Abstract
Cancer stem cells (CSCs)/cancer-initiating cells (CICs) are characterized as a small population of cancer cells that have high tumor-initiating ability. CSCs/CICs are resistant to several cancer therapies, and eradication of CSCs/CICs is essential to cure cancer. How can we eradicate CSCs/CICs? Cytotoxic T lymphocytes (CTLs) might be a promising answer.
Keywords: antigenic peptide, cancer stem cell, CTL, immunotherapy, tumor antigen
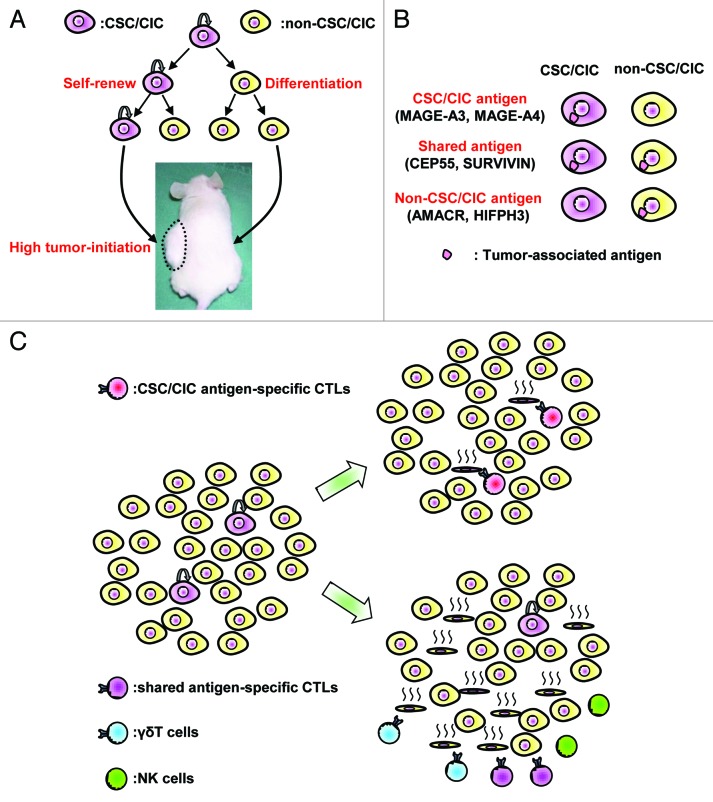
Cancer stem-like cells (CSCs)/cancer-initiating cells (CICs) are defined as a small population of cancer cells that have (1) high tumor-initiating ability, (2) self-renewal ability and (3) differentiation ability (Fig. 1A).1 In recent studies, CSCs/CICs have shown to be resistant to cancer therapies by their senescence state, high expression of transporters to efflux anti-cancer drugs, high expression of apoptosis inhibitors, low expression of reactive oxygen species.2 Thus, the action of CSCs/CICs are regarded as major mechanisms of cancer recurrence, distant metastasis and treatment resistance. However, effective cancer treatment targeting CSCs/CICs effectively have not been reported so far.
Figure 1. CSC/CIC targeting immunotherapy. (A) Characters of CSC/CIC. CSC/CIC has three distinct characteristics: (1) high tumor-initiating ability, (2) self-renewal ability and (3) differentiation ability. (B) Three groups of tumor-associated antigens. Tumor-associated antigens can be classified into 3 groups according to the expression in CSC/CIC and non-CSC/CIC: (1) CSC/CIC antigens, which are expressed in CSCs/CICs but not in non-CSCs/CICs (e.g., MAGE-A3 and MAGE-A4); (2) shared antigens, which are expressed in both CSCs/CICs and non-CSCs/CICs (e.g., CEP55, SURVIVIN); and (3) non-CSC/CIC antigens, which are expressed in only non-CSCs/CICs but not in CSCs/CICs (e.g., AMACR, HIFPH3). (C) CSC/CIC-targeting immunotherapy. CSC/CIC antigen specific CTLs recognize only higher tumorigenic CSCs/CICs, whereas shared antigen specific CTLs, NK cells and γδT cells recognize both CSCs/CICs and non-CSCs/CICs. CSCs/CICs might be eliminated most efficiently by CSC/CIC antigen-specific CTLs.
The prominent nature of the acquired immune system is its antigen specificity due to antigen-specific receptors including T cell receptors and B cell receptors, and isolation of human tumor-associated antigens (TAAs) has enabled us to target caner cells specifically in an antigen-specific manner.3 Cancer immunotherapy trials using TAAs have recently been performed in several facilities and significant results have been obtained.4 However, it is still not clear whether the immune system can recognize therapy-resistant CSCs/CICs or not. Some reports on immunity and CSCs/CICs have recently been published, and natural killer (NK) cells and γδT cells have been shown to recognize CSCs/CICs derived from human colon cancer and gliomas; however CTLs, which are a major component of the acquired immune system, have not been characterized yet.5
We analyzed the relation between CTLs and CSCs/CICs.6 We isolated CSCs/CICs from human colon cancer cells using a side population (SP) technique. Since CTLs recognize antigenic peptides derived from TAAs, we evaluated the expression of TAAs in colon CSCs/CICs and non-CSCs/CICs. Colon CSCs/CICs expressed CEP55, one of the TAAs, at the same level as did non-CSCs/CICs. In a further study, we evaluated the expression of several TAAs in both CSCs/CICs and non-CSCs/CICs, and we found that the expression pattern can be classified into the following groups (Fig. 1B, unpublished data): (1) CSC/CIC antigens, which are expressed in CSCs/CICs but not in non-CSCs/CICs (e.g., MAGE-A3 and MAGE-A4); (2) shared antigens, which are expressed in both CSCs/CICs and non-CSCs/CICs (e.g., CEP55, SURVIVIN); and (3) non-CSC/CIC antigens, which are expressed in only non-CSCs/CICs but not in CSCs/CICs (e.g., AMACR, HIFPH3). Therefore, CEP55 is one of the (2) shared antigens.
