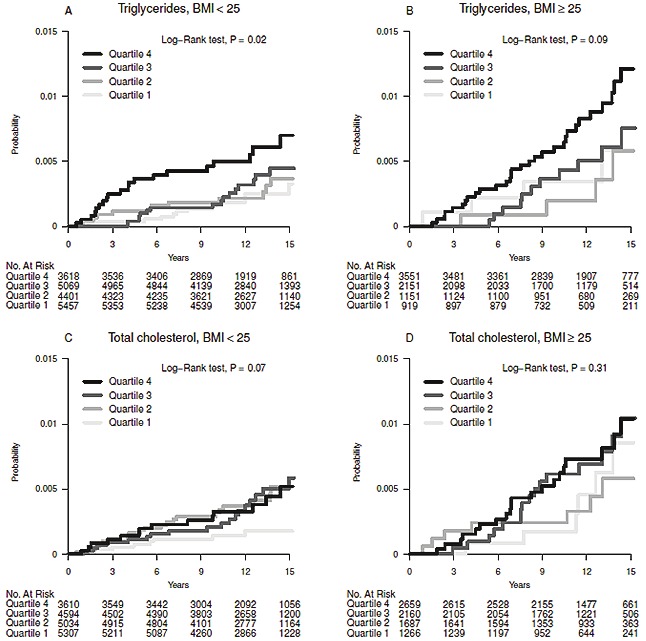
Cumulative incidence of endometrial cancer for quartiles of triglycerides and total cholesterol, stratified by levels of BMI (</≥ 25kg/m2).
Next, we determined how parity or menopausal status may alter associations between lipids and EC risk (Table 5). While confidence intervals overlapped where there were lower levels of TC, EC risk was positively associated with high levels of TG for both categories of parity, with the risk being slightly higher among nulliparous women. In the group of pre-menopausal women, HR for EC was only statistically significant for the highest quartile of TG levels, but for the post-menopausal women it was statistically significant for both the 3rd and 4th quartiles of TG (Table 5).
Finally, excluding those with follow-up time < 3 years, in order to assess reverse causality, did not affect the above findings (results not shown).
Discussion
This study found consistent evidence for a positive association between elevated TG levels and EC risk, both when using quartiles and dichotomous values of TG as well as when conducting stratified analysis by parity or menopausal status.
A series of contradictory observations have emerged regarding the relation between lipids and EC. In a prospective study of 5,209 subjects, no association was found between TC and any female cancer, while others have found both negative and positive associations between TC and EC risk [26-28]. Most recently, a prospective cohort study including 31,473 women found no association between TC, LDL, or HDL and EC risk, but there was a positive association of TG levels and EC risk (RR: 2.34 (95% CI: 1.04-5.28)) [6].
Our findings of an association between TG and EC risk corroborate this most recent study, but we also found an association between TC and EC, albeit not when using medical cut-offs [6]. Certain nuclear receptors, such as perioxisome activated receptors (PPAR), have been found to modulate lipid levels [6,29,30]. One form of PPAR, PPAR gamma, is expressed in adipose tissue and is activated by endogenous ligands, such as fatty acids, and is involved in lipid metabolism [31]. In EC, as among several other cancers, it has been noted that PPAR gamma expression is downregulated, which suggests that, since PPAR-gamma has also been found to regulate expression of p21 in EC cells, down-regulation of PPAR-gamma supports EC while preventing the clearing of TG [30,32]. Such a mechanism would suggest that increased triglyceride levels may possibly cause a decrease in PPAR-gamma levels, causing adverse effects on regulation of cell cycle, as is present in cancerous cells. PPAR-alpha, another form of PPAR, is also expressed in adipose tissue and facilitates catabolism of TG [33]. Furthermore, in mice fed ligands for PPAR-alpha, increased expression of proteins such as CDK-1 and CDK-4 in murine hepatic cells was noted, which are over-expressed in cancer cells [31]. As fatty acids are recognized ligands for PPAR-alpha and it has been noted that PPAR-alpha levels are elevated in endometrial cancer, it could be speculated that PPAR-alpha may be a possible explanation to the association between TG and EC risk [31,34].
