Abstract
The recent findings on NK activation indicate that these cells are important antitumor effectors. NK cells participate in the graft-vs.-leukemia effect to control the relapse in leukemic patients transplanted with allogeneic hematopoietic stem cells. In various tumors, correlation between NK cell infiltrates and prognosis were reported. However, tumor-infiltrating NK cells are yet poorly characterized. We here summarize our results and the recent studies of the literature on tumor-infiltrating NK cells, and discuss the impact of these novel insights into NK cell responses against tumors for the design of NK cell-based therapies.
Keywords: chemotherapy, NK cells, tumors
Antitumor Effects of NK Cells
NK cells were first identified as a distinct sub-population of lymphocytes endowed with the spontaneous capacity to in vitro kill tumor cells.1,2 Since then, the increased knowledge of NK cell biology has provided strong arguments for their role in immune response against infections and tumors.
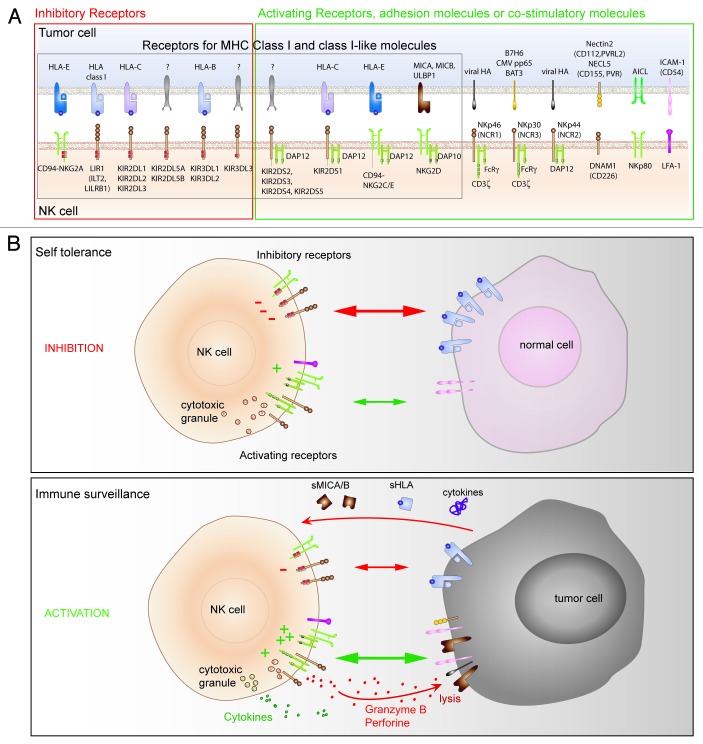
Natural Killer cell activation depends on the intricate balance between activating and inhibitory signals derived from receptors. Most inhibitory receptors (KIR, NKG2A, LIR) are specific for different HLA-I molecules. In normal conditions, the low engagement of activating receptors counterbalanced by high inhibitory signals triggered by HLA-I molecules on normal cells avoids self reactivity of NK cells (Fig. 1). In the context of tumor, NK cell activation relies on the expression of NK ligands by target cells. To be “seen” by NK cells, cancer cells have to express ligands for activating receptors, while the low expression of HLA-I molecules attenuates the triggering of inhibitory receptors. Cancer cells can also secrete immuno-modulating molecules inducing immune cell anergy. Thus, tumor-related parameters strongly control NK cell activation and any factor that modifies the expression of NK ligands on tumor cells may thus affect the activation of NK cells.
Figure 1. Regulation of NK cell activation. (A) Main NK receptors (lower line) and respective ligands on target cells (upper line) implicated in NK cell triggering and inhibition. (B) Functions of NK cells (cytotoxicity and cytokine secretion) depend on a balance between opposing signals derived from activating and inhibitory receptors. The presence and density of ligands dictate whether the target cell will be susceptible (immune surveillance) or not (self tolerance) to NK cell lysis.
