Abstract
Thrombocytopenia-induced tumor hemorrhage improves drug delivery to tumors. This phenomenon presents a new way to increase drug efficacy with minimal side effects. Combining anti-platelet treatment with therapeutic drugs may help us in the search for more effective ways to fight cancer.
Keywords: bleeding, inflammation, platelets, thrombocytopenia, tumor, vascular permeability
We recently showed that platelet depletion increased the efficacy of chemotherapy and reduced tumor growth in mice.1 Platelets are the cellular orchestrators of primary hemostasis. They have long been known to prevent bleeding upon injury by their ability to induce coagulation and thrombus formation. Through expression of adhesion molecules and release of their granule contents, they modulate the immune system and preserve vascular integrity.2 Platelets also have a protective role during the inflammatory response. Our group has shown, that in thrombocytopenic animals, induction of local inflammation results in severe bleeding originating from capillaries and post-capillary venules at the inflamed site.3 The observed hemorrhage correlates with the presence of inflammatory cells suggesting that platelets protect blood vessels from injurious leukocytes.
In cancer, platelets have been known for their potential to promote metastasis. They have been shown to support angiogenesis, and promote cancer cell survival and adherence to the endothelium, all of which contributes to the spreading of cancer to distant sites.4In the primary tumor, platelets may modulate the growth of tumor vessels by differential release of pro- and anti-angiogenic factors. Recently, we found a new role for platelets in cancer: the maintenance of tumor vessel integrity.5 We showed that injection of platelet-depleting antibody in tumor-bearing mice induced rapid bleeding in and surrounding the tumor without affecting vessels elsewhere in the body.1,5 Since only tumor vessels were affected by the low platelet count, we hypothesized that opening of the tumor vasculature would promote the delivery of circulating drugs to the tumor. We thus combined platelet depletion with the chemotherapeutic agent paclitaxel and showed a significant reduction in tumor growth compared with paclitaxel alone.1 This combined treatment increased the accumulation of the drug at the tumor site thereby increasing its efficacy, as observed by increased tumor cell apoptosis and reduced proliferation, without increasing toxic side-effects to other organs. Thus, thrombocytopenia increased the efficacy of the chemotherapeutic treatment by causing a higher quantity of drug to be delivered specifically to the tumor site.
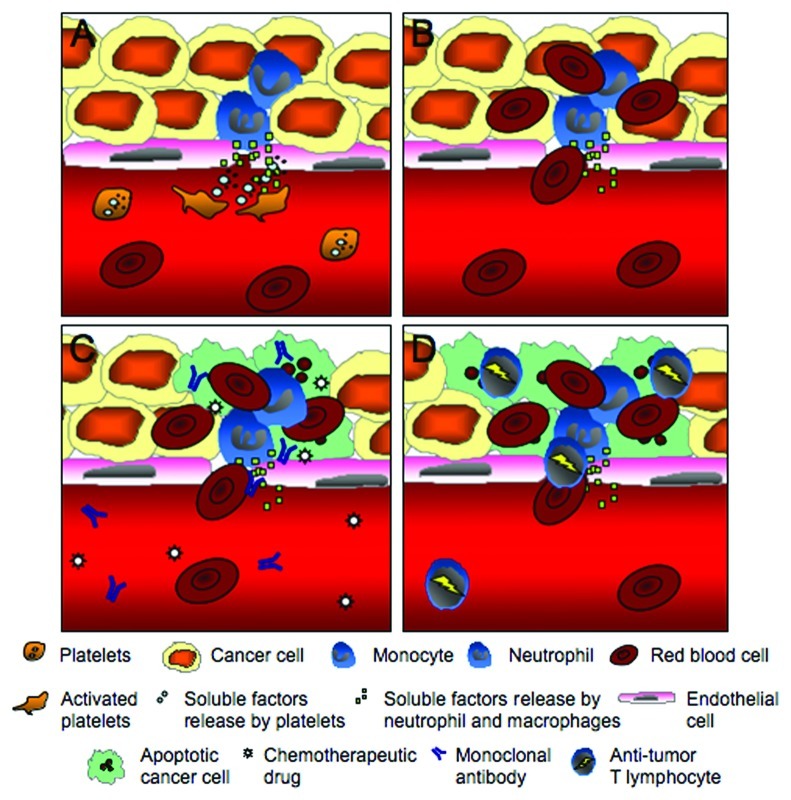
Using low platelet counts to improve drug delivery to tumors provides a new way to increase drug access and efficacy that could be applied to a variety of tumors. Tumor hemorrhage was observed upon platelet depletion in several different tumor types, such as subcutaneous lung carcinoma, mammary carcinoma, and melanoma as well as established lung metastasis in mice.1,5,6 Moreover, similar to the localized bleeding upon inflammatory challenge, the tumor hemorrhage associated with platelet depletion occurred at sites of neutrophil and macrophage accumulation.6 Induction of thrombocytopenia in mice deficient in leukocyte adhesion molecules β2 and β3 integrins, which are characterized by a reduction in infiltrating macrophages and neutrophils in the tumor stroma, results in a significant reduction in tumor hemorrhage. This strongly suggests that, in the tumor, the inflammatory cells are responsible for the bleeding associated with low platelet counts (Fig. 1A and B). Because platelets prevent tumor vessel injuries caused by infiltrating leukocytes, and most tumors contain inflammatory leukocytes,7 lowering platelet count to improve drug delivery should be feasible in a large variety of tumors.
