Abstract
The matrix metalloproteinase-2 (MMP-2), overexpressed in most cancers, induces TH2 polarization by conditioning dendritic cells to over-express OX40L and downregulate IL-12p70 through the degradation of the type-I IFN receptor IFNAR1. Elucidating mechanisms underlying detrimental tumor-associated type-2 responses represent a crucial step in designing effective immune therapies to treat cancer patients.
Keywords: CD4+ T cell differentiation, CD4+ T cells, dendritic cells, IFNARI, IL-12, matrix metalloproteinase-2, melanoma, OX40L, STAT1
As CD4 help appears crucial for generating effective anti-tumor immunity, we investigated the existence of MMP-2-specific CD4+ T cells. We detected such cells in healthy donors and more importantly at a much higher frequency in over 40% of melanoma patients tested. We cloned CD4+ T cells specific for 11 novel MMP-2-derived epitopes. Strikingly, these cells displayed an inflammatory TH2 phenotype, i.e., mainly secreting TNFα, IL-4 and IL-13 and expressing GATA3. Whereas IFNγ-secreting TH1 cells appear to exhibit effective anti-tumor properties, TH2 cells are often described as detrimental in this function.4-6 Despite a lack of statistical significance, we also observed a trend toward a poorer clinical outcome/survival for patients with detectable MMP-2-specific TH2 cells, suggesting the significance of these cells and/or MMP-2 itself in melanoma progression.
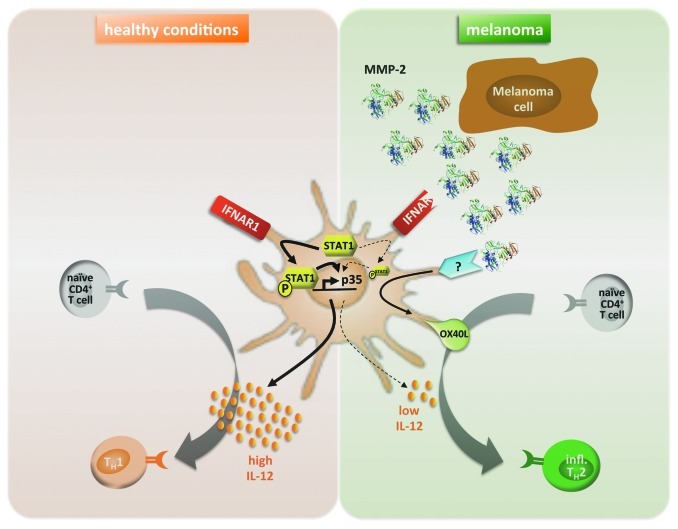
We investigated the mechanism underlying the observed TH2 skewing and found that MMP-2-conditioned dendritic cells (DCs) preferentially prime inflammatory TH2 cells. Of note, MMP-2 enzyme in its active conformation was the strongest inducer of TH2 responses. While, according to the literature, TH1 differentiation reliably depends on IL-12, the generation of TH2 cells is not fully understood and/or may differ from one model to another. IL-4, through GATA3 induction, is described as critical for TH2 differentiation in most studies. However, IL-4 did not seem to be involved in our system. A default mechanism could also explain TH2 differentiation, where the lack of TH1-polarizing signal, namely IL-12, would be sufficient and/or necessary.7 Strikingly, we found that DCs exposed to active MMP-2 lost their ability to produce IL-12p70. Moreover, we demonstrated that this lack of IL-12p70 actually played a major role in the MMP-2-dependent TH2 polarization. Indeed, DCs exposed to active MMP-2 lost their ability to prime TH2 cells when rhIL-12p70 was supplemented in the culture. Rather, CD4+ T cells differentiated into TH1-like cells. We characterized the mechanism behind MMP-2-dependent inhibition of IL-12p70: by degrading the type I IFN receptor (IFNAR1) on DCs, and subsequently preventing STAT1 phosphorylation, MMP-2 inhibits IL-12p35 subunit transcription (Fig. 1).
Figure 1.
