Abstract
In our recent publication (Zheng et al., PLoS ONE) we described the identification of annexin A2 as a new pancreatic cancer associated tumor antigen. Its involvement in pancreatic cancer progression and metastases supports its role as an antigenic target for the development of both therapeutic antibody and T cell immunotherapy.
Keywords: annexin A2, pancreatic cancer, therapeutic target, tumor-associated antigen, vaccine
Identification of Annexin A2 (ANXA2) as a Pancreatic Cancer Antigen
Immune-based therapy has become a new modality of treatment for pancreatic ductal adenocarcinoma (PDA), the most common type of pancreatic cancer, and is being tested in clinical trials.1 The rationale for an immune-based approach to the treatment of cancer rests on the supposition that immune effector mechanisms can be induced and directed toward antigens preferentially expressed or overexpressed by tumor cells. The antigens previously targeted by PDA-specific immune based approaches include carcinoembyonic antigen, mutated KRAS, mucin-1 and gastrin. These antigens were identified over 10 y ago by expression analysis and have been shown to be weak immunogens.2 These findings are stressing the importance of selecting antigenic determinants based on their immunogenicity, rather than solely selecting them a priori based upon their overexpression in tumors.
Mesothelin, was the first PDA antigen to be defined using a functional approach. This approach combined the results of a SAGE database that identified genes that were overexpressed in PDA relative to normal tissue, with a biologically relevant and non-biased screening assay that allowed immunized T cells to prioritize the best antigens for further testing.3 This approach identified mesothelin as one of the targets recognized by immunized lymphocytes from patients receiving an allogeneic GM-CSF secreting tumor vaccine (GVAX) who demonstrated improved disease-free survival (DFS). Mesothelin was subsequently validated to be a target of the immune response and the induction of T cell responses were shown to be associated with prolonged DFS and overall survival (OS) in two prospective clinical trials.4,5
The above functional genomic approach utilized immunized lymphocytes to screen for T cell recognized antigens. Although this approach identified a candidate new antigen that has so far shown to be a target of the antitumor immune response, this approach is tedious and requires significant quantities of patient lymphocytes. In addition, it requires HLA reagents specific for each individual patient which is not always available. A number of studies have demonstrated the feasibility of utilizing sera to identify proteins expressed in cancers. We therefore utilized serum from patients who demonstrated both evidence of post-vaccination mesothelin-specific T cell responses and prolonged DSF in a phase 2 clinical study.5 Protein extracts from the pancreatic tumor cell lines used to create the pancreatic cancer GVAX were separated by 2-dimensional electrophoresis. Subsequently, immunoblot analysis was performed to compare antigen recognition by pre- and post-vaccination sera. Proteins recognized by post-vaccination sera relative to pre-vaccination sera were identified by mass spectrometry. ANXA2 was one of the proteins identified on the post-vaccination sera immunoblots.6 To further evaluate the prevalence of post vaccination humoral responses to ANXA2, purified recombinant ANXA2 was used to screen pre-vaccination and post-vaccination sera from 16 additional patients by both ELISA and protein gel blot. Vaccine-induced anti-ANXA2 antibodies, measured by an ELISA, were detected in 6 of 7 patients who demonstrated a DFS greater than 36 mo, and only in 1 of the other 9 patients who did not demonstrate long-term DFS.6 Thus, our data suggested that ANXA2 is an antigenic target of vaccine induced humoral responses in pancreatic cancer patients.
Evidence of Both Autoantibody and T Cell Responses to ANXA2
We found that some of our pancreatic cancer patients had anti-ANXA2 antibodies at their baseline prior to the vaccination.6 The prevalence of anti-ANXA2 auto-antibodies in human PDA patients remains to be investigated. However, accumulated evidence revealed auto-antibodies against ANXA2 in patients with diseases other than cancer. Anti-ANXA2 antibodies were first reported in patients with lupus and rheumatoid arthritis. Further investigation thus found anti-ANXA2 antibodies were significantly more prevalent in patients with antiphospholipid syndrome than healthy individuals, patients with non-autoimmune thrombosis, or patients with lupus without thrombosis.7
ANXA2 specific antibodies have also been detected at baseline in cancer patients. Sera from 60% of patients with lung adenocarcinoma and 33% of patients with squamous cell lung carcinoma, but none of the noncancer controls, exhibited IgG-based reactivity against proteins identified as glycosylated annexins A1 and/or A2. IL-6 levels were significantly higher in sera of antibody-positive lung cancer patients compared with antibody-negative patients and controls.8 Nonetheless, the prognostic value of the presence of anti-ANXA2 antibodies for lung cancers remains to be explored.
To validate that ANXA2 is also an antigenic target of T cell immunity, we are in the process of identifying epitopes within the ANXA2 protein against which T cells were induced in patients who received the pancreatic cancer GVAX. Indeed, there has been evidence of natural T cell response to ANXA2. A T cell epitope of ANXA2 was isolated during a search for tumor-specific MHC-class-II-restricted antigens in melanoma patients. The immunogenicity of endogenous peptides that had been eluted from HLA-DR molecules on the human melanoma cell line FM3 contained a 16-mer representing ANXA2 positions 208–223.9 This epitope bound well to isolated DRB1*0401 molecules. Artificial antigen presenting cells pulsed with this ANXA2 peptide were able to stimulate HLA-DR-matched normal donors T cells, which in turn can respond to naïve autologous melanoma cells specifically.9 Additional studies are warranted to determine the extent to which ANXA2 serves as a tumor rejection target of T cells.
