Abstract
Our recent data and that of others demonstrate that both tumor and host CD73-generated adenosine promote tumor growth and metastasis in a multifactorial manner. Results with small molecule inhibitors or monoclonal antibodies against CD73 in multiple tumor models suggest that CD73 is a previously unappreciated important target for effective cancer therapy.
Keywords: Adenosine, Adenosine receptors, CD73, metastasis, tumor immunotherapy
Evading immune destruction has recently emerged as one of the “hallmarks of cancer.”1 The identification of multiple mechanisms of tumor-induced immune evasion provides an array of novel targets for new cancer therapies.2 Given the well-established immune effects of CD39/CD73 and the A2A adenosine receptor (A2AR) on cancer growth and metastasis, the phosphohydrolysis of extracellular ATP to adenosine can now be viewed as one of the most important immunosuppressive regulatory pathways in the tumor microenvironment. The focus of this review is to specifically discuss the newly available experimental evidence demonstrating a pivotal role of tumor and host CD73-mediated adenosinergic effects on tumor growth and metastasis.
Tumor CD73 Promotes Tumor Growth and Metastasis
CD73, or ecto-5′-nucleotidase (ecto-5′-NT, EC 3.1.3.5), is a cell surface enzyme that catalyzes the dephosphorylation of extracellular AMP to adenosine. It is broadly expressed in many types of cancer3-6 and has been associated with a pro-metastatic phenotype in melanoma and breast cancer, has prognostic value for colon cancer patients, and has been suggested as a diagnostic aid in papillary thyroid carcinomas. The biological function of CD73 is primarily a consequence of the regulated enzymatic phosphohydrolytic activity on extracellular nucleotides. This ecto-enzymatic cascade in tandem with CD39 (ecto-ATPase) generates adenosine from ATP that in turn signals through adenosine receptors. Previous studies reported that CD73 participates in cell–cell and cell–matrix interactions7,8 and implicated CD73 in drug resistance9 and tumor-promotion.10,11 We12 and another group13 have recently shown that CD73 expressed by tumor cells significantly impairs adaptive antitumor immune responses through its ecto-enzymatic activity, suggesting that tumor CD73-generated adenosine blocks T-cell immunosurveillance of murine tumors. These results strongly support and extend the concept proposed that extracellular adenosine and the A2AR comprise a pivotal axis in tumor immune escape.14
Using an immune-competent breast cancer model, Stagg and his colleagues have identified tumor-derived CD73 as contributor to tumor-induced immune suppression.13 Notably, the therapeutic efficacy observed with an anti-CD73 monoclonal antibody (mAb) as a single agent is largely dependent on the induction of adaptive anti-tumor immune responses, and is comparable with the efficacy of other means of immune-stimulating antibodies such as anti-CD40 mAb or anti-41BB mAb against breast tumors. We tested the functional relevance of tumor CD73 in an immune-competent ovarian cancer model.12 In a series of in vitro and in vivo experiments, we found that knockdown of CD73 on ovarian cancer cells increased tumor-specific T cell activation and expansion, and decreased T cell apoptosis. Strikingly, adoptive transfer of antigen specific T cells cured all mice bearing CD73-knockdown ovarian tumors, while CD73 competent tumors could not be cleared. Therefore, inhibition of CD73 could be a therapeutic adjuvant to improve cancer immunotherapy.
The clinical importance of CD73 in ovarian cancer is further confirmed by a recent study15 revealing the expression and putative immunosuppressive function of CD39 and CD73 in human ovarian cancer specimens ex vivo and cell lines in vitro. Interestingly, a reporter gene assay employing A2AR-transfected HEK-293 ‘sensor’ cells was developed and indicated that ovarian cancer cells produced far greater amounts of adenosine than resting or activated Tregs.16 Although sample numbers were too low to reliably assess correlations between CD73/CD39 expression and immune cell infiltration, Hausler and his colleagues,15 on a functional level, demonstrated that siRNA against, or small molecule inhibitors of, CD39 and CD73 promoted allogeneic human CD4+ T cell proliferation, NK cell-mediated lysis and cytotoxic T cell priming against recall antigens in co-culture with primary ovarian cancer cells and cell lines. As expected, similar affects were observed by the blockade of the A2AR.15 We also validated expression and enzymatic activity of CD73 in a small number of human ovarian cancer specimens. Importantly, blockade of CD73 using the selective inhibitor APCP (α,β-methylene adenosine 5′-diphosphate) augmented the in vivo antitumor effects of T cells and increased overall survival in a mouse xenograft model of human epithelial ovarian cancer (unpublished data). In addition, we found that CD73 was highly expressed in melanoma lesions compared with halo nevi (common benign skin lesions) (unpublished data). Therefore, these data establish a potential and feasible strategy of targeted CD73 therapy for cancer that can be readily translated into the clinical arena.
