Abstract
The evaluation of proteins using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis is a common technique used by biochemistry and molecular biology researchers1-4. For laboratories that perform daily analyses of proteins, the cost of commercially available polyacrylamide gels (˜$10/gel) can be considerable over time. To mitigate this cost, some researchers prepare their own polyacrylamide gels. Traditional methods of pouring these gels typically utilize specialized equipment and glass gel plates that can be expensive and preclude pouring many gels and storing them for future use. Furthermore, handling of glass plates during cleaning or gel pouring can result in accidental breakage creating a safety hazard, which may preclude their use in undergraduate laboratory classes. Our protocol demonstrates how to pour multiple protein gels simultaneously by recycling Invitrogen Nupage Novex minigel cassettes, and inexpensive materials purchased at a home improvement store. This economical and streamlined method includes a way to store the gels at 4°C for a few weeks. By re-using the plastic gel cassettes from commercially available gels, labs that run frequent protein gels can save significant costs and help the environment. In addition, plastic gel cassettes are extremely resistant to breakage, which makes them ideal for undergraduate laboratory classrooms.
Keywords: Basic Protocols, Issue 60, Molecular Biology, minigel, cassettes, protein, gel, electrophoresis
Protocol
1. Preparing the cassettes
Clean front and back plates of a used Invitrogen Nupage Novex minigel cassette, or other commercial minigel cassette.
Prepare approximately 1-3 ml of plastic epoxy and mix well according to manufacturer's instructions.
Apply the epoxy to the inside edge of the front plate, where the two plates will come in contact when the cassette is assembled.
Slide a piece of filter paper or cardboard through the bottom slit of the back plate when assembling the cassette to prevent excess epoxy from sealing the opening. Remove the filter paper as soon as the two plates are assembled.
Place binder clips along the edges for a tight seal, and let the cassettes sit overnight for the epoxy to fully cure.
Seal the slit on the bottom of the back plate with electrical tape.
2. Pouring the resolving gel
To a 125 ml side arm flask, add the following: 2.25 ml of 4X Tris-Base Resolving gel buffer pH 8.70, 3.50 ml of 30.8% acrylamide/bisacrylamide, 3.25 ml of Type I reagent grade water (IRG-H2O) for a total volume of 9 ml.
De-gas the solution for 10 min by attaching the side arm of the flask to a vacuum apparatus, and placing an inverted stopper on the top of the flask.
Transfer the 9 ml of de-gassed solution to a 50 ml disposable polypropylene centrifuge tube and add 30 μl of 10% ammounium persulfate (APS) and 6 μl of N, N, N', N',-tetramethylethylenediamine (TEMED) and mix well by swirling the solution to avoid introducing bubbles.
Immediately pour the solution into the cassette, allowing it to run down the side of the cassette to prevent any bubbles from forming between the gel plates. Fill the cassette to approximately 2 cm from the top edge of the front (shorter) plate.
Gently add 0.5 ml of 1-butanol saturated with 1X resolving gel buffer and let the gel polymerize for 1 hour.
3. Pouring the stacking gel
Add the following to a 125 ml side arm flask: 0.75 ml of 4X Tris-Base Stacking gel buffer pH 6.80, 0.40 ml of 30.8% acrylamide/bisacrylamide, 1.85 ml of IRG-H2O for a total volume of 3 ml.
De-gas the solution for 10 min as described in step 2.
While the stacking gel solution is degassing, pour off the 1-butanol saturated with 1X resolving gel buffer from the top of the resolving gel. Rinse the top of the resolving gel once with IRG-H2O. Pour off the water and use a bibulous or Whatman filter paper to absorb any remaining liquid.
Remove 3 ml of your degassed solution and add 9 μl of 10% APS and 2 μl of TEMED and mix well.
Quickly pour the solution in the cassette, allowing it to run down the side of the cassette until it is near the top edge of the shorter front gel plate.