Following the studies investigating the association between the metabolic syndrome and EC risk [35-37], we performed a glucose-stratified analysis. Despite the rather consistent positive association between serum glucose levels and EC risk, our study did not find evidence for effect modification. The association between TG and EC remained for those with normal glucose levels, but the lack of an association among those with high glucose is likely due to the small number of cases with high glucose levels. Besides the PPAR mechanism mentioned above, it was recently suggested that dendritic cells, responsible for initiation and maintenance of immune response, from tumour-bearing mice and persons with cancer have high amounts of TG compared with those from tumour free mice and healthy individuals [38]. These findings suggest that manipulating lipid levels in dendritic cells may improve immune responses in cancer and illustrate the importance of assessing reverse causality when assessing the link between lipid components and risk of cancer. We did not find evidence for reverse causation, however to verify the findings by Herber et al. [38], we lack information on tumour stage in this database.
In our study, upon stratification by BMI, the positive association between TG and EC risk was attenuated for overweight women; this may be due to the difference in baseline levels of triglyceride-associated EC risk between overweight and non-overweight individuals. Our results have to be interpreted carefully, as there is already a higher EC risk associated with obese individuals, which is not represented in our calculated hazard ratios, but can be seen in Figure 2.
We found that the association between TG and EC risk was slightly greater in women who were nulli-parous at baseline, which may suggest that parity may affect the association of TG and EC. However, as this was observed for only one quartile, it may not be conclusive and TG may be a proxy of another metabolic marker such as obesity. Several studies have noted the protective effect of parity on EC risk [39,40]. As EC is associated with high levels of unopposed estrogen, the protective effect of parity may be due to the high levels of progesterone that are present during the course of pregnancy [19]. Protection due to parity may also arise from the shedding of malignant cells through childbirth [40]. Experiments in mice have shown a decrease in elevated TG levels in mice injected with progesterone, which while speculative, could explain the protective effect of parity on the association of TG with greater EC risk [41].
Additionally, we noted a stronger association between TG and EC risk for postmenopausal women compared to premenopausal women. However, this observation was limited to the highest quartile of TG. In addition, one has to be aware that we did not have detailed information on menopausal status and used age as a proxy. Among premenopausal women, obesity is related to an increased number of anovulatory menstrual cycles [42]. Our observation of an association between menopause and EC risk via TG is in line with previous research, which found menopause to be positively associated with increased TG, possibly related to increased hepatic production of TG-rich particles [43,44]. The increase in TG and possible subsequent downstream increases in PPAR-alpha and cancer-associated proteins is a potential mechanism of association between possible effect-modification of menopause status at baseline and association between TG and EC risk.
The large number of women with prospective measurements of lipid biomarkers, all measured at the same clinical laboratory, is a major advantage of our analysis. The AMORIS population covers a range of SES and ethnicity similar to that of the general working population of Stockholm county. During the study period the population selected for AMORIS had an all-cause mortality about 14% lower than in the general population of Stockholm county when taking age, gender, and calendar year into account [45], but this does not affect the internal validity of our study. Another strength of this prospective study was its ability to account for parity, menopausal status, and BMI as potential confounders. However, only limited data on BMI was available which confined the statistical power when also studying BMI (n=31,792) and age was used as a proxy for menopausal status. The latter approximation has been used previously and is based on a previously estimated median age at menopause of 50 [12,21]. All models were adjusted for fasting status, however it was missing for about 10% of the study population. Lack of possible confounding variables, such as age at menarche, oral contraceptive use, measurements of different hormone levels, and hormonal use are limitations of this study.
Conclusion
Triglycerides were consistently associated with a greater EC risk in this prospective study. These results suggest that lipid metabolism, particularly TG, is a possible mechanism through which obesity is linked to EC. Future research should continue to analyze the metabolic pathway and its relation to EC risk, as a pathway to further understand the relation of obesity and disease.
