Abstract
Tumor-induced myeloid-derived suppressor cells (MDSCs) are a critical barrier to effective immunotherapy of cancer. We identified that Docetaxel and a natural compound, Icariin, can target MDSCs with preferential apoptosis of M2 cells and polarization of the surviving cells towards M1 cells. Such strategic targeting of MDSCs restored T cell function accompanied by tumor retardation in vivo.
Keywords: Apoptosis, chemoimmunotherapy, Docetaxel, Icariin, myeloid-derived suppressor Cells, tumor microenvironment, tumor-associated macrophages
Adaptive T cell immunity is crucial for cancer immunity, not only in direct elimination of the growing tumor cells but also in generating memory responses to prevent tumor recurrence. The drawback of immunotherapy and vaccine therapy of cancer has been the realization that an active immunosuppressive environment is induced by the growing tumor and that it can neutralize the effectiveness of the immune-enhancing regimen. In order to develop strategies to overcome such blockade, it is necessary to identify the mechanisms associated with it. Myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages TAMs) are implicated in this process. Growing evidence points to an accumulation of granulocytic (G-MDSCs) and monocytic (M-MDSCs) subsets accompanied by a dynamic change in macrophages that enter the tumor site toward a predominant IL10-producing M2 phenotype, all of which have the potency to induce T regulatory cells and/or directly suppress T cell function against the tumor. Additionally, M-MDSCs are apparently a source of the M2 cells and factors produced by tumor cells may guide this polarization. We thus set out to identify means to target these immunosuppressive phenotypes and to generate IL12-producing M1 cells that can restore T cell function.
Traditional chemotherapeutic agents offer an attractive solution if they might be found to target MDSCs because of the possibility of rapid translation into clinic practice. Using the 4T1 breast cancer model, we demonstrated that Docetaxel can serve such a purpose.5 Docetaxel treatment of mice bearing 8 mm2 tumors effectively blocked tumor progression which corresponded to a dramatic reduction of CD11b+Gr1+ MDSCs in the spleen. Of these MDSCs, careful analysis indicated that Ly6G+ G-MDSCs were preferentially lost with little effect on Ly6C+ M-MDSCs. More importantly, splenic CD4+ and CD8+ T cells isolated directly from Docetaxel-treated mice spontaneously displayed more IFNγ and tumor-specific cytoxicity respectively, compared with the same T cells from untreated tumor-bearing mice. This suggested that Docetaxel may have modified MDSCs toward M1 macrophages that can present antigen to T cells in vivo. Indeed, MDSCs isolated from Docetaxel-treated mice could effectively activate OVA323–329 peptide-specific CD4+ T cells from OTII transgenic mice. To directly analyze the effect of Docetaxel on macrophage polarization, we chose to detect M1 and M2 cells via CCR7 and MR (CD206) surface expression respectively. In vivo, tumor bearers treated with Docetaxel had a marked increase in CCR7+ M1 cells but a significant loss in MR+ M2 cells within the splenic MDSCs. Other markers of M1 differentiation were also noted to be upregulated in MDSCs of drug-treated mice, including CD11c, MHC-Class II, iNOS. In vitro, culture of MDSCs isolated from tumor bearers with Docetaxel for 24 hours caused a high level of apoptosis which occurred primarily in the MR+ cells, leading to survival and preferential increase in CCR7+ cells with heightened IL12 production and correspondingly less IL10. In probing what mechanism might be at play for Docetaxel to polarize MDSCs toward CCR7+ M1 cells, we uncovered that STAT3, known to be critical for function and maintenance of the immunosuppressive MDSCs, was targeted by Docetaxel. In comparison, STAT1 function was untouched by Docetaxel treatment. Thus, we elucidated a novel process that can be attributed to Docetaxel in selective apoptotic induction in G-MDSCs and MR+ M2 cells, sparing M-MDSCs with the accumulation of CCR7+ M1 cells.