Insert a clean gel comb into the top of the gel cassette, place a binder clip on the cassette over the comb to hold it in place. Let the stacking gel polymerize for 1 hour to overnight.
4. Storing the gel
When the gel has completely polymerized, remove the binder clips and place the cassette in a heat-sealable plastic pouch, or a re-sealable plastic bag.
Pour 20 ml of 1X stacking gel buffer that contains 0.1% sodium azide into the pouch.
Heat seal the pouch for 5 sec and make sure there are no leaks in the bag.
Store the gel at 4°C.
5. Preparing the Samples
In a thin-walled microcentrifuge tube, add up to 6.5 μl of protein sample, 7.5 μl of 2x Novex Tris-Glycine SDS sample buffer, 1 μl of 100 mM dithiothreitol (DTT) and IRG-H2O (if necessary) for a total volume of 15 μl.
Mix the sample by vortexing, and briefly centrifuge the sample to bring contents to the bottom of the tube.
Denature the sample at 70°C for 10 min.
6. Running the gel
Set up the Novex gel apparatus.
Add 200 ml of Tris-Glycine Running buffer that contains 500 μl of Nupage antioxidant to the inner chamber, and 300 ml of Tris-Glycine Running buffer to the outer chamber.
Load the denatured protein samples (15 μl) into the wells using a gel loading pipette tip.
Run the gel at 200 V for 60-70 min.
7. Staining the gel
Remove the cassette from the gel apparatus and break the epoxy seal by prying the two plastic gel plates apart using a gel knife or a stiff putty knife. Carefully separate the gel from the plates, taking care to not tear the gel.
Rinse the gel with IRG-H2O three times with gentle agitation.
Stain the gel with Invitrogen SimplyBlue SafeStain for 1 hour with gentle agitation.
Pour off the Staining solution and destain the gel with IRG-H2O.
8. Cleaning the cassettes
Use a metal spatula to scrape or peel off all the epoxy on the cassettes so they can be reused again.
Wash each of the plastic gel plates with a mild detergent solution, and rinse well with tap water followed by distilled water.
9. Representative Results
Because epoxy will form a water-tight seal, (Figure 1), the cassettes will not leak when the acrylamide solution is poured into them. In addition, the gel cassettes can be easily opened to retrieve the gel for staining, and the epoxy can be peeled off of the cassettes to allow their continued re-use. As shown in Figure 2, the gel shows clear separation of protein markers and sample bands suitable for routine protein electrophoresis.
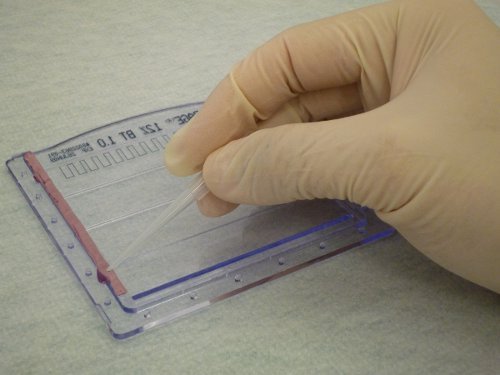 Figure 1. Application of epoxy to seal minigel cassettes. A thin layer of epoxy is applied to the edge of one of the cassettes prior to assembly. To highlight the epoxy, it was false-colored red in this image.
Figure 1. Application of epoxy to seal minigel cassettes. A thin layer of epoxy is applied to the edge of one of the cassettes prior to assembly. To highlight the epoxy, it was false-colored red in this image.