MMP-2-dependent mechanism licensing DCs to prime inflammatory TH2 cells. The left panel (orange) represents « healthy conditions »: activated DCs produce IL-12p35 through IFNAR1 and STAT1 signaling. DCs secrete high levels of IL-12p70 necessary for priming naïve CD4+ T cells into TH1 cells. In the context of melanoma (right panel, green), tumor cells produce and activate MMP-2. Active MMP-2 degrades IFNAR1 on DCs, leading to low levels of STAT1 phosphorylation preventing transcription of IL-12p35. Furthermore, MMP-2 in both active and inactive conformations induces OX40L overexpression on the surface of DCs through triggering of a receptor yet to be determined. Both lack of IL-12p70 and OX40L overexpression by MMP-2-exposed DCs are responsible for priming naïve CD4+ T cells into inflammatory TH2 cells.
Furthermore, we established that MMP-2 induced DCs to overexpress OX40L, which was also involved in the observed TH2 skewing, as shown by performing the same priming experiments in the presence of a blocking antibody for OX40L: naïve CD4+ T cells primed against MMP-2 when OX40L is being blocked, differentiated into IFNγ-secreting TH1-like cells (Fig. 1). Despite evidence suggesting that OX40 signaling in CD4+ T cells can directly induce type-2 lineage commitment by inducing NFATc1, which triggers IL-4 production and subsequent GATA3 expression,8 the role of OX40L in TH2 differentiation is not yet clearly understood. MMP-2-induced type-2 differentiation likely works differently as we showed that IL-4 was not a major determinant in this model. Thymic stromal lymphopoietin (TSLP) can also promote TH2 differentiation through OX40L-expressing DCs. Interestingly, MMP-2 did not induce DCs to produce TSLP, implying that MMP-2-induced expression of OX40L does not depend on TSLP. TSLP-dependent TH2 differentiation is believed to involve basophils in several models,9,10 but we could not detect any basophil activation by MMP-2. We are now exploring how MMP-2 induces OX40L expression and are trying to identify the responsible receptor(s). Surface molecules such as αvβ3, CD91 or MT1-MMP are known to bind MMP-2. However, we excluded any role for these receptors in OX40L induction.
Together, our priming results suggest that both the lack of IL-12 and OX40L overexpression play distinct and additive roles in our model. Moreover, OX40L blockade had an effect even in the presence of IL-12p70, indicating that the absence of IL-12 is not strictly required for TH2 polarization in this model.
While OX40L expression is induced after exposure to MMP-2 in both active and inactive conformations, IL-12p70 blockade only occurs in the presence of active MMP-2. Aggressive tumors display high levels of activated MMP-2 and therefore should license in situ DCs to express OX40L in the absence of IL-12, creating, an ideal environment to generate TH2 cells, according to our model. Whether TH2 polarization in tumors is only a consequence of the environment or actually plays a part in tumor aggressiveness by restricting immune control still needs to be determined.
An additional major finding of our study is that MMP-2 acts as a type-2 conditioner for CD4+ T cells specific for other tumor antigens, as we showed for Melan-A/MART-1 and NY-ESO-1. One could imagine that MMP-2-exposed DCs would turn naïve CD4+ T cells of any specificity into type-2 cells. This would imply the generation/accumulation of a local type-2 tumor micro-environment, potentially reinforcing itself through second-hand IL-4 secreted by resident T cells.
Unraveling the mechanisms underlying tumor-associated TH2 polarization, including the one we characterized in this study, opens the way to developing new therapeutic strategies for cancer patients able to induce effective type-1 responses and limit tumor cells escaping the immune system.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/17994
References
- 1.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. J Invest Dermatol. 2000;115:337–44. doi: 10.1046/j.1523-1747.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 3.Godefroy E, Manches O, Dréno B, Hochman T, Rolnitzky L, Labarrière N, et al. Matrix metalloproteinase-2 conditions human dendritic cells to prime inflammatory T(H)2 cells via an IL-12- and OX40L-dependent pathway. Cancer Cell. 2011;19:333–46. doi: 10.1016/j.ccr.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauerova L, Dusek L, Simickova M, Kocák I, Vagundová M, Zaloudík J, et al. Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma. 2002;49:159–66. [PubMed] [Google Scholar]
- 5.Botella-Estrada R, Escudero M, O’Connor JE, Nagore E, Fenollosa B, Sanmartín O, et al. Cytokine production by peripheral lymphocytes in melanoma. Eur Cytokine Netw. 2005;16:47–55. [PubMed] [Google Scholar]
- 6.Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–28. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 8.So T, Song J, Sugie K, Altman A, Croft M. Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc Natl Acad Sci U S A. 2006;103:3740–5. doi: 10.1073/pnas.0600205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]