Abstract
The mechanism of tumor cell death after treatment with DNA alkylating agents in vivo previously remained largely unknown. We demonstrate that tumor regression after chemotherapy occurs via sporadic necrosis and relies on activation of innate immunity in a manner dependent on high mobility group box 1 protein (HMGB1).
Keywords: chemotherapy, HMGB1, innate immunity, necrosis, tumor associated macrophage
The ability of a cell to evade apoptosis drives tumorigenesis and resistance to anti-cancer therapy. While tremendous effort has been made to design therapies which target specific components of the apoptotic machinery, understanding the molecular basis underlying nonspecific yet clinically effective chemotherapy will accelerate the design of strategies with improved efficacy and reduced adverse effects. Although chemotherapy has long been known to induce cancer cell death, thought to occur via apoptosis mediated by p53 and/or the mitochondrial apoptotic machinery, the mode of cell death remains increasingly confusing, and the data as a whole suggest that apoptosis is not the only or even major mechanism that is responsible for chemotherapy-induced tumor regression. Most of the mechanistic studies interrogating cell death mechanisms of chemotherapeutic agents have been conducted using cultured cells. Although cell culture studies have generated copious amounts of information, a comprehensive understanding of the in vivo role of tumor cell death during chemotherapy has been lacking, largely due to the complex tumor response to chemotherapy. In addition, chemotherapy may activate multiple cell death pathways simultaneously, and crosstalk between them complicates the identification of a precise role for specific molecular pathways in the overall tumor response.
Using an allograft mouse tumor model engineered with cells genetically deficient in mitochondrial apoptosis, we have recently shown that p53 and the mitochondrial apoptotic pathway are not absolutely required for a complete tumor regression in response to DNA alkylation therapy.1 In this study, tumorigenic cells were generated by oncogenic transformation (K-RasG12D and E1A) of mouse embryonic fibroblasts (MEFs) deficient in p53 or the mitochondrial pro-apoptotic proteins Bax and Bak. These tumorigenic cells formed fibrosarcomas when implanted into athymic nude mice. When treated with cyclophosphamide (CP), a DNA alkylating agent that is clinically prescribed as a neoadjuvant agent, both the p53-null and Bax/Bak-deficient tumors completely regressed, albeit with a delay compared with the wild-type tumors. Pathological analysis of the treated tumor tissue revealed signs of necrosis. This CP-induced necrosis differs from necrosis that is often observed in solid tumors with overgrowth. The latter constitutes the “necrotic centers” comprised of large amounts of necrotic cells resulting from limited oxygen and nutrient supplies due to tumor overgrowth and lack of vascularization. In contrast, CP-induced necrotic cells were scattered and evenly distributed throughout the tumor mass, thus referred to as “sporadic necrosis.”1 In addition, features of other cytocidal and cytostatic mechanisms including autophagy, mitotic catastrophe, and premature senescence were also observed in our study, as they have been suggested to contribute to the anti-cancer effect of chemotherapy.2-4