Numerous data demonstrate the implication of NK cells in tumor control and support a potential benefit of their usage in cancer immunotherapy. Clinical evidence for the benefit of in vivo NK cell targeting of human tumors has come from allogenic hematopoietic stem cell transplantation (HSCT) to treat the relapse in patients with acute myeloid leukemia (AML). Infusions of donor alloreactive NK cells (presenting at least one KIR/HLA mismatch with the recipient) participate in graft-vs.-leukemia (GvL) effect and correlate with relapse-free survival.3-5 An 11 y-follow up of 3625 residents of a Japanese population showed a negative correlation between NK cell cytotoxic activity from blood and cancer incidence.6 Other arguments for the role of NK cells in tumor control rely on the presence of NK cell infiltrates in diverse solid tumors.
Different strategies aimed at increasing NK cell lytic activity against tumor cells and/or to enhance their homing at the tumor site have been evaluated: cytokine-mediated activation of endogenous NK cells (IL2, IFNα), NK infusions, anti-KIR mAb, ADCC-promoting therapeutic Abs, Bispecific Ab (CD16/CD30) or combined therapies.7,8 Preclinical and clinical studies emphasize that NK-mediated immunotherapy would be efficient in treating minimal residual disease. On the other hand, accumulating evidences indicate that, even when conventional therapies apparently result in complete remission, micrometastases of residual tumor cells can lead to tumor relapse. Thus, it is conceivable to associate conventional cancer therapies, reducing tumor size, with immunotherapy treatments for the complete and durable eradication of tumors.9,10
However, different mechanisms concerning the anti-tumor function of NK cells remain partially understood: the in vivo NK cell activation during tumor progression, the influence of tumor microenvironment on NK cells, and how the treatments interfere with NK cell effector functions in cancer patients.
This review summarizes our results on NK cells in metastatic renal cell carcinoma (RCC) and melanoma, and recent studies in other solid tumors. In particular, we discuss the role of some important tumor parameters that interfere with NK cell activation and subsequent recognition of cancer cells. We especially analyze the mechanisms involved in tumor immunogenicity and how tumor stage and patient therapies modulate the functional status of NK cells. Finally, we discuss the impact of these novel insights on the design of anti-tumor NK cell-based therapies.
Oncogenic Events are Recognized by NK Cells
Malignant tumors are proliferating cells that have accumulated mutations in genes regulating important biological pathways as, for example, proliferation, apoptosis, angiogenesis and migration. Oncogenic mutations often lead to the aberrant activation of signaling pathways and to important modifications in protein expression by cancer cells. Compared with normal cells, cancer cells can overexpress stress-induced molecules, bear constitutive activation of growth factors, downregulate MHC-I molecules, or express specific tumor-associated antigens. Immune cells detect tumors through receptors that recognize the altered expression of these molecules. Thus, an oncogenic event can influence tumor immunogenicity when it directly or indirectly modulates the expression of those molecules recognized by immune cells and involved in their activation.
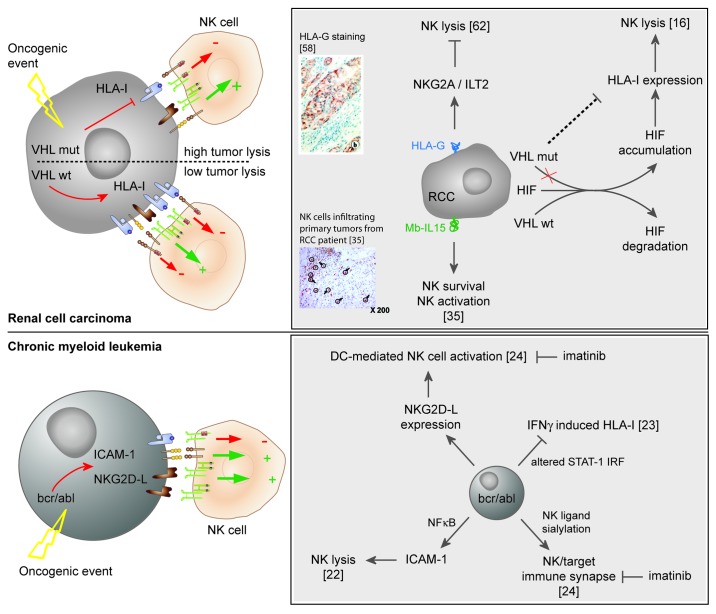
