Abstract
Th2-type inflammation has been proposed to facilitate tumor growth. In De Monte et al. (J Exp Med 208:469-478, 2011) we identify in pancreatic cancer a complex cytokine/chemokine cross-talk within the tumor microenvironment mediating Th2 immune-deviation and show that the ratio of Th2/Th1 tumor infiltrating lymphocytes is an independent predictive marker of patients survival.
Keywords: cancer associated fibroblasts, dendritic cells, pancreatic cancer, Th2-type inflammation, thymic stromal lyphopoietin
Pancreatic cancer is a very aggressive disease with prominent fibrosis and dismal prognosis.1 We previously reported2 in patients undergoing surgical resection for pancreatic cancer the presence in the blood of tumor-antigen specific CD4+ Th2 cells that correlated at the tumor site with a predominant GATA-3+ (Th2) over T-bet+ (Th1) immune infiltrate. The same patients showed conserved anti-viral Th1 immunity2 and thus we hypothesized that local rather than systemic immunomodulatory factor(s) might have determined the anti-tumor specific Th2 immune-deviation.
Th2-type inflammation has been proposed to facilitate tumor growth.3 In De Monte et al.4 we addressed whether Th2 cells present at the tumor site had any role in disease progression and the mechanism responsible for Th2 immune-deviation. We analyzed tumor samples from 69 patients and identified the ratio of GATA-3+/T-bet+ tumor infiltrating lymphoid cells as an independent predictive marker of patients survival. Indeed, when grouped according to the ratio patients with a value inferior to the median survived significantly longer. To address the mechanism we performed in vitro and ex vivo analyses using surgical samples from primary tumors, tumor and stromal cells isolated by laser capture microdissection and in vitro established tumor and cancer associated fibroblast (CAF) cell lines.
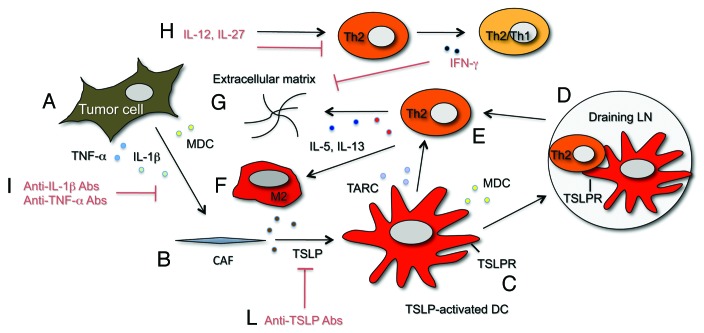
A model of the cross-talk within the tumor microenvironment mediating Th2 immune-deviation in pancreatic cancer, is depicted in Figure 1. We reasoned that the thymic stromal lymphopoietin (TSLP), an IL-7-like cytokine known to drive Th2 cell differentiation through dendritic cells (DCs) conditioning,5 could be implicated in our system. Indeed, we found that tumor samples express TSLP and that CAFs but not tumor cells are responsible for its expression. We then showed that TSLP secretion by CAFs is driven by activation with TNF-α (produced by tumor cells) and IL-1β (produced by tumor and stromal cells)(Fig. 1A and 1B). We further demonstrated in vitro that the supernatant of pro-inflammatory cytokine-treated CAFs induces activation and maturation of myeloid DCs, which are endowed with TSLP-dependent Th2 polarizing capability. The presence in vivo of TSLP-conditioned DCs (CD11c+TSLPR+) was confirmed in tumor tissues (Fig. 1C) and in draining (Fig. 1D) but not in non-draining lymph nodes (LNs). We hypothesized that DCs after uptake of released tumor antigens and conditioning by CAFs-derived TSLP migrate to the draining LNs where they activate tumor-antigen specific CD4+ T cells toward a Th2 phenotype (Fig. 1D). Th2 cells would then home to the tumor under the influence of Th2 chemoattractants (Fig. 1E). Indeed, we found in vitro that DCs conditioned by the CAFs supernatant release the Th2 attractant chemokines thymus and activation-regulated chemokine (TARC/CCL17) and macrophage derived chemokine (MDC/CCL22) and confirmed their expression in vivo where TARC/CCL17 was expressed by cells present in the stroma and MDC/CCL22 by tumor and stromal cells. At the tumor site Th2 cells release IL-5 and IL-13, cytokines known to contribute to fibrosis by promoting collagen synthesis6 (Fig. 1G), and possibly induce differentiation of alternatively activated macrophages (M2), whose presence in pancreatic cancer stroma has been detected (CD68+CD163+ cells, data not shown)(Fig. 1F).
Figure 1.
