Abstract
We recently described the upregulation of HLA-E in ovarian and cervical cancers. Instead of interacting with natural killer cells, HLA-E appeared to inhibit intratumoral cytotoxic T lymphocytes (CTL) via the receptor CD94/NKG2A. Strikingly, the survival benefit of intraepithelial infiltrating CTL was lost in those cancers with high HLA-E expression.
Keywords: CD94/NKG2A, CTL, gynecological cancers, HLA-E, infiltration
The interaction between cancer cells and the immune system has been studied since the 1890s.1 In the 20th century, extensive research revealed that the immune system is actually capable of mounting an immune response to tumors. In recent years, focus shifted to the identification of mechanisms responsible for the magnitude and efficacy of antitumor immune responses, such as FoxP3+ regulatory T lymphocytes (Treg), and downregulation of classical Major Histocompatibility Complex (MHC) class I molecules.2 We reported in the Proceedings of the National Academy of Sciences that HLA-E is an additional element greatly influencing the ability of CD8+ cytotoxic T lymphocytes (CTL) to respond to cancer cells.3
HLA-E is a non-classical MHC class I molecule, which can bind a very limited variety of peptides, mainly derived from signal sequences of classical MHC class I peptides. HLA-E/peptide complexes bind CD94, which forms heterodimers with the inhibitory NKG2A or stimulatory NKG2C. CD94/NKG2 molecules are predominantly expressed on natural killer (NK) cells, but also on some activated CTL. The affinity of HLA-E for the inhibiting CD94/NKG2A is about eight times higher than for CD94/NKG2C.4 The effect of HLA-E on immune cells is of great importance during the resolving stages of infections, to prevent excessive tissue damage, and in trophoblast invasion, to prevent rejection of the fetal allograft.5,6
HLA-E had not been studied extensively in large cohorts of cancer patients. Therefore, we set out to determine the expression of HLA-E in ovarian and cervical cancer, as well as its association with clinical characteristics and interaction with intratumoral CTL. By performing immunohistochemical stainings on 150 cervical and 270 ovarian cancer samples and healthy counterpart tissues, we found that HLA-E is overexpressed in respectively 89% and 83% of tumors. This phenomenon was previously explained by Malmberg et al.,7 who found that cytokines such as interferon-γ can induce HLA-E on ovarian cancer cell lines.
Interestingly, upregulation was not associated with clinicopathological factors, such as stage and grade, nor with survival. However, we found strong positive associations with components of the antigen processing and presentation pathway and classical MHC molecules, especially in ovarian cancer. These results are consistent with the fact that HLA-E binds peptides derived from these classical molecules.
Since the receptors for HLA-E are mainly expressed on NK cells, we determined whether these innate cells were present in the tumors. Surprisingly, both ovarian and cervical cancer contained hardly any NKp46+ NK cells, and NK cell infiltration was not related to HLA-E. Therefore, we hypothesized that HLA-E in gynecological cancers is not expressed to inhibit NK cells, but to hinder the subset of CD94/NKG2 positive CTL. We then determined the expression of CD94, NKG2A, and NKG2C on intatumoral CD4+ and CD8+ T lymphocytes via flow cytometry analysis on fresh surgical samples. Importantly, tumor-infiltrating CTL displayed CD94 and the inhibiting NKG2A chain, but not the activating NKG2C chain. Moreover, the frequency of CD94/NKG2A+ CTL in tumor was significantly higher than in blood from age-matched healthy controls. CD4+ T cells were largely devoid of both HLA-E interacting receptors.
To substantiate these results, we also performed triple fluorescent staining for CD3, CD94 and NKG2A on cryosections and found that these markers are indeed co-expressed. Even more interestingly, CD94/NKG2A expression was predominantly present on CTL in tumor nests, even though the majority of T cells resided in tumor stroma (CD94/NKG2A on 6% of stromal CTL vs. 48% of intratumoral CTL). These results indicate that a) instead of NK cells, CTL are the likely target of HLA-E, b) especially intratumoral CTL are susceptible to being inhibited by HLA-E.
This raises the question how CD94/NKG2A is being expressed on CTL and why this is so location dependent. Previous work by others showed that cytokines like interleukin-15 and transforming growth factor β play a major role in this phenomenon.8 Together with HLA-E expression being related to IFN-γ, these results underline the concept described in recent literature,9 considering an intratumoral inflammatory milieu (promoting either tumor progression or inhibition) a hallmark of cancer.
Since we previously found that CTL infiltration is associated with improved survival in ovarian cancer,10 we wondered whether this would be affected by HLA-E expression. After all, when CTL are inhibited by HLA-E and CD94/NKG2A, they are unable to exert antitumor effects, thereby reducing the prognostic benefit of CTL infiltration. To this end, we stratified our cohort by HLA-E expression. In patients with low HLA-E expression, CTL infiltration still showed an association with improved survival (hazard ratio (HR) 0.53, 95% confidence interval (CI) 0.36–0.78, p = 0.001). On the contrary, patients with high HLA-E expression did not benefit at all from CTL infiltration (HR 0.97, 95% CI 0.77–1.22, p = 0.816). This effect was not observed in cervical cancers, where CTL were not predictive of survival in the first place. However, cervical cancers did contain over three times as many CTL as ovarian cancer (median 95.3 ± 221.6/mm2; ovarian cancer: 28.3 ± 120/mm2; p < 0.001). This is most likely due to the viral nature of cervical cancers. Since ovarian cancer might not be as immunogenic, the impact of already low numbers of CTL might be reduced below a critical level more easily.