While this tumor allograft study provides in vivo evidence that non-apoptotic cytotoxic/cytostatic responses are induced during chemotherapy, a related question emerged as to what role the immune response associated with these non-apoptotic events plays in tumor regression. Necrosis, autophagy, and senescence have each been shown to stimulate a pro-inflammatory response, via passive release or active secretion of immune-stimulating molecules. HMGB1 is one of the cellular molecules that can be released into the extracellular environment during necrosis via plasma membrane rupture or during autophagy via an unknown mechanism.1,5,6 In our allograft tumor model, CP treatment induced extracellular release of HMGB1, which was accompanied by infiltration of innate immune cells including macrophages, neutrophils, and natural killer (NK) cells.1 To address whether the therapy-induced infiltration of leukocytes was mediated by HMGB1, and if so how the HMGB1-mediated pro-inflammatory response may influence the outcome of chemotherapy, we generated tumor cells using HMGB1-null MEFs. Strikingly, while HMGB1-deficient tumor cells did not have a survival advantage in response to DNA alkylating agents in cell culture, they were resistant to chemotherapy in vivo. The resistance correlates with impaired innate immune activation, indicated by lower levels of infiltration of macrophages, neutrophils, and NK cells into the HMGB1-deficient tumor tissues upon CP treatment.7 This finding indicates that HMGB1 is required for chemotherapy-induced tumor regression, and suggests that HMGB1-mediated activation of the innate immune system may play a role. Indeed, preventing the infiltration of macrophages, neutrophils, or NK cells, using depleting antibodies or pharmacological inhibitors leads to a failure in tumor regression.7
While our findings highlight an important role for the innate immune response in therapy-induced tumor clearance, the mechanism by which the innate immune system kills cancer cells remains to be determined. The end outcome of tumor regression may result from a combined effect of phagocytosis and cytotoxicity conferred by innate immune cells. At least one mechanism may involve NK cell recruitment and activation, as markedly lower levels of perforin and granzyme B were detected in hmgb1−/− tumors, suggesting that perforin/granzyme-mediated cytotoxicity may contribute to tumor cell death. This may also explain why tumor cells deficient in mitochondrial apoptosis are susceptible, as perforin and granzymes are known to mediate cell death independent of the mitochondrial apoptotic pathway.8
Another important clue arising from our study is that the M1/M2 switch of tumor-associated macrophages (TAMs) plays an important role during chemotherapy, and may be regulated by HMGB1. Macrophages represent up to 50% of a human tumor mass9 and represent a heterogeneous population of cells. The heterogeneity reflects the plasticity and versatility of these cells in response to exposure to various environmental signals. M1 macrophages are considered classically activated and are potent effector cells that kill microorganisms and tumor cells and produce pro-inflammatory cytokines. M2 macrophages are considered alternatively activated and promote angiogenesis, tissue remodeling and repair, and secrete anti-inflammatory cytokines. It is generally accepted that TAMs are a polarized M2 macrophage population and show pro-tumor functions, promoting tumor survival, proliferation, and dissemination.9 In our study, macrophages were recruited into both HMGB1-deficient and proficient CP-treated tumor tissue, and recruitment was associated with tumor resistance or regression to CP-therapy, respectively.7 HMGB1-deficient tumors had high levels of anti-inflammatory (pro-tumor) cytokines (IL-4, -10, and -13); whereas HMGB1-expressing tumors had markedly decreased levels of these cytokines and showed an increase in pro-inflammatory (anti-tumor) cytokines (IL-1β and TNFα). Therefore, HMGB1 released from tumor cells may facilitate tumor regression in response to chemotherapy by suppressing an M2 macrophage response and instead promoting an M1 anti-tumor response. As other work suggests that blocking TAM recruitment to tumor tissue with a CSF1R-antagonist enhances current anti-tumor therapy,10 our results indicate that re-polarizing M2 TAMs toward an M1 phenotype may also be beneficial during anti-cancer treatment.