References
- 1.Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011;205:518–525. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald PC, Edman CD, Hemsell DL, Porter JC, Siiteri PK. Effect of obesity on conversion of plasma androstenedione to estrone in postmenopausal women with and without endometrial cancer. Am J Obstet Gynecol. 1978;130:448–455. doi: 10.1016/0002-9378(78)90287-9. [DOI] [PubMed] [Google Scholar]
- 4.Savona-Ventura C, Grima S, Buttigieg GG. Metabolic carcinogenesis in the Maltese population. Exp Clin Endocrinol Diabetes. 2009;117:78–82. doi: 10.1055/s-2008-1078732. [DOI] [PubMed] [Google Scholar]
- 5.Yoon M. The role of PPARalpha in lipid metabolism and obesity: focusing on the effects of estrogen on PPARalpha actions. Pharmacol Res. 2009;60:151–159. doi: 10.1016/j.phrs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Lindemann K, Vatten LJ, Ellstrom-Engh M, Eskild A. Serum lipids and endometrial cancer risk: results from the HUNT-II study. Int J Cancer. 2009;124:2938–2941. doi: 10.1002/ijc.24285. [DOI] [PubMed] [Google Scholar]
- 7.Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Tjonneland A, Olsen A, Overvad K, Jakobsen MU, Chajes V, Clavel-Chapelon F, Boutron-Ruault MC, Linseisen J, Lukanova A, Boeing H, Pischon T, Trichopoulou A, Christina B, Trichopoulos D, Palli D, Berrino F, Panico S, Tumino R, Sacerdote C, Gram IT, Lund E, Quiros JR, Travier N, Martinez-Garcia C, Larranaga N, Chirlaque MD, Ardanaz E, Berglund G, Lundin E, Bueno-de-Mesquita HB, van Duijnhoven FJ, Peeters PH, Bingham S, Khaw KT, Allen N, Key T, Ferrari P, Rinaldi S, Slimani N, Riboli E. Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2007;14:755–767. doi: 10.1677/ERC-07-0132. [DOI] [PubMed] [Google Scholar]
- 8.Swanson CA, Potischman N, Barrett RJ, Berman ML, Mortel R, Twiggs LB, Wilbanks GD, Hoover RN, Brinton LA. Endometrial cancer risk in relation to serum lipids and lipoprotein levels. Cancer Epidemiol Biomarkers Prev. 1994;3:575–581. [PubMed] [Google Scholar]
- 9.Jungner I, Marcovina SM, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I values in 147576 Swedish males and females, standardized according to the World Health Organization-International Federation of Clinical Chemistry First International Reference Materials. Clin Chem. 1998;44:1641–1649. [PubMed] [Google Scholar]
- 10.Jungner I, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I in relation to serum cholesterol and triglycerides in 43,000 Swedish males and females. Int J Clin Lab Res. 1992;21:247–255. doi: 10.1007/BF02591655. [DOI] [PubMed] [Google Scholar]
- 11.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 12.Lambe M, Wigertz A, Garmo H, Walldius G, Jungner I, Hammar N. Impaired glucose metabolism and diabetes and the risk of breast, endometrial, and ovarian cancer. Cancer Causes Control. 2011;22:1163–1171. doi: 10.1007/s10552-011-9794-8. [DOI] [PubMed] [Google Scholar]
- 13.Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register. Non-notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol. 1984;23:305–313. doi: 10.3109/02841868409136026. [DOI] [PubMed] [Google Scholar]
- 14.Van Hemelrijck M, Garmo H, Binda E, Hayday A, Karagiannis SN, Hammar N, Walldius G, Lambe M, Jungner I, Holmberg L. Immunoglobulin E and cancer: a meta-analysis and a large Swedish cohort study. Cancer Causes Control. 2010;21:1657–67. doi: 10.1007/s10552-010-9594-6. [DOI] [PubMed] [Google Scholar]