In conclusion, HLA-E is frequently upregulated in ovarian and cervical cancer. Instead of inhibiting NK cells, its role is most likely inhibition of CTL. In ovarian cancer, the presence of HLA-E is able to neutralize the protective role of the relatively scarce intratumoral CTL.
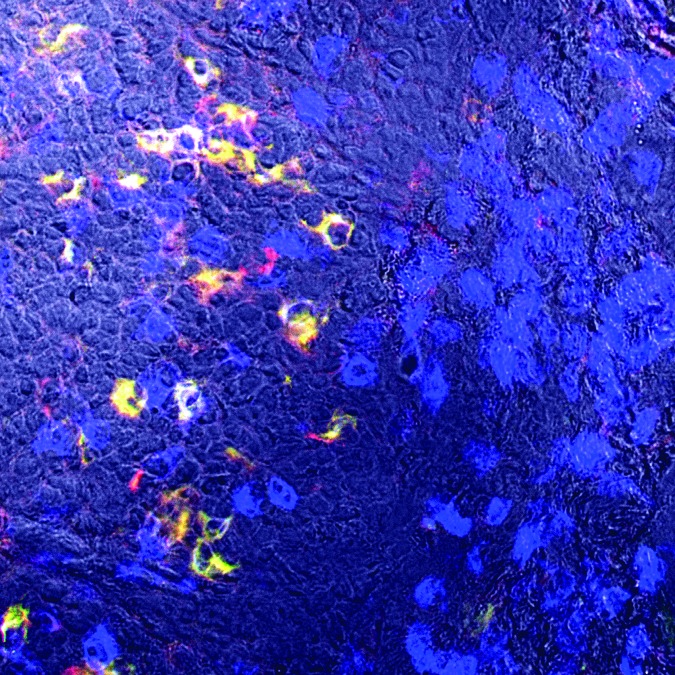
See Figure 1.
Figure 1.
Immunohistochemistry staining of cervical cancer shows the expression of CD94, NKG2A and CD3 on intratumoral lymphocytes. The stroma tissue at the right side of the picture gives an impression on the high degree of T cell infiltration (CD3 in blue) in cervical cancers. The co-expression of CD94 and NKG2A is predominantly observed on intraepithelial T cells in tumor nests, as can be seen at the left side of the picture. Dr. E. Jordanova is acknowledged for providing this photograph.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/17961
References
- 1.Parish CR. Cancer immunotherapy: The past, the present and the future. Immunol Cell Biol. 2003;81:106–13. doi: 10.1046/j.0818-9641.2003.01151.x. [DOI] [PubMed] [Google Scholar]
- 2.Steer HJ, Lake RA, Nowak AK, Robinson BW. Harnessing the immune response to treat cancer. Oncogene. 2010;29:6301–13. doi: 10.1038/onc.2010.437. [DOI] [PubMed] [Google Scholar]
- 3.Gooden M, Lampen M, Jordanova ES, Leffers N, Trimbos JB, van der Burg SH, et al. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8 T lymphocytes. Proc Natl Acad Sci USA. 2011;108:10656–61. doi: 10.1073/pnas.1100354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan LC, Clements CS, Rossjohn J, Brooks AG. The major histocompatibility complex class ib molecule HLA-E at the interface between innate and adaptive immunity. Tissue Antigens. 2008;72:415–24. doi: 10.1111/j.1399-0039.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Matsuoka M, Cantor H, Homer R, Enelow RI. Cutting edge: Engagement of NKG2A on CD8+ effector T cells limits immunopathology in influenza pneumonia. J Immunol. 2008;180:25–9. doi: 10.4049/jimmunol.180.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Tilburgs T, van der Mast BJ, Nagtzaam NM, Roelen DL, Scherjon SA, Claas FH. Expression of NK cell receptors on decidual T cells in human pregnancy. J Reprod Immunol. 2009;80:22–32. doi: 10.1016/j.jri.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Malmberg KJ, Levitsky V, Norell H, de Matos CT, Carlsten M, Schedvins K, et al. IFN-gamma protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J Clin Invest. 2002;110:1515–23. doi: 10.1172/JCI15564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheu BC, Chiou SH, Lin HH, Chow SN, Huang SC, Ho HN, et al. Up-regulation of inhibitory natural killer receptors CD94/NKG2A with suppressed intracellular perforin expression of tumor-infiltrating CD8+ T lymphocytes in human cervical carcinoma. Cancer Res. 2005;65:2921–9. doi: 10.1158/0008-5472.CAN-04-2108. [DOI] [PubMed] [Google Scholar]
- 9.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 10.Leffers N, Gooden MJ, De Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–59. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]