Apart from cancer cells themselves, it has been demonstrated that exosomes secreted by diverse cancer cells are capable of dephosphorylating exogenous ATP and AMP to generate adenosine17 due in part to expression of functional CD39 and CD73. Similar enzymatic activity was observed with exosomes from pleural effusions of mesothelioma patients. Furthermore, conversion of 5′-AMP to adenosine by exosome-derived CD73 inhibited T cell activation independently of T cell CD73 expression. These results suggest a previously unrecognized role of exosome-derived CD73 in tumor immune escape via rising adenosine levels within the microenvironment that mimics the paracrine secretion of adenosine described above for cancer cells themselves.
Host CD73 Promotes Tumor Growth and Metastasis
Although CD73 is expressed on many host cell types, including subsets of lymphocytes, endothelial cells (EC), and dendritic cells (DC), the role of host CD73 expression and activity in cancer progression has been less appreciated. Using the same CD73 deficient mice, three independent groups18-20 including ours simultaneously studied the contribution of host CD73 to tumor growth and metastasis in several tumor models including transplantable CD73-negative B16-F10 melanoma. The results obtained from these three independent groups are incredibly similar and complementary to one another and are summarized below.
First, all 3 groups demonstrated that CD73-deficient mice are resistant to primary growth and metastasis of B16-F10 melanoma. Stagg et al.19 found that CD73 ablation also significantly suppressed the growth of ovalbumin-expressing MC38 colon cancer, EG7 lymphoma, and AT-3 mammary tumors. We found a tumor-inhibiting advantage of host CD73 deficiency in mice bearing s.c. EL4 lymphoma, or peritoneal ID8 ovarian tumor.20 In addition, CD73 deficiency was more effective in inhibiting the growth of immunogenic EG7 or B16-SIY (B16 derivative expressing SIY antigen) cells than parental tumor EL4 or B16F10 cells, indicating that the efficacy of host CD73 deficiency in boosting antitumor immunity appears to depend in part on tumor immunogenicity. Both we20 and Stagg et al.19 showed that the protective effect of host CD73 deficiency on primary tumors was dependent on CD8+ T cells and associated with increased endogenous antitumor T cell immunity. In line with these results, Salmi’s group18 and ours20 found that loss of CD73 expression in mice resulted in increased infiltration of CD8+ T cells into tumor tissue.
Second, CD73 deficiency on both hematopoietic and nonhematopoietic cells was required to limit tumor growth effectively. We20 and Stagg et al.19 both showed that in the hematopoietic compartment, the pro-tumorigenic effect of CD4+ CD25+ regulatory T cells (Tregs) was in part dependent on their expression of CD73, which is consistent with previous studies.21 Beyond expectation, Stagg et al.19 found that the pro-metastatic effect of host-derived CD73 was dependent on CD73 expression on nonhematopoietic cells, most likely endothelial cells, in a manner independent from immunoregulatory effects. Further study is needed to clarify the mechanism by which nonhematopoietic CD73 promotes lung metastasis. This work will be facilitated by the availability of mice with tissue-specific deletion/overexpression of CD73 (e.g., in endothelial cells). Nevertheless, CD73 expression on nonhematopoietic cells may directly influence tumor-reactive CD8+ T cells at the tumor site. Indeed, we found that host CD73 deficiency or blockade increased tumor antigen–specific T cell homing to tumors, consistent with the fact that endothelial CD73 is known to inhibit T cell endothelial adhesion and migration.22 Because CD73 expression on hematopoietic cells, and not on nonhematopoietic cells, significantly influenced systemic antitumor CD8+ T cell immunity, we conclude that CD73 has distinct roles in hematopoietic and nonhematopoietic cells in limiting adaptive immune responses. Another unique mechanism has been proposed by Salmi’s group18 in which altered purinergic signaling in the absence of CD73 affects intratumoral infiltration of Tregs and intratumoral differentiation of type 1 macrophages (M1) into tumor promoting type 2 macrophages (M2), which are two key events in this tumor immune evasion process. This particular mechanism is likely important to our understanding of the diverse CD73-mediated adenosinergic effects in the tumor microenvironment, although the overall pathophysiological significance of the ratio of adenosine to ATP regulated by CD73 remains to be resolved. See Figure 1.