- 15.Van Hemelrijck M, Garmo H, Holmberg L, Walldius G, Jungner I, Hammar N, Lambe M. Prostate cancer risk in the Swedish AMORIS study: the interplay among triglycerides, total cholesterol, and glucose. Cancer. 2011;117:2086–95. doi: 10.1002/cncr.25758. [DOI] [PubMed] [Google Scholar]
- 16.Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy--a review of the evidence. J Intern Med. 2006;259:493–519. doi: 10.1111/j.1365-2796.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 17.Millan J, Pinto X, Munoz A, Zuniga M, Rubies-Prat J, Pallardo LF, Massana L, Mangas A, Hernandez Mijares A, Gonzalez-Santos P, Ascaso JF, Pedro-Botet J. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
- 18.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.Hinkula M, Pukkala E, Kyyronen P, Kauppila A. Grand multiparity and incidence of endometrial cancer: a population-based study in Finland. Int J Cancer. 2002;98:912–915. doi: 10.1002/ijc.10267. [DOI] [PubMed] [Google Scholar]
- 20.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women's Health Across the Nation. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bengtsson C, Lindquist O, Redvall L. Menstrual status and menopausal age of middle-aged Swedish women. A population study of women in Goteborg 1968--69 and 1974--75. Acta Obstet Gynecol Scand. 1981;60:269–275. doi: 10.3109/00016348109158130. [DOI] [PubMed] [Google Scholar]
- 22.Ahn J, Lim U, Weinstein SJ, Schatzkin A, Hayes RB, Virtamo J, Albanes D. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2814–2821. doi: 10.1158/1055-9965.EPI-08-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Hemelrijck M, Garmo H, Holmberg L, Walldius G, Jungner I, Hammar N, Lambe M. Prostate cancer risk in the Swedish AMORIS study: the interplay among triglycerides, total cholesterol, and glucose. Cancer. 2011;117:2086–2095. doi: 10.1002/cncr.25758. [DOI] [PubMed] [Google Scholar]
- 24.Van Hemelrijck M, Walldius G, Jungner I, Hammar N, Garmo H, Binda E, Hayday A, Lambe M, Holmberg L. Low levels of apolipoprotein A-I and HDL are associated with risk of prostate cancer in the Swedish AMORIS study. Cancer Causes Control. 2011;22:1011–1019. doi: 10.1007/s10552-011-9774-z. [DOI] [PubMed] [Google Scholar]
- 25.Van Hemelrijck M, Garmo H, Hammar N, Jungner I, Walldius G, Lambe M, Holmberg L. The interplay between lipid profiles, glucose, BMI and risk of kidney cancer in the Swedish AMORIS study. Int J Cancer. 2012;130:2118–2128. doi: 10.1002/ijc.26212. [DOI] [PubMed] [Google Scholar]
- 26.Kark JD, Smith AH, Hames CG. The relationship of serum cholesterol to the incidence of cancer in Evans County, Georgia. J Chronic Dis. 1980;33:311–332. doi: 10.1016/0021-9681(80)90026-0. [DOI] [PubMed] [Google Scholar]
- 27.Williams RR, Sorlie PD, Feinleib M, McNamara PM, Kannel WB, Dawber TR. Cancer incidence by levels of cholesterol. JAMA. 1981;245:247–252. [PubMed] [Google Scholar]
- 28.Wallace RB, Rost C, Burmeister LF, Pomrehn PR. Cancer incidence in humans: relationship to plasma lipids and relative weight. J Natl Cancer Inst. 1982;68:915–918. [PubMed] [Google Scholar]
- 29.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 30.Ota K, Ito K, Suzuki T, Saito S, Tamura M, Hayashi S, Okamura K, Sasano H, Yaegashi N. Peroxisome proliferator-activated receptor gamma and growth inhibition by its ligands in uterine endometrial carcinoma. Clin Cancer Res. 2006;12:4200–4208. doi: 10.1158/1078-0432.CCR-05-1833. [DOI] [PubMed] [Google Scholar]