Reduction of MDSCs or their function has also been recently reported with use of sunitinib, sildenafil or gemcitabine, resulting in improved immunotherapy of cancer6-8. Another strategy to target MDSCs might be through natural compounds which are gaining renewed interest as alternative less toxic agents that could be used to prevent or treat cancer. We have identified that Icariin found in the family of Herba Epimedii plants can mimick Docetaxel in its potency to block MDSC function in vivo and delay tumor progression.9 It and its derivative, 3, 5, 7-Trihydroxy-4'-methoxy-8-(3-hydroxy-3- methylbutyl)–flavone (ICT), can reduce splenic MDSCs in 4T1 tumor bearing mice accompanied by a restoration of IFNγ production in the CD8+T cells. In vitro treatment of splenic MDSCs from tumor bearers with these compounds also reduced the percentage of MDSCs with concomitant differentiation toward M1 cells. More impressively, these compounds specifically repressed S100A8/9 expression which is linked to the immunosuppressive state of MDSCs.10
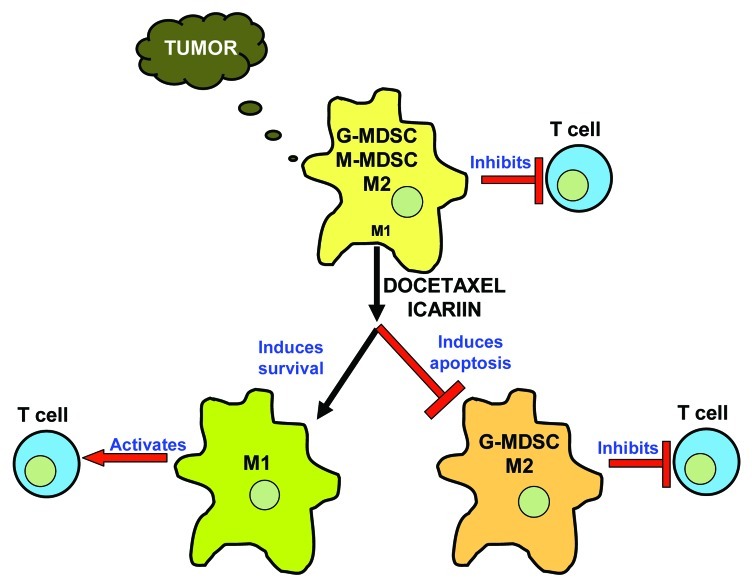
Based on our accumulative data and those reported in the literature, we propose the following pathway using Docetaxel or Icariin (Fig. 1). A growing tumor attracts both G-MDSCs and M-MDSCs together with a predominance of M2 type macrophages, all produced by factors in the tumor microenvironment and possessing highly immunosuppressive function that inhibits T cells. Upon Docetaxel or Icariin treatment, the G-MDSCs and M2 cells are eliminated via induction of apoptosis, while CCR7+ M1 cells are spared. It is also possible that M-MDSCs survive and differentiate toward CCR7+ M1 cells thus increasing their pool. An intriguing observation is the number of cytotoxic agents besides Docetaxel and Icariin that can preferentially target MDSCs,. It is unclear whether there are intrinsic differences between the MR+ and CCR7+ MDSCs that affect their response to therapeutic agents. A higher complexicity may also be encountered in vivo where the stroma and tumor cells can be modulated by chemotherapy to have a secondary effect on MDSCs. This area needs further exploration and a thorough understanding could refine therapeutic targeting to the tumor microenvironment as an adjuvant to current immunotherapeutic strategies.
Figure 1.
Docetaxel or Icariin targets Myeloid-Derived Suppressor Cells (MDSCs) and restores T cell function against cancer. Progressing tumors release factors that attract and maintain immunosuppressive MDSCs that contain two subsets, granulocytic G-MDSCs and monocytic M-MDSCs. Moreover, macrophages with the inhibitory M2 phenotype dominate over M1 in the tumor microenvironment. Docetaxel or Icariin selectively induces apoptosis in G-MDSCs and M2 cells, while sparing M1 cells and M-MDSCs, which may be further polarized toward M1 cells to activate T cells.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18074
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 4.Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Hegmans JP, et al. Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma. Br J Cancer. 2010;103:629–41. doi: 10.1038/sj.bjc.6605814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–94. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–22. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Wu J, Chen X, Fortenbery N, Eksioglu E, Kodumudi KN, et al. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol. 2011;11:890–8. doi: 10.1016/j.intimp.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]