Abstract
Despite intensive study, the role of CCR5 in cancer remains elusive. We showed that CCR5 expression by both CD4+ and CD8+ T cells is necessary to boost anti-tumor responses by optimizing helper-dependent CD8+ T cell priming. Our findings could have implications for cancer treatment in patients with defective CCR5 expression.
Keywords: activation, antigen-presenting cell, cancer, CCR5, CD4 help, chemokines, cross-priming, immunotherapy, maturation, T cell, TLR
Chemokines and their receptors were classically established as key molecules that control immunity by orchestrating leukocyte trafficking into and from lymphoid organs and tissues.1 The CCR7/CCL19–21 pair regulates naïve T cell encounter with mature antigen-presenting cells (APC) in lymph nodes (LN); the CXCR5-CXCL13 system controls lymphocyte migration into and within lymphoid tissues, and the so-called inflammatory chemokines guide activated leukocytes to peripheral tissues, enabling pathogen eradication and tissue repair. Given the relevance of chemokines in immune and inflammatory responses, their association to cancer is not surprising, as they both promote and restrict tumor onset and/or progression. CCR5, the receptor for the chemokines CCL3 (MIP1α), CCL4 (MIP1β) and CCL5 (RANTES), exemplifies this paradox; whereas some reports suggest that CCR5 activation fosters tumor growth, angiogenesis, metastasis and immune evasion, studies in mouse and humans justify the use of CCR5 agonists as adjuvants to bolster anti-tumor immune responses.2 Indeed, CCR5 ligands can recruit both effector and immunosuppressive cells to tumors.3 Based on our results in a number of transplanted and spontaneous neoplasia models in mice,4 we propose that CCR5 is central to maximization of the immune response to tumors.
Since its discovery in 1996 as a HIV-1 coreceptor, CCR5 has been implicated in immune-associated processes that include allograft rejection, autoimmunity and clearance of viral infections. CCR5 function in these pathologies might be not limited to chemoattraction of specific leukocyte subtypes. Evidence indicates that CCR5 is a regulatory molecule in T cell activation, where it acts as a costimulatory receptor for CD4+ lymphocytes in a migration-independent manner.5 CCR5 also participates in helper-dependent CD8+ T cell activation, although its role is not well defined; whereas CCR5 was proposed to steer APC to CCL5-producing CD4+ T cells for in situ CD40L-mediated APC licensing,6 an intravital two-photon study suggested that CCR5 is required to guide “preactivated” naive CD8+ cells to CCL3- and CCL4-secreting DC-CD4+ T cell complexes.7 In both studies, CCR5 expression in CD4+ lymphocytes was apparently dispensable for helper-dependent CD8+ T cell activation.
What is the role of CCR5 in CD4+ helper function in the context of anti-tumor responses? We compared the effectiveness of tumor rejection after adoptive transfer of tumor-specific, CCR5-expressing or -deficient CD4+ and CD8+ T cells into tumor-bearing mice. Potent CD8+ T cell responses require CD4+ T cell help8 and, accordingly, we observed effective tumor rejection when recipients were co-transferred with CD4+ and CD8+ T cells.4We found that CCR5 expression on CD8+ T cells was necessary for their efficient activation and migration to the tumor site and for tumor killing; importantly, CCR5 must also be expressed by CD4+ T lymphocytes to achieve maximal CD8+ T cell effector function. We found a CCR5-dependent enhancement of CCL3, CCL4 and CCL5 secretion by CD4+-APC complexes, which correlated with increased CD8+ T cell priming. High CD40L expression in CD4+ cells was CCR5-dependent, and led to enhanced upregulation of CD80, CD86 and MHC-II in APC.4 We propose that CCR5 and its ligands regulate communication between CD4+, APC and CD8+ cells in the draining LN, not only by steering encounter of these three partners, but also through the CCR5-mediated upregulation of CD40L on CD4+ T cells, which triggers optimal APC maturation for efficient CD8+ T lymphocyte priming (Fig. 1).
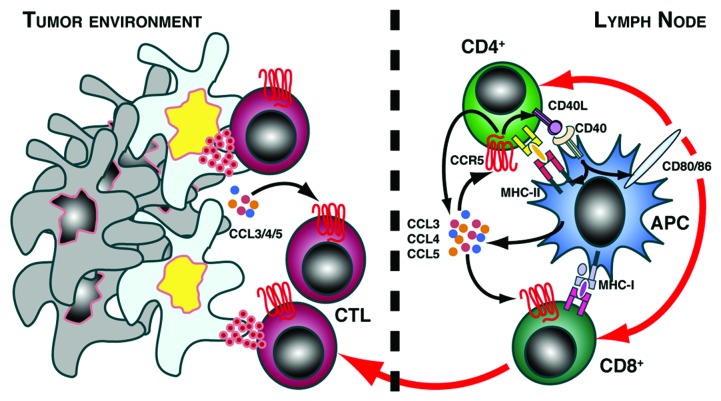
Figure 1.
CCR5 potentiates CD4+ T cell help to boost anti-tumor responses. Interaction between CD4+ T cells and antigen-loaded APC typically causes CCR5 agonist secretion and CD40L upregulation on the helper cell. We found that CCR5 expression on CD4+ cells increases agonist secretion and CD40L by these cells, in turn promoting full APC maturation with optimal expression of MHC-II and costimulatory molecules CD80 and CD86. This enhances the crosspriming of CCR5-expressing CD8+ T lymphocytes, which then exit the draining lymph node and migrate to the tumor site, where they exert their cytotoxic function on cancer cells. Lack of CCR5 expression on CD4+ T cells causes inefficient CD8+ T cell priming and tumor progression.