Since we have established CTL clone #41 which is specific for CEP55-derived antigenic peptide,7,8 we evaluated the reactivity of CTL clone #41 for colon CSCs/CICs and non-CSCs/CICs. Interestingly, CTL clone #41 recognized both colon CSCs/CICs and non-CSCs/CICs at the same level in vitro. Furthermore, CTL clone #41 inhibited the tumor-initiating ability of colon CSCs/CICs in vivo. These findings clearly indicate that treatment-resistant colon CSCs/CICs, as well as non-CSCs/CICs are sensitive to CTLs. Therefore, CTL-based immunotherapy is a promising approach to target CSCs/CICs.
In the next stage, another question has emerged. Which are the best TAAs for CSC/CIC-targeting cancer immunotherapy: (1) CSC/CIC antigens, (2) shared antigens or (3) non-CSC/CIC antigens? Non-CSC/CIC antigens do not seem to be suitable for targeting CSCs/CICs since they are not expressed in CSCs/CICs. Further analyses are under way to address thes questions, and we have found that targeting CSC/CIC antigens was more effective than targeting shared antigens in a CTL adoptive transfer model and a DNA vaccination model (unpublished data). Both CSC/CIC antigens and shared antigens are expressed in CSCs/CICs; however, the anti-tumor effects are different. We are not sure about the exact mechanisms and we are now analyzing; however, these data indicate that targeting CSC/CIC specific antigens is more effective than targeting shared antigens.
The numbers of CTL clones are very restricted and limited in vivo, and the maximum numbers of one CTL clone might be about 107 to 108 cells in the whole body. On the other hand, cancer tissues contain 5 × 108 cancer cells per gram,9 and advanced cancer tissues may therefore contain more than 1010 cancer cells. It is easy to imagine the difficulty in eliminating all cancer cells with such a limited number of CTLs (Estimated effector/target ratio is about 0.001 in the case of 107 CTL and 1010 cancer cells.). On the other hand, if we focus on just CSCs/CICs targeting CSC/CIC antigens, the situation will be improved (Estimated effector/target ratio is about 0.1 in the case of 107 CTL, 1010 cancer cells and 1% frequency of CSCs/CICs.). Therefore, targeting CSC/CIC antigens might be a more effective approach to eradicate higher tumorigenic CSCs/CICs and may bring about greater anti-tumor effects (Fig. 1C).
As stated above, NK cells and γδT cells have been reported to recognize CSSs/CICs. However, these immune cells belong to the innate immune system and do not recognize target cells in an antigen-specific manner. Thus, activation of these cells in vivo may not be more effective than CSC/CIC antigen-specific CTLs (Fig. 1C). CTL adoptive transfer therapy has recently been described in detail,10 and huge numbers of CTLs can be obtained by in vitro culture. Therefore, (2) shared antigens may also be suitable candidates for CTL adoptive transfer therapy using high numbers of CTLs.
In summary, CTLs can recognize CSCs/CICs as well as non-CSCs/CICs, and targeting CSC/CIC antigens with CTLs may be a reasonable approach for CSC/CIC targeting therapy.
Glossary
Abbreviations:
- CSC
cancer stem-like cell
- CIC
cancer-initiating cell
- CTL
cytotoxic T lymphocyte
- TAA
tumor-associated antigen
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18075
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Park CY, Tseng D, Weissman IL. Cancer stem cell-directed therapies: recent data from the laboratory and clinic. Mol Ther. 2009;17:219–30. doi: 10.1038/mt.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 4.Hirohashi Y, Torigoe T, Inoda S, Kobayasi J, Nakatsugawa M, Mori T, et al. The functioning antigens: beyond just as the immunological targets. Cancer Sci. 2009;100:798–806. doi: 10.1111/j.1349-7006.2009.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirohashi Y, Torigoe T, Inoda S, Takahashi A, Morita R, Nishizawa S, et al. Immune response against tumor antigens expressed on human cancer stem-like cells/tumor-initiating cells. Immunotherapy. 2010;2:201–11. doi: 10.2217/imt.10.10. [DOI] [PubMed] [Google Scholar]
- 6.Inoda S, Hirohashi Y, Torigoe T, Morita R, Takahashi A, Asanuma H, et al. Cytotoxic T lymphocytes efficiently recognize human colon cancer stem-like cells. Am J Pathol. 2011;178:1805–13. doi: 10.1016/j.ajpath.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoda S, Hirohashi Y, Torigoe T, Nakatsugawa M, Kiriyama K, Nakazawa E, et al. Cep55/c10orf3, a tumor antigen derived from a centrosome residing protein in breast carcinoma. J Immunother. 2009;32:474–85. doi: 10.1097/CJI.0b013e3181a1d109. [DOI] [PubMed] [Google Scholar]
- 8.Inoda S, Morita R, Hirohashi Y, Torigoe T, Asanuma H, Nakazawa E, et al. The feasibility of Cep55/c10orf3 derived peptide vaccine therapy for colorectal carcinoma. Exp Mol Pathol. 2011;90:55–60. doi: 10.1016/j.yexmp.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Laird AK. Cell fractionation of normal and malignant tissues. Exp Cell Res. 1954;6:30–44. doi: 10.1016/0014-4827(54)90145-7. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]