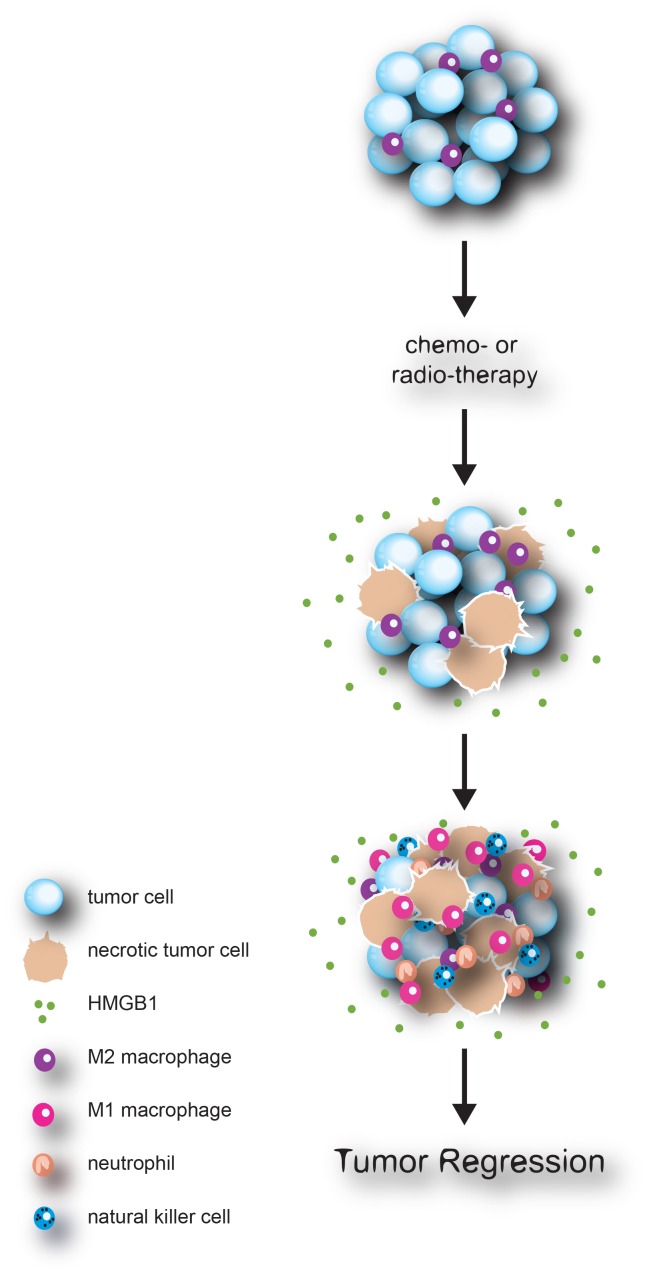
Taken together, our study provides an explanation for why CP, which is also used as an immunosuppressant, works efficiently in cancer treatment through activation of the innate immune response in an HMGB1-dependent manner. We propose a model whereby the initial cytotoxic/cytostatic response to chemotherapy (or radiation therapy) leads to the extracellular release of immune-stimulating molecules such as HMGB1, which in turn activate innate immune cells and leads to subsequent tumor killing and clearance (Fig. 1). A number of questions/issues remain to be addressed. For instance, what is the precise contribution of various forms of cytotoxic/static events on tumor regression? How is adaptive immunity involved? How might this model be applied to other contexts, such as various types of cancer, chemotherapeutic agents, and the immune status of the host? Nevertheless, our study suggests that modulating non-apoptotic pathways and the innate immune system should be considered as strategies for adjuvant treatment of human cancer.
Figure 1.
Chemotherapy induces HMGB1-mediated anti-tumor innate immune response. Upon chemotherapy, tumor cells undergo an array of cell death including sporadic necrosis, through which pro-inflammatory molecules, such as HMGB1, are released into the extracellular environment. HMGB1 polarizes tumor associated M2 macrophages toward an anti-tumor M1 type, and recruits innate immune cells such as NK cells and neutrophils that contribute to an anti-tumor response.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/17885
References
- 1.Guerriero JL, Ditsworth D, Fan Y, Zhao F, Crawford HC, Zong WX. Chemotherapy induces tumor clearance independent of apoptosis. Cancer Res. 2008;68:9595–600. doi: 10.1158/0008-5472.CAN-08-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–7. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 3.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–95. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 4.Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–9. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 5.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 6.Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2009;16:175–83. doi: 10.1038/cdd.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerriero JL, Ditsworth D, Catanzaro JM, Sabino G, Furie MB, Kew RR, et al. DNA Alkylating Therapy Induces Tumor Regression through an HMGB1-Mediated Activation of Innate Immunity. J Immunol. 2011;186:3517–26. doi: 10.4049/jimmunol.1003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol. 2008;26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 10.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]