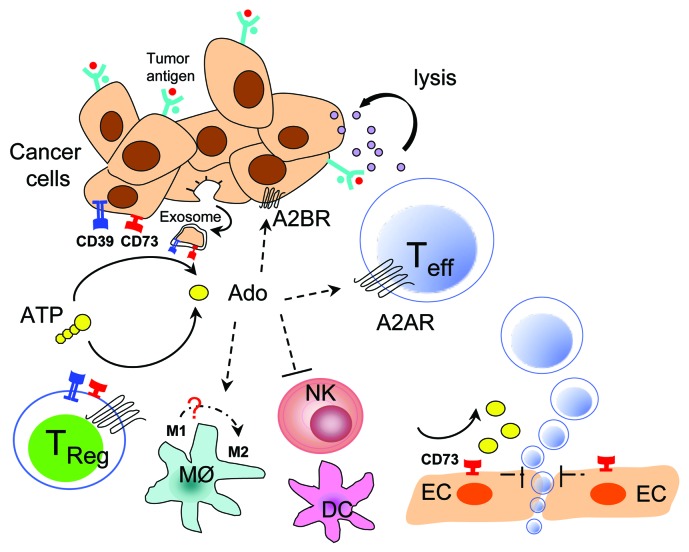
Figure 1.
Immune actions of CD73-generated adenosine in the tumor microenvironment. The sequential action of cellular ecto-ATPase (CD39) and/or ecto-5′-nucleotidase (CD73) represents a primary pathway of extracellular adenosine generation in the tumor microenvironment. Recent studies including our own work suggest that both tumor and host CD73 promote tumor growth and metastasis. CD73 has distinct roles in hematopoietic and nonhematopoietic cells in limiting antitumor T cell immunity through its etco-enzymatic activity. The mechanism by which nonhematopoietic CD73 promotes lung metastasis remains unclear. Meanwhile, the impact of CD73-generated adenosine on differentiation and function of dendritic cells (DCs) and macrophages (MØ), and natural killer (NK) cell proliferation and cytolytic function requires further investigation.
Last, in vivo pharmacological blockade of CD73 with a selective inhibitor APCP or anti-CD73 monoclonal antibody significantly reduced tumor growth and metastasis.19,20 Furthermore, we demonstrated that the optimal antitumor effect of CD73 blockade requires inhibiting both tumor and host CD73.20 Combining APCP or anti-CD73 monoclonal antibody treatment with adoptive T cell therapy resulted in tumor regression. Therefore, all three independent groups came to the same conclusion that lack of CD73 activity in host cells is a novel mechanism controlling anti-tumor immunity and tumor progression, and that targeting CD73 can be manipulated therapeutically to inhibit tumor growth and metastasis.