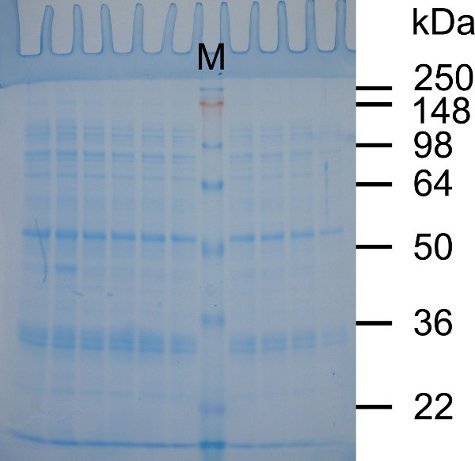 Figure 2. Separation of proteins on an SDS-Tris-glycine gel poured using re-used minigel cassettes. SDS-PAGE was performed as described above. Following electrophoresis the gel was removed from the minigel cassette, stained in SimplyBlue SafeStain as per manufacturer's instructions, and photographed using transmitted white light. Lane M contains SeeBlue Plus2 pre-stained protein standards. The molecular weights of the proteins in lane M are indicated on the right side of the gel in kilodaltons (kDa). The other lanes show proteins from a cleared lysate from bacteria expressing a glutathione-S-transferase (GST)-tagged protein. The proteins shown are those washed from the glutathione beads prior to elution of the GST-tagged protein.
Figure 2. Separation of proteins on an SDS-Tris-glycine gel poured using re-used minigel cassettes. SDS-PAGE was performed as described above. Following electrophoresis the gel was removed from the minigel cassette, stained in SimplyBlue SafeStain as per manufacturer's instructions, and photographed using transmitted white light. Lane M contains SeeBlue Plus2 pre-stained protein standards. The molecular weights of the proteins in lane M are indicated on the right side of the gel in kilodaltons (kDa). The other lanes show proteins from a cleared lysate from bacteria expressing a glutathione-S-transferase (GST)-tagged protein. The proteins shown are those washed from the glutathione beads prior to elution of the GST-tagged protein.
Discussion
Protein gel electrophoresis is an indispensible method for most molecular biology laboratories. Commercially available minigels provide a high level of convenience, but can be costly. Here we show how to re-use the minigel cassettes from a popular brand of commercially available minigels. This method retains the convenience of having a ready-made source of gels, but at a fraction of the cost of commercially available minigels. A key step in this procedure is applying the correct amount of epoxy to completely seal the cassettes prior to adding the unpolymerized acrylamide to prevent leakage. Use only epoxy that is rated for plastic, and allow enough time for polymerization of the epoxy as described in the manufacturer’s instructions. For added convenience, multiple cassettes can be sealed and stored prior to pouring the gels. When pouring the resolving gel, be sure to leave enough room for the stacking gel, typically about 0.5 cm more than the height of the comb used to form the wells. During polymerization of the resolving gel, keep the gel upright such that the buffer-saturated 1-butanol will form a straight, flat surface to form on the top of the resolving gel. If the gel does not polymerize within 30 min, the amount of 10% APS and TEMED may be increased. Once the gel has finished running, it is important to carefully break the epoxy seal to prevent ripping the thin gel. The method reported here of re-using commercial gel cassettes can save significant costs associated with frequent SDS-PAGE use. The same cassettes may be reused for years and we have no evidence that repeated use of the same cassette reduces their usability. In addition, multiple gels can be prepared and stored for weeks in a polyester barrier film bag containing a small amount of 1X stacking gel buffer containing 0.1% sodium azide.
Abbreviations:
Dithiothreitol (DTT ), N, N, N', N',-tetramethylethylenediamine (TEMED), Type I reagent grade water (IRG-H2O), ammounium persulfate (APS), sodium dodecyl sulfate (SDS), Tris (hydroxymethyl) aminomethane (Tris-base), N,N'-methylene-bis-acrylamide (bis-acrylamide)
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors thank the Howard Hughes Medical Institute for a UF Science for Life award to A.H.
References
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Raymond S, Weintraub L. Acrylamide gel as a supporting medium for zone electrophoresis. Science. 1959;130:711–711. doi: 10.1126/science.130.3377.711. [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shapiro AL, Viñuela E, Maizel JV. Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem. Biophys. Res. Commun. 1967;28:815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]