In RCC, we have shown that loss-of-function mutations of Von Hippel Lindau (VHL) gene may be targeted by NK cells (Fig. 2). This oncogenic event is important for the development of these tumors and 80% of RCC bear mutations in VHL gene leading to HIF (Hypoxia-inducible factor)-1 accumulation and increased activation of downstream signaling pathways.11,12 Previous studies including ours showed that NK cells lyse RCC cell lines and that LFA-1/ICAM-1 and HLA-I/NKR interactions are important in RCC recognition by NK cells.13-15 We have shown that certain loss-of-function VHL mutations correlate with a reduced expression of classical HLA-I molecules via a partially HIF-1α-dependent mechanism and with higher RCC lysis by NK cells.16 These results corroborate previous findings reporting that VHL controls the constitutive expression of STAT-1 and LMP2, involved in MHC-I dependent antigen presentation, probably via the downregulation of STRA-13.17 HLA-E molecules, ligands of the inhibitory receptor NKG2A, are also decreased in VHL-mutated cells. HLA-E promoter also contains STAT-1 binding site.18 HLA-I molecules are often downregulated in tumors and their modulation is considered a common mechanism of tumor escape from T cell immune response.19 Conversely, low HLA-I expression may shift the balance toward activation in NK cells. In non-tumor pathology and upon hypoxia-dependent HIF accumulation, the expression of MICA/B molecules, stress-induced ligands of the activating receptor NKG2D, was found augmented and correlated with increased NK cytotoxicity.20,21 In our studies, the expression of NKG2D ligands was not controlled by VHL mutation.
Figure 2. Tumors parameters implicated in the activation of NK cells. In RCC, VHL mutations induce constitutive activation and accumulation of Hypoxia-inducible factor (HIF). Certain VHL mutations correlate with low HLA-I molecules expression via a partially HIF-dependent pathway. The low expression of HLA-I molecules by VHL-mutated RCC cells reduces the engagement of NKG2A inhibitory receptor, shifting the balance toward NK cell activation. Membrane HLA-G molecules upregulate inhibitory receptors (NKG2A,and ILT2) on NK cells. Membrane-bound IL15 (Mb-IL15) is involved in NK cell activation and survival. In CML, the oncogeneic protein bcr/abl induces the expression of ICAM-1 and NKG2D ligands on myeloid cells favoring NK/target conjugates and lysis. Altered IFNγ signaling in bcr/abl target maintains a low HLA-I molecules expression. Bcr/abl dendritic cells activate NK cells via NKG2D receptor. Imatinib mesylate interferes with NK/leukemic targets.24 DC: Dendritic cell; IM: Imatinib Mesylate; IRF: Interferon regulatory factor; STAT-1: Signal Transducer and Activator of Transcription factor-1.
Data on VHL-dependent RCC susceptibility to NK cell lysis are reminiscent of previous studies of the lab on Chronic Myeloid Leukemia (CML), as summarized in Figure 2. We showed that the high expression of bcr/abl oncoprotein in leukemic cells increased NK cell-mediated lysis through the NF-κB activation dependent induction of ICAM-1 expression on tumor cells.22 In addition, we reported an altered IFNγ pathway consequent to bcr/abl expression; HLA-I molecules were not induced by IFNγ treatment, thus preserving leukemic cell susceptibility to NK cell lysis.23,24 Finally, we showed that the overexpression of bcr/abl oncogenic protein in DCs promotes DC-mediated NK cell activation via the upregulation of NKG2D ligands.25
Triggering of NK cell activation by oncogenic-induced changes in the expression of NK ligands on tumor cells may be a common phenomenon. In melanoma, BRAF is frequently mutated in tumor tissue and new therapies targeting the activation of its signaling pathway have been recently developed for the treatment of patients. Moreover, other genes implicated in the familial occurrence of melanoma (i.e., germline mutations of CDKN2A) were identified.26 It will be interesting to study whether mutations in BRAF, or in other genes involved in melanoma development and progression, determine changes in melanoma immunogenicity (expression of NK ligands), thus modulating NK activation.
Whether NK cells are able to indirectly target oncogenic defects, or to recognize the constitutive overactivation of membrane receptors in tumors, are important issues and may contribute to develop complementary targeted therapies based on these cytotoxic cells.