Implications and Future Directions
The present data support the theory that the hypoxia triggers a pathway that leads to upregulation of CD73 via HIF-1α and ultimately to generation of adenosine from AMP in the tumor.12,23 At present, it is difficult to estimate the relative contribution of cancer cells compared with other cells or exosomes contributing to adenosine production in the tumor microenvironment in vivo. Nevertheless, our findings and those of others suggest strongly that CD73 functions at multiple levels to limit antitumor effects. Adenosine generated by both tumor and host CD73 impacts cellular antitumor immune responses in a fascinatingly multifactorial manner by inhibiting the activation, clonal expansion and homing of tumor-specific T cells with helper and cytolytic effector function, impairing tumor cell killing by cytolytic effector T lymphocytes (CTL), decreasing the survival of CTL, altering Treg activity, and enhancing the conversion of type 1 macrophages into tumor promoting type 2 macrophages. In addition to the immunoregulatory roles of CD73, the participation of CD73 per se as a proliferative factor, being involved in the control of cell growth, differentiation, invasion, migration and metastasis processes has been established.10,11 Moreover, CD73-derived adenosine can directly enhance tumor cell adhesion7,8 and/or chemotaxis.19 It is also intriguing that CD73 may contribute to tumor angiogenesis.3 The main proangiogenic actions of adenosine could be attributed to its ability to stimulate endothelial cell migration and proliferation, and to modulate the production of proangiogenic substances such as vascular endothelial growth factor from vascular cells and immune cells within the hypoxic tumor tissues.24 Given both immune and non-immune actions of CD73-generated adenosine on the tumor microenvironment, the actual in vivo effects of a therapeutic approach that uses CD73 as a molecular target for cancer treatment may far exceed the expectations raised by our published experimental data.
From a translational perspective, small molecule inhibitors and monoclonal antibodies against CD73 are available and well-tolerated in mice, and have already been used in vivo to treat cancer in various mouse tumor models.18-20 The development and clinical applications of CD73 blockade are thus very promising. However, it is important to note that inhibiting CD73 alone fails to cure cancer. We think that counteracting the immunosuppressive adenosinergic effects of CD73 in the tumor microenvironment must be complemented by other approaches directed at improving the development and function of antitumor T cells, such as adoptive T-cell therapy and dendritic cell vaccines in order to achieve an optimal anti-tumor strategy.
Of note, targeted CD73 therapy is not limited to specific types of solid tumors but is of more general significance. In particular, host CD73 becomes a potential therapeutic target for limiting tumor progression also in those cases in which cancer cells themselves lack or lose CD73 expression.
In summary, we have made the novel discovery that CD73 has distinct roles in the regulation of antitumor immunity in both tumor and host cells. Similar to CD73, a recent series of studies investigated the role of host-derived CD39,25-27 A2AR14 and A2BR28 on tumor growth and metastasis. In fact, all 4 adenosine receptor subtypes participate in controlling tumor progression.29 Combined with previous work conducted by others, it now appears that tumor growth and metastasis are significantly impacted by CD73 mediated adenosinergic effects, working in a coordinated fashion involving indirect and direct actions on various cellular components such as cancer cells, endothelial cells, and immune cells. These results support the feasibility of potent strategies to harness antitumor immune responses by targeting the important axis of CD39/CD73-adenosine receptors (ARs) in the tumor. Whether inhibition of CD73 and blockade of CD39 or ARs might be better than either treatment alone remains to be explored. The possibility of unintended tissue damage and/or abnormal tissue remodeling resulting from the therapeutic targeting of ARs has been raised.30 Therefore, special caution may be required in the timing and intensity of anti-adenosinergic treatment based upon the specific disease pathogenesis and the stage of inflammation.
Disclosure of potential conflicts of interest
The authors have no competing financial interests. Due to space limitations, I regret that I could not cite many worthy references in this field from my colleagues.
Acknowledgments
I thank Dr. Linda F. Thompson for her critical reading of the manuscript. Financial assistance was provided by National Institutes of Health grant CA149669, Ovarian Cancer Research Foundation Founds (LT/UTHSC/01.2011), CTSA grant (UL1RR025767), and the Cancer Therapy and Research Center (2P30 CA054174–17).