- 31.Roberts-Thomson SJ. Peroxisome proliferator-activated receptors in tumorigenesis: targets of tumour promotion and treatment. Immunol Cell Biol. 2000;78:436–441. doi: 10.1046/j.1440-1711.2000.00921.x. [DOI] [PubMed] [Google Scholar]
- 32.Saidi SA, Holland CM, Charnock-Jones DS, Smith SK. In vitro and in vivo effects of the PPAR-alpha agonists fenofibrate and retinoic acid in endometrial cancer. Mol Cancer. 2006;5:13. doi: 10.1186/1476-4598-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Raalte DH, Li M, Pritchard PH, Wasan KM. Peroxisome proliferator-activated receptor (PPAR)-alpha: a pharmacological target with a promising future. Pharm Res. 2004;21:1531–1538. doi: 10.1023/b:pham.0000041444.06122.8d. [DOI] [PubMed] [Google Scholar]
- 34.Holland CM, Saidi SA, Evans AL, Sharkey AM, Latimer JA, Crawford RA, Charnock-Jones DS, Print CG, Smith SK. Transcriptome analysis of endometrial cancer identifies peroxisome proliferator-activated receptors as potential therapeutic targets. Mol Cancer Ther. 2004;3:993–1001. [PubMed] [Google Scholar]
- 35.Bjorge T, Stocks T, Lukanova A, Tretli S, Selmer R, Manjer J, Rapp K, Ulmer H, Almquist M, Concin H, Hallmans G, Jonsson H, Stattin P, Engeland A. Metabolic syndrome and endometrial carcinoma. Am J Epidemiol. 2010;171:892–902. doi: 10.1093/aje/kwq006. [DOI] [PubMed] [Google Scholar]
- 36.Friedenreich CM, Biel RK, Lau DC, Csizmadi I, Courneya KS, Magliocco AM, Yasui Y, Cook LS. Case-control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2384–2395. doi: 10.1158/1055-9965.EPI-11-0715. [DOI] [PubMed] [Google Scholar]
- 37.Rosato V, Zucchetto A, Bosetti C, Dal Maso L, Montella M, Pelucchi C, Negri E, Franceschi S, La Vecchia C. Metabolic syndrome and endometrial cancer risk. Ann Oncol. 2011;22:884–889. doi: 10.1093/annonc/mdq464. [DOI] [PubMed] [Google Scholar]
- 38.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, Knight SC, Padhya T, McCaffrey TV, McCaffrey JC, Antonia S, Fishman M, Ferris RL, Kagan VE, Gabrilovich DI. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elwood JM, Cole P, Rothman KJ, Kaplan SD. Epidemiology of endometrial cancer. J Natl Cancer Inst. 1977;59:1055–1060. doi: 10.1093/jnci/59.4.1055. [DOI] [PubMed] [Google Scholar]
- 40.Lambe M, Wuu J, Weiderpass E, Hsieh CC. Childbearing at older age and endometrial cancer risk (Sweden) Cancer Causes Control. 1999;10:43–49. doi: 10.1023/a:1008860615584. [DOI] [PubMed] [Google Scholar]
- 41.Valette A, Verine A, Varesi L, Boyer J. Effects of ethynyl estradiol and progesterone on triglyceride metabolism in the female rat. Endocrinology. 1978;103:1647–1653. doi: 10.1210/endo-103-5-1647. [DOI] [PubMed] [Google Scholar]
- 42.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 43.Bonithon-Kopp C, Scarabin PY, Darne B, Malmejac A, Guize L. Menopause-related changes in lipoproteins and some other cardiovascular risk factors. Int J Epidemiol. 1990;19:42–48. doi: 10.1093/ije/19.1.42. [DOI] [PubMed] [Google Scholar]
- 44.Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325:1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 45.Holzmann M, Jungner I, Walldius G, Ivert I, Nordqvist T, Östergren J. Apolipoproteins B and AI, standard lipid measures and incidence of myocardial infarction in men and women, with or without chronic kidney disease. Study IV in Thesis for doctorial degree (PhD) In: Holzmann M, editor. Renal insufficiency, mortality and myocardial infarction. Stockholm: Karolinska Institutet; 2008. [Google Scholar]