NK Cell Alterations in Cancer Patients: What are the Direct and/or Indirect Effects of Tumor Cells?
The interactions between NK and tumor cells could be influenced by the site of their encounter. Well characterized in blood, NK cells are also present in various tissues and different sites of NK development and maturation have been discovered. On the other hand, cancer cells develop from and disseminate to distinct sites depending on tumor type and on the course of the disease. Thus, when studying tumor-infiltrating NK cells, it is important to consider the tissue resident NK cells and the site of metastases.
(1) Circulating NK cells
The first ex vivo evaluation of anti-tumor NK function was done on circulating NK cells from leukemia patients. Due to the easier access, NK cells from blood are also the best characterized in patients with solid tumors. However, in these patients, blood is probably not the most relevant compartment for the investigation of NK cells.
Numerous studies have reported functional defects of blood NK cells from most cancer patients and the severity of these deficiencies varies among different types of tumor. Profound alterations of NK differentiation and function were found in CML, AML and myelodysplastic patients,27-29 whereas in patients with solid tumors defects on blood NK cells are generally mild and often associated with advanced tumor stage.
In patients with invasive cervical carcinoma and premalignant lesions, the expression of NKp30 and NKp46 was found downregulated on blood NK cells and correlated with a reduced cytolytic activity and with the clinical stage of patients. In invasive cervical carcinoma, the expression of NKG2D was also decreased.30 We recently showed that circulating NK cells from stage IV melanoma patients present a unique NKp46low/NKG2Alow expression profile that correlates with high lytic efficiency toward melanoma cell lines.31 Our preliminary results indicate that NK cells from stage III melanoma patients are less affected, suggesting that NK alterations may change during disease progression (unpublished data). Other groups also reported a reduced expression of NKG2D and NCR receptors in NK cells from melanoma patients.32,33 Moreover, altered activating NK receptors and dysfunctions of circulating NK cells were recently reported from a large series of patients with breast cancer; the severity of the defects was clearly increased with the progression of the disease.34
(2) Tumor-infiltrating NK cells
Numerous reports show that NK cells infiltrate tumors of different origins. However, the detection of NK cells has been done using different antibodies. In some cases, double staining with CD3 and CD56 or CD57 specific antibodies have been performed, whereas more recent studies used the direct staining with anti-NKp46 antibody. Several groups (including ours), reported that NK cells infiltrate clear-cell RCC.15,35,36 NK cells were described in melanoma,37 non small cell lung cancer,38,39 gastrointestinal stromal tumors (GIST)40 and colon-rectal carcinoma (CRC), even if in lower proportion than T cells.41