ANXA2 is Functionally Important for PDA Development and Metastases
ANXA2 is reported to be overexpressed in a variety of cancers including PDA, lung cancer, colon cancer, gastric cancer, hepatocellular carcinoma, and malignant glioma, when compared with normal tissues.10 Overexpression of ANXA2 has already been demonstrated to be a poor prognostic factor for some of these cancers. Through immunohistochemistry analysis, we and others showed that normal pancreatic epithelial ductal cells display weak cytoplasmic and luminal staining, and that cell-surface localized ANXA2 increases with progression from pancreatic intraepithelial neoplasia (PanINs) to invasive PDA. We showed that 39 (75%) of 52 fresh pancreatic tumor tissue samples have increased cell surface expression of ANXA2 and that essentially all the metastases strongly expressed ANXA2 on the cell surface.6
More recently, ANXA2 was shown to translocate from the cytosol to the cell surface in a tyrosine 23 (Tyr23) phosphorylation-dependent manner (Fig. 1). The translocation is critical for PDA cell invasion and TGFβ-Rho mediated epithelial to mesenchymal transition (EMT) in PDA.6 Knock-down of ANXA2 by siRNA, blockade by anti-ANXA2 antibodies, or by post-treatment sera from patients who demonstrated prolonged survival, suppresses PDA invasion in an in vitro invasion assay. Using mouse PDA models, we showed that shRNA knock-down of ANXA2, a mutation at Tyr23, or anti-ANXA2 antibodies, inhibit PDA metastases and prolong mouse survival,6 supporting ANXA2 as a target for PDA therapeutic development.
Figure 1.
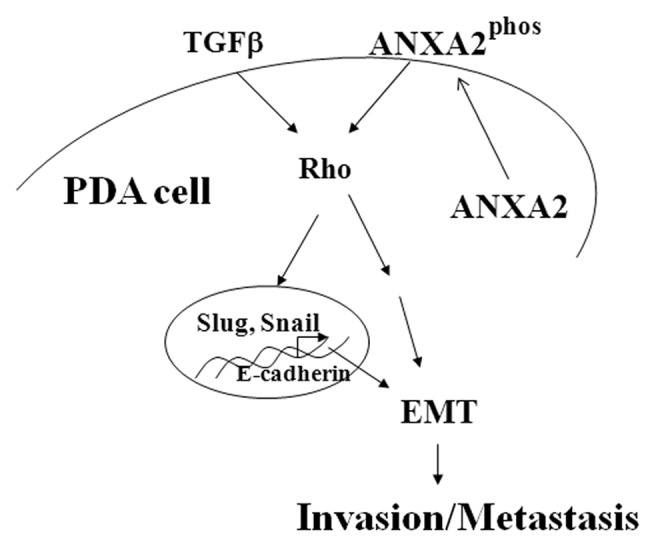
Annexin A2 pathway in pancreatic cancer invasion and metastasis.
Prospective on the Development of ANXA2-Targeting Immunotherapy
Since the ANXA2-specific antibodies detected in patients’ sera by ELISA are of the IgG isotype whose production requires T cell help, it can be assumed that ANXA2–specific T cells were also induced in these patients, making ANXA2 an attractive candidate antigen for both antibody and T cell immunotherapy. Overexpression and cell surface translocation of ANXA2 during PDA pathogenesis suggests that ANXA2 is a pancreatic cancer specific target, and this specificity is desirable to avoid autoimmunity. The involvement of ANXA2 in PDA tumor progression and metastasis are welcomed features during immunotherapy, as it reduces the chances for ANXA2 to be lost during cancer progression. Importantly, cancer patients typically die of metastases, further providing support for targeting ANXA2, a protein that promotes metastases. Therefore, we are currently developing both anti-ANXA2 therapeutic antibodies and vaccines for future testing in patients with pancreatic cancer.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18017
References
- 1.Zheng L, Jaffee EM. Vaccine Therapy and Immunotherapy for Pancreatic Cancer. In: Neoptolemos JP, Urrutia R, Abbruzzese JL, Buchler MW, ed. Handbook of Pancreatic Cancer. New York, NY: Springer, 2009:1269-318. [Google Scholar]
- 2.Dodson LF, Hawkins WG, Goedegebuure P. Potential targets for pancreatic cancer immunotherapeutics. Immunotherapy. 2011;3:517–37. doi: 10.2217/imt.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng L, Foley K, Huang L, Leubner A, Mo G, Olino K, et al. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS ONE. 2011;6:e19390. doi: 10.1371/journal.pone.0019390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesarman-Maus G, Rios-Luna NP, Deora AB, Huang B, Villa R, Cravioto Mdel C, et al. Autoantibodies against the fibrinolytic receptor, annexin 2, in antiphospholipid syndrome. Blood. 2006;107:4375–82. doi: 10.1182/blood-2005-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brichory FM, Misek DE, Yim AM, Krause MC, Giordano TJ, Beer DG, et al. An immune response manifested by the common occurrence of annexins I and II autoantibodies and high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci USA. 2001;98:9824–9. doi: 10.1073/pnas.171320598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halder T, Pawelec G, Kirkin AF, Zeuthen J, Meyer HE, Kun L, et al. Isolation of novel HLA-DR restricted potential tumor-associated antigens from the melanoma cell line FM3. Cancer Res. 1997;57:3238–44. [PubMed] [Google Scholar]
- 10.Sharma MC, Sharma M. The role of annexin II in angiogenesis and tumor progression: a potential therapeutic target. Curr Pharm Des. 2007;13:3568–75. doi: 10.2174/138161207782794167. [DOI] [PubMed] [Google Scholar]