Figure 1.
Thrombocytopenia-induced tumor hemorrhage improves drug delivery to tumors. (A) By releasing their granule contents, platelets counteract the injurious effect(s) on the vasculature induced by neutrophils and monocytes infiltrating the tumor. (B) In the absence of platelets, tumor hemorrhage is observed. (C) Generation of openings in the tumor vasculature during thrombocytopenia provides a better access for circulating drug to enter and accumulate in the tumor. The vessels’ breaches would also allow a higher tumor antigen visibility and the use of tumor specific agents such as monoclonal antibodies could result in an even higher accumulation and efficacy. (D) Immunotherapies inducing anti-tumor specific T lymphocyte activation could also potentially be improved during thrombocytopenia. A greater access and visibility of the tumor antigen would result in an increase in intratumoral accumulation of activated anti-tumor cells and improve treatment efficacy.
In addition, low platelet counts could be used for the delivery of a wide range of therapeutic drugs (Fig. 1C). The drugs evaluated in our study are not tumor specific and thus accumulate in the tumor through passive permeability.1 Platelet depletion induces breaches in the tumor vasculature enabling more drug to cross the endothelium. In addition to accumulation of a chemotherapeutic drug, we showed that fluorescently-labeled 1 μM microspheres infused i.v. was accumulating in the hemorrhagic tumor.1 This suggests that the delivery of drug-containing nanoparticles allowing a slow release of the drug can also be improved by pairing with thrombocytopenia. The use of antigen- specific therapy, such as monoclonal antibodies and antibody-enzyme fusion proteins, may have an even bigger effect on tumor growth when paired with platelet depletion. The combination of direct specificity for the tumor with a better access to the target would certainly be beneficial. Even the combination of low platelet counts with the newest immunotherapeuticals strategies, such as dendritic cell vaccines and adoptive T cell transfer, would be applicable.8,9 Induction of activated tumor-specific cytotoxic T lymphocytes combined with an increased exposure of tumor antigen via breaches of blood vessels in absence of platelets would again allow the immune system to more efficiently reach its target, increasing treatment efficacy (Fig. 1D).
It will be important to identify the factor(s) released by platelets that constitutively prevent tumor hemorrhage and learn how they could be inhibited. Employing specific inhibitors of platelet function or neutralizing agents would enable the development of new treatments without the need for lowering platelet count. Such anti-platelet treatment combined with chemotherapy or immunotherapy would enhance their delivery to the tumor and may represent a significant improvement in our ability to fight cancer with minimal side effects and without increasing the risk for bleeding.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest and no competing financial interests.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health grants P01 HL056949 and R01 HL041002 (to D.D.W.), and the Terry Fox Foundation through the Canadian Cancer Society (to MD).
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/17962
References
- 1.Demers M, Ho-Tin-Noe B, Schatzberg D, Yang JJ, Wagner DD. Increased efficacy of breast cancer chemotherapy in thrombocytopenic mice. Cancer Res. 2011;71:1540–9. doi: 10.1158/0008-5472.CAN-10-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho-Tin-Noé B, Demers M, Wagner DD. How platelets safeguard vascular integrity. J Thromb Haemost. 2011;9(Suppl 1):56–65. doi: 10.1111/j.1538-7836.2011.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goerge T, Ho-Tin-Noé B, Carbo C, Benarafa C, Remold-O'Donnell E, Zhao BQ, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood. 2008;111:4958–64. doi: 10.1182/blood-2007-11-123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–34. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho-Tin-Noé B, Goerge T, Cifuni SM, Duerschmied D, Wagner DD. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res. 2008;68:6851–8. doi: 10.1158/0008-5472.CAN-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho-Tin-Noé B, Carbo C, Demers M, Cifuni SM, Goerge T, Wagner DD. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am J Pathol. 2009;175:1699–708. doi: 10.2353/ajpath.2009.090460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–83. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]