In several tumors, NK cell infiltration has been associated with clinical outcome. A positive correlation was reported in RCC between the numbers of tumor-infiltrating NK cells (NK-TILs), the expression of CD16 receptor and NK cytotoxic activity.35 In a study including 150 patients, the count of intra-tumor NK cells (anti-CD57 staining) in resected lung adenocarcinomas was associated with the control of tumor progression, representing a likely prognostic marker in patients with lung adenocarcinoma.42 Correlation between intra-tumor NK cell counts and tumor control was also reported in patients with squamous cell lung cancer.43 The prognostic value of CD57+ NK-TILs was observed in gastric carcinoma44 and colorectal carcinoma.45 In prostate cancer, elevated counts of CD56+ NK cells were associated with a lower risk of progression.46 Moreover, a recent study showed a positive association between numbers of NK-TILs (anti-CD56 staining) and regression of melanocytic lesions.47
Compared with blood NK cells, only a limited amount of information is available on tissue infiltrating NK cells. NK-TILs usually present more severe phenotypic and functional alterations compared with circulating or non-cancerous tissue-infiltrating NK counterparts. There is a marked decreased expression of NKp30 receptor in GIST.40 Furthermore, NK cells show tissue specific patterns of expression. In few studies, NK-TILs and non-cancerous tissue specific NK cells were analyzed in parallel. In non small cell lung cancer (NSCLC), Carrega and colleagues showed that although peritumoral NK cells were similar in phenotype and function to blood NK cells, intratumoral NK cells were CD56brightCD16low, expressed NKp44 and HLA-DR, lacked perforin, expressed high levels of inhibitory receptors and were impaired in cytotoxic function but secrete cytokines (IFNγ, TNFα).38 A recent study further showed profound reduction in the expression of a cluster of NK receptors (NKp30, NKp80, DNAM-1, CD16 and ILT-2) and functional anergy in intratumoral NK cells compared with blood and lung-derived NK cells.39 Moreover, a distinct NK cell distribution was shown between malignant (MLTA) and non malignant lung tissues (NMLTA). NK cells were abundant in non malignant lung tissues and displayed high cytotoxic activity compared with NK cells in MLTA.48 A similar alteration in NK cell distribution was also recently found between normal liver tissue and liver metastasis of CRC.41 In breast cancers, the number of NK cells in malignant tissue is reduced and includes a higher percentage of CD56bright compared with healthy mammary tissue. These NK-TILs display reduced activities compared with paired circulating NK cells.34
In RCC tumors, NK-TILs differ from autologous blood NK cells regarding the expression of inhibitory receptors, which may contribute to functional deficits within tumor tissues. However, in some RCC highly infiltrated by NK cells, NK function could be restored after stimulation with low-dose IL2 and the cytotoxic activity of NK-TILs resided in the CD16dim/NKG2Ahigh NK cell population.15,35
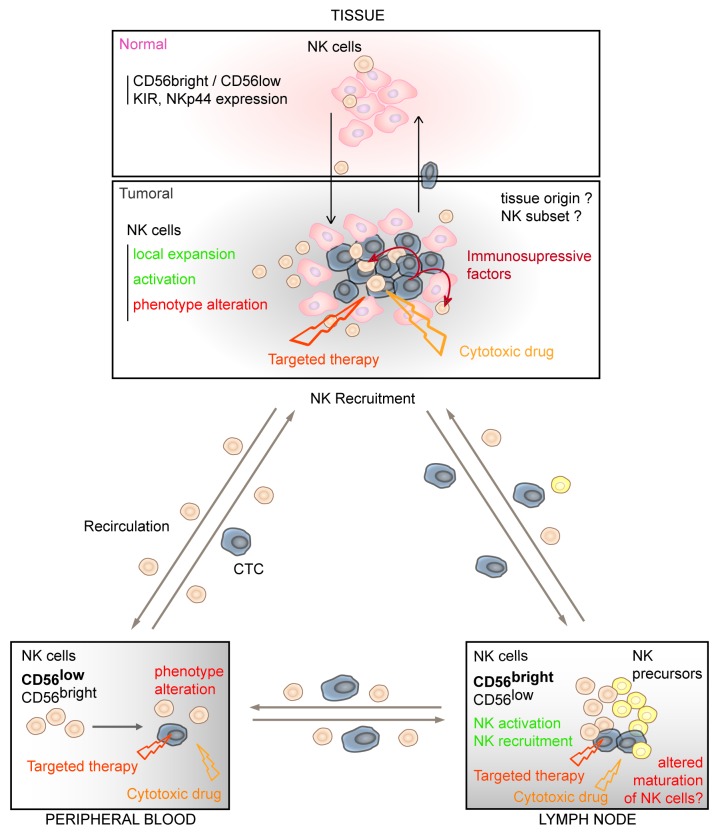
An open question concerns the origin of the NK-TILs for which several hypotheses may be proposed. The activation of tissue resident NK cells could occur although the marked differences between peri- and intratumoral NK cells do not favor this hypothesis. Moreover, NK precursors are present in certain tissues, like lymph nodes (LN),49 and within these sites, tumor cells may interfere with NK maturation and contribute to the altered phenotype observed in blood NK cells. Our preliminary results indicate that, compared with normal LNs, NK cells from melanoma metastatic LNs are functionally defective and displayed a co-reduced expression of a pattern of activating receptors (CD16, NKG2D, DNAM-1 and NKp30) close to that described in NK-TILs from ovarian carcinoma and NSCLC.39,50 Alternatively, NK-TILs may be recruited from the circulation through chemokine gradients produced by the tumor.