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18068
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spychala J. Tumor-promoting functions of adenosine. Pharmacol Ther. 2000;87:161–73. doi: 10.1016/S0163-7258(00)00053-X. [DOI] [PubMed] [Google Scholar]
- 4.Zhang B. CD73: A Novel Target for Cancer Immunotherapy. Cancer Res. 2010;70:6407–11. doi: 10.1158/0008-5472.CAN-10-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–58. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 6.Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells. Int J Oncol. 2008;32:527–35. [Review] [PubMed] [Google Scholar]
- 7.Sadej R, Spychala J, Skladanowski AC. Ecto-5′-nucleotidase (eN, CD73) is coexpressed with metastasis promoting antigens in human melanoma cells. Nucleosides Nucleotides Nucleic Acids. 2006;25:1119–23. doi: 10.1080/15257770600894188. [DOI] [PubMed] [Google Scholar]
- 8.Sadej R, Inai K, Rajfur Z, Ostapkowicz A, Kohler J, Skladanowski AC, Mitchell BS, Spychala J. Tenascin C interacts with ecto-5′-nucleotidase (eN) and regulates adenosine generation in cancer cells. . Biochim Biophys Acta. 2008;1782:35–40. doi: 10.1016/j.bbadis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Ujházy P, Berleth ES, Pietkiewicz JM, Kitano H, Skaar JR, Ehrke MJ, et al. Evidence for the involvement of ecto-5′-nucleotidase (CD73) in drug resistance. Int J Cancer. 1996;68:493–500. doi: 10.1002/(SICI)1097-0215(19961115)68:4<493::AID-IJC15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Bavaresco L, Bernardi A, Braganhol E, Cappellari AR, Rockenbach L, Farias PF, et al. The role of ecto-5′-nucleotidase/CD73 in glioma cell line proliferation. Mol Cell Biochem. 2008;319:61–8. doi: 10.1007/s11010-008-9877-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Zhou X, Zhou T, Ma D, Chen S, Zhi X, et al. Ecto-5′-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol. 2008;134:365–72. doi: 10.1007/s00432-007-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–55. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci USA. 2010;107:1547–52. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–7. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hausler SF, Montalban Del Barrio I, Strohschein J, Anoop Chandran P, Engel JB, Honig A, Ossadnik M, Horn E, Fischer B, Krockenberger M, Heuer S, Seida AA, Junker M, Kneitz H, Kloor D, Klotz KN, Dietl J, Wischhusen J. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. . Cancer Immunol Immunother. 2011 doi: 10.1007/s00262-011-1040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Häusler SF, Ossadnik M, Horn E, Heuer S, Dietl J, Wischhusen J. A cell-based luciferase-dependent assay for the quantitative determination of free extracellular adenosine with paracrine signaling activity. J Immunol Methods. 2010;361:51–6. doi: 10.1016/j.jim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–83. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 18.Yegutkin GG, Marttila-Ichihara F, Karikoski M, Niemela J, Laurila JP, Elima K, et al. Altered purinergic signaling in CD73-deficient mice inhibits tumor progression. Eur J Immunol. 2011;41:1231–41. doi: 10.1002/eji.201041292. [DOI] [PubMed] [Google Scholar]
- 19.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, et al. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71:2892–900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, et al. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest. 2011;121:2371–82. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ålgars A, Karikoski M, Yegutkin GG, Stoitzner P, Niemela J, Salmi M, et al. Different role of CD73 in leukocyte trafficking via blood and lymph vessels. Blood. 2011;117:4387–93. doi: 10.1182/blood-2010-11-321646. [DOI] [PubMed] [Google Scholar]
- 23.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14:5947–52. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 24.Auchampach JA. Adenosine receptors and angiogenesis. Circ Res. 2007;101:1075–7. doi: 10.1161/CIRCRESAHA.107.165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–7. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Muller CE, et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–40. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Künzli BM, Bernlochner MI, Rath S, Kaser S, Csizmadia E, Enjyoji K, et al. Impact of CD39 and purinergic signalling on the growth and metastasis of colorectal cancer. Purinergic Signal. 2011;7:231–41. doi: 10.1007/s11302-011-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, et al. Host A(2B) adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–95. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. Biochim Biophys Acta. 2011;1808:1400–12. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Ohta A, Sitkovsky M. Caveats and cautions for the therapeutic targeting of the anti inflammatory A2 adenosine receptors: The notes of caution. Nat Rev Drug Discov. 2006:5. [Google Scholar]