It is intriguing to ask whether other chemokine receptors can replace CCR5 function in T cells. Redundancy is a characteristic of the chemokine system in vitro; CCL3 and CCL5 are ligands for CCR1, a receptor also expressed on effector and helper T cells. Although we cannot formally exclude a role for CCR1 in helper-dependent CD8+ T cell activation, we found that inhibition of subcutaneous tumors overexpressing CCL5 was CCR5-dependent but CCR1-independent,4 suggesting that CCR1 cannot substitute for CCR5 in this context. This finding reinforces the concept of in vivo chemokine receptor functional specificity.
Given the relevance of CCR5 in T cell activation, a pertinent question is whether CCR5 has a role in tumor immune surveillance. In this regard, CCR5 deficiency enhanced incidence and accelerated the onset of 3-methylcholantrene-induced sarcomas. Nonetheless, CCR5 showed neither a protective nor a detrimental effect in the onset of spontaneous breast cancers in MMTV-neu mice. These differences might be explained by the distinct tumor immunogenicity in each model; MMTV-neu mice are functionally tolerized to neu antigens,9 resembling the situation of most human tumors in which tumor-reactive T cells are anergized by central or peripheral mechanisms.
Even so, clinical evidence indicates that the remaining low-avidity, tumor-specific T cell pool that escapes negative selection can be reactivated to restrict cancer growth. CCR5 might be central to this reactivation process, as shown in the tolerogenic MMTV-neu model. We found that intratumor injection of CpG-ODN, a TLR9 agonist, caused a significant reduction of breast tumor growth in wild-type but not in CCR5-deficient MMTV-neu mice, and that TLR9-mediated tumor growth inhibition was associated with enhanced expansion and effector activity of neu-specific CD8+ T cells in CCR5-expressing mice.4 Strikingly, CCR5 was also shown to prevent the anti-tumor effect elicited by a TLR3 agonist in combination with chemotherapy.3 These results suggest intricate crosstalk circuits for the combinatorial control of CCR5 responses by TLR.
In summary, our results, together with other studies recently published, implicate CCR5 and its ligands as key elements in maximizing T cell-mediated responses, of potential importance in tumor elimination and pathogen clearance.4,10 Deciphering how the inflammatory environment affects CCR5 function, determining the role of CCR5 in acute vs. chronic immune responses, and the planning of clinical trials to determine the therapeutic response of cancer patients with polymorphisms in CCR5 and/or CCR5 ligand genes, are challenges that, if resolved, could have considerable impact on the design and/or improvement of cancer therapies.
Acknowledgments
We thank lab members for discussion, and C. Mark for editorial assistance. We would like to dedicate this work to the memory of our collaborator Joseph Lustgarten. Our apologies to colleagues who were not cited due to space restrictions. This work was supported in part by the Spanish Ministry of Science and Innovation (SAF2011–24453), the Carlos III Health Institute (RIER Network, RD08/0075), and the Comunidad de Madrid (IMMUNOTHERCAN; S2011/BMD-2326).
Glossary
Abbreviations:
- APC
antigen-presenting cells
- CpG-ODN
CpG oligodeoxynucleotides
- LN
lymph node
- MMTV
mouse mammary tumor virus
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/17995
References
- 1.Viola A, Contento RL, Molon B. T cells and their partners: The chemokine dating agency. Trends Immunol. 2006;27:421–7. doi: 10.1016/j.it.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Lapteva N, Huang XF. CCL5 as an adjuvant for cancer immunotherapy. Expert Opin Biol Ther. 2010;10:725–33. doi: 10.1517/14712591003657128. [DOI] [PubMed] [Google Scholar]
- 3.Conforti R, Ma Y, Morel Y, Paturel C, Terme M, Viaud S, et al. Opposing effects of toll-like receptor (TLR3) signaling in tumors can be therapeutically uncoupled to optimize the anticancer efficacy of TLR3 ligands. Cancer Res. 2010;70:490–500. doi: 10.1158/0008-5472.CAN-09-1890. [DOI] [PubMed] [Google Scholar]
- 4.González-Martín A, Gómez L, Lustgarten J, Mira E. Mañes S. Maximal T cell-mediated antitumor responses rely upon CCR5 expression in both CD4+ and CD8+ T cells. Cancer Res. 2011;71:5455–66. doi: 10.1158/0008-5472.CAN-11-1687. [DOI] [PubMed] [Google Scholar]
- 5.Molon B, Gri G, Bettella M, Gómez-Moutón C, Lanzavecchia A, Martinez-A C, et al. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6:465–71. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 6.Nesbeth YC, Martinez DG, Toraya S, Scarlett UK, Cubillos-Ruiz JR, Rutkowski MR, et al. CD4+ T cells elicit host immune responses to MHC class II- ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells. J Immunol. 2010;184:5654–62. doi: 10.4049/jimmunol.0903247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–5. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 8.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–83. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Lustgarten J, Dominguez AL, Cuadros C. The CD8+ T cell repertoire against Her-2/neu antigens in neu transgenic mice is of low avidity with antitumor activity. Eur J Immunol. 2004;34:752–61. doi: 10.1002/eji.200324427. [DOI] [PubMed] [Google Scholar]
- 10.Crawford A, Angelosanto JM, Nadwodny KL, Blackburn SD, Wherry EJ. A role for the chemokine RANTES in regulating CD8 T Cell responses during chronic viral infection. PLoS Pathog. 2011;7:e1002098. doi: 10.1371/journal.ppat.1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]