These different hypotheses are not exclusive and the source of NK cells may vary as a result of changes in the tumor microenvironment (Fig. 3). The determination of the transcription factors and/or the immune related genes expressed by NK cells from different compartments will be a valuable strategy to precise the origin of NK-TILs. For instance, in the case of metastatic LNs, it will be likely useful to compare the transcriptional profiles of NK cells derived from metastatic LNs from diverse cancers (i.e., breast cancer, melanoma).
Figure 3. Proposed hypotheses to explain the alterations of NK cells in cancers patients. NK cells are present in different body compartments (peripheral, blood, secondary lymphoid organs and tissues) and NK defects may depend on where NK and tumor cells encounter: (1) Circulating tumor cells (CTC) may directly affect the phenotype and function of blood NK cells; (2) metastatic cells invading certain sites of NK cell maturation (i.e., LN) can influence final differentiation of NK cell subsets; (3) Compared with NK cells in normal tissue, NK-TILs are dysfunctional. Cancer cells can directly affect the functional status of tissue resident NK cells or of recruited NK-TILs by cell-to-cell contacts or by the secretion of soluble immunosuppressive factors; (4) NK-TILs may return to blood contributing to the altered phenotype observed on circulating NK cells. Finally, cytotoxic drugs and targeted therapies can interfere with NK cell activation, affecting (directly or indirectly) their phenotype or signaling pathways.
Mechanisms Underlying Alterations of NKs in Cancer Patients
In cancer patients, the defects of blood NK cells depend of the tumor type and are more severe in advanced stages, and NK TILs are more affected than peritumoral NK cells.
Several mechanisms may contribute to the alterations of NK cells in cancer patients depending on where NK and tumor cells encounter (Fig. 3).
Circulating tumor cells (CTC) have been detected in melanoma and other tumors51-54 and could directly modulate blood NK cells. It would be interesting to evaluate whether the alterations in blood NKs correlate with CTC quantity.
It can be postulated that NK cells are modulated at the tumor site and then recirculate to peripheral blood. Little is known about NK cell recirculation, but chemokine receptors and selectins are considered good candidates for the orchestration of NK trafficking. While NK recruitment at site of inflammation was described, the data concerning a possible recirculation of these cells to blood are scarce.55
Independently of the site, tumor cells may affect NK functions in a direct or indirect manner, involving membrane and/or soluble factors expressed and released by tumor cells. The functional consequences will depend on the nature and the density of NK ligands expressed by tumor cells. Cancer cells express ligands for activating NK receptors and in solid tumors, NKG2DL, DNAM-1L, as well as adhesion molecules are important determinants. Thus, in situ, the downregulation/shedding of activating NK receptors may be caused by the constitutive expression of NK ligands or through the release of soluble factors by tumor cells in the microenvironment. Soluble MICA/B molecules were shown to downregulate NKG2D.56,57 However, in our study, there was no correlation between the phenotype of NK cells (NKG2D) and seric levels of MICA/B in patients.
On the other hand, MHC-I molecules control the activation of NK cells. Membrane HLA-G is expressed by RCC and melanoma cells and participates in immune surveillance escape.58-61 In particular, HLA-G has been described to upregulate inhibitory receptors on NK cells.62 Soluble forms of non classical HLA-I molecules were also implicated in tumor evasion from immune response.63 We have detected elevated seric levels of HLA-E in melanoma patients.64
In RCC, prostate cancer and melanoma, a membrane-bound IL15 has been described.65 Endowed with particular properties on tumor cells, tumor IL15 is involved in NK cell activation and survival36 (Fig. 2). It can exert reverse signaling and favor tumor progression.66,67
TGFβ is a major immunosuppressive molecule, known to downregulate the expression of NKp30 and NKG2D.68 TGFβ expression was upregulated in metastatic LN from patients with cervical cancer69 and anti-TGFβ restores NK cell reactivity in breast cancer patients.34 Supporting the hypothesis of a modulation of NK status by soluble factors, changes in immune function were shown to precede metastasis in melanoma draining LN.70
In addition, the particular phenotype of NK cells in cancer patients may be the result of NK cell interactions with other tumor-modulated cells (DC, T cell subsets, fibroblasts) present at the sites of NK maturation, and/or tumor microenvironment. In melanoma, tumor-associated fibroblasts have been described to directly suppress the function of IL2-activated blood NK cells and to inhibit the cytokine-induced expression of NKp44, NKp30 and DNAM-1 via cell-to-cell contacts.71
Developing from different tissues and expressing specific pattern of molecules, each tumor type is unique and characterized by the capacity to metastasize in preferential sites. Differences observed in NK cells (blood NKs vs. NK-TILs) from patients with different cancers likely reflect intrinsic characteristics of the tumor that must be taken into account in the investigation of NK cells in cancer patients.