Schematic representation of cytokine/chemokine and cell networks involved in and proposed therapeutic interventions to interfere with Th2 immune-deviation in pancreatic cancer. Tumor cells under the influence of yet unknown stimuli release pro-inflammatory cytokines (TNFα and IL-1β)(A) that induce TSLP release by CAFs (B). Resident DCs are activated/matured by TSLP, express the TSLPR (C) and migrate to draining LNs where they prime Th2 cells (D). Th2 cells are then recruited at the tumor site (E) by Th2 attracting chemokines (TARC and MDC) released by TSLP-activated DCs and tumor cells. Th2 cells release Th2 cytokines (IL-5 and IL-13) that further foster fibrosis by increasing extracellular matrix deposition (G) and possibly influence the development of M2 macrophages (F). In red are indicated potential therapeutic interventions to counteract at different levels the cytokine/chemokine network driving Th2 type inflammation. Anti-IL-1β and TNF-α Abs may be used to interfere with CAFs activation (I), anti-TSLP Abs to interfere with DCs conditioning (L). IL-27 and IL-12 were shown7 to inhibit Th2 cytokines and induce IFNγ production by Th2 cells (Th2/Th1 cells) (H). IFN-γ directly suppress collagen synthesis by fibroblasts6 (G). Cytokines/chemokines color code: IL-1β (light green), TNFα (light blue), IL-5 (dark blue), IL-13 (red), TSLP (brown), TARC (light violet), MDC (yellow).
This model of cross-talk within the pancreatic cancer microenvironment favoring Th2-type inflammation supports the design of innovative therapeutic strategies by delivering of cytokines reported to modulate the phenotype of Th2 cells7 and by targeting mediators involved in their differentiation. We previously reported7 that in vitro combined treatment of tumor-antigen specific CD4+ Th2 cells from pancreatic cancer patients with IL-12 and IL-27 strongly induce IFNγ while inhibiting IL-5 and IL-13 secretion. These data suggest that Th2 cells are functionally plastic and support the development of IL-12 and IL-27 delivery systems based on loco-regional administration or targeted therapies with antibodies or molecules directed to the tumor stroma for manipulation of the pattern of cytokines secreted by Th2 cells (Fig. 1H). Based on our ex vivo data4 on tumor samples, manipulation of the balance of Th2/Th1 cytokines produced by CD4+ T cells present in the tumor microenvironment should favorably impact on pancreatic cancer patients overall survival and might directly hamper fibrosis due to the opposing effect of IFNγ and IL-5/IL-13 on extracellular matrix deposition.6 Other approaches aimed at interfering with the mechanism implicated in Th2 immune-deviation should target tumor-derived pro-inflammatory cytokines responsible for CAFs activation and TSLP. To this aim clinical grade anti-TNFα and anti-IL-1β antibodies (Fig. 1I) are already available in the clinic3 and anti-TSLP antibodies (Fig. 1L) are ready to be tested8 and should be available for clinical applications in the near future. As a role for TSLP in driving Th2-type inflammation and tumor progression has been also demonstrated in breast cancer9,10 and likely will be in other tumors, the use of anti-TSLP antibodies as anti-tumor agent is of particular interest.
Clinical outcome in pancreatic cancer even in patients with resectable tumor is still poor.1 In De Monte et al.4 we have identified a complex negative cross-talk among tumor cells, CAFs, DCs and Th2 cells. We propose that based on these findings conventional chemotherapies as well as experimental immunotherapeutics combined with the approaches detailed above (Fig. 1H, 1I and 1J) should be implemented in the treatment of pancreatic cancer patients possibly in a neo-adjuvant setting. Manipulation of the balance of Th2 and Th1 cytokines in the tumor microenvironment should redirect the immune system toward efficacious Th1-type inflammation while reducing fibrosis and therefore positively impact on patients survival.
Glossary
Abbreviations:
- CAFs
cancer associated fibroblasts
- DCs
dendritic cells
- LNs
lymph nodes
- MDC/CCL22
Macrophage derived chemokine
- TARC/CCL17
Thymus and activation-regulated chemokine
- TSLP
thymic stromal lymphopoietin
- TSLPR
TSLP receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/17939
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Tassi E, Gavazzi F, Albarello L, Senyukov V, Longhi R, Dellabona P, et al. Carcinoembryonic antigen-specific but not antiviral CD4+ T cell immunity is impaired in pancreatic carcinoma patients. J Immunol. 2008;181:6595–603. doi: 10.4049/jimmunol.181.9.6595. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–78. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 6.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tassi E, Braga M, Longhi R, Gavazzi F, Parmiani G, Di Carlo V, et al. Non-redundant role for IL-12 and IL-27 in modulating Th2 polarization of carcinoembryonic antigen specific CD4 T cells from pancreatic cancer patients. PLoS ONE. 2009;4:e7234. doi: 10.1371/journal.pone.0007234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards MJ. Therapy directed against thymic stromal lymphopoietin. Drug News Perspect. 2008;21:312–6. doi: 10.1358/dnp.2008.21.6.1246830. [DOI] [PubMed] [Google Scholar]
- 9.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208:479–90. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol. 2011;186:5656–62. doi: 10.4049/jimmunol.1100463. [DOI] [PMC free article] [PubMed] [Google Scholar]