Treatments Affect NK Cell Phenotype and Functional Status
Another important issue from our recent studies is that conventional chemotherapy affects circulating NK cells in melanoma patients. Post-chemotherapy NK cells displayed an induced expression of NKG2A and NKp46 receptors compared with pre-chemotherapy patients and donors.31 This profile is compatible with a less mature phenotype, and was associated with a reduced NK activation toward melanoma cells. The treatment-induced modulation of NK cell status was not direct because blood samples were collected at least four weeks after the last course of chemotherapy. In addition, using global transcriptome analysis and immunohistochemistry, we showed that dacarbazine induced stromal and immune related genes in responsive cutaneous metastatic lesions.72 These data further indicate that cytotoxic drugs, in addition to their direct cytotoxicity to tumor cells, may also influence changes in the tumor microenvironment. Thus, in vivo, chemotherapy acts on tumor cells and on other immune cells causing the release of cytokines or soluble factors that in turns modulate NK status.
It is likely that compared with chemotherapy, “targeted therapy,” which consists of a novel group of antibodies or small kinase inhibitors targeting specific growth signaling pathways in cancer cells, will exert slight effects on immune cells. However, certain targeted therapies have shown to affect pathways implicated in the activation of immune cells. A recent study has compared the effects of sunitinib and Sorafenib, two kinase inhibitors adopted for the treatment of RCC, on circulating NK cells.73 They showed that NK cell function in RCC patients is inhibited by Sorafenib as a consequence of an impaired phosphorylation of PI3K and ERK, which directly control NK cell reactivity. In contrast, pharmacological doses of Sunitinib do not affect NK cell function. Thus, new therapies when targeting signaling pathways shared by NK cells (and in general in immune cells) can likely affect their function. In melanoma, BRAF inhibitors have been developed for the treatment of patients. It will be interesting to evaluate their effect on NK cells. Another promising therapeutic approach in melanoma uses anti-CTLA4 mAb to block an inhibitory receptor expressed by activated immune cells (T cells). The effect of such mAbs on NK cells deserves precise investigation.
NK cell activation by cancer therapy does occur and the underlying mechanisms have been investigated. In GIST, gain-of-function mutations of c-kit are found in 85% of tumors and treatment by Imatinib, a c-kit inhibitor, is highly effective. In certain patients, the efficacy of Imatinib was not associated with c-kit mutation in tumor, but dependent on IFNγ production by NK cells upon DC-mediated activation.74
Treatments can also modulate the expression of NK ligands on tumor cells. Cisplatin and 5-fluorouracil were described to induce the expression of NKG2D ligands,75 likely augmenting tumor cell susceptibility to NK lysis. IFNα, used as adjuvant for the treatment of melanoma and RCC, increases the expression of HLA molecules, thus inhibiting NK cell activation toward cancer cells.
All together these studies indicate that anti-cancer therapy (conventional, targeted therapies) can exert important effects on the innate immune response and particularly affect NK cell activation. When NK-based adjuvant therapies are planned to be used, it is important to evaluate whether and how they will be compatible with the first line treatment. A clear example concerns breast cancer patients for which responses to adjuvant therapy by trastuzumab (a Her2-targeting monoclonal Ab) positively correlate with NK cell activity76 and for which chemotherapy was shown to affect NK function.77,78
Conclusion
In melanoma and RCC patients, complete responses have been obtained by high doses of IL2 and first trials of allogenic NK cell infusions in these patients demonstrated that the treatment is safe.79 These clinical evidences indicated that NK-based immunotherapy approach may represent a good strategy for the adjuvant treatment of cancer. However, only limited objective responses have been achieved using the different NK-based protocols thus far developed. These approaches were aimed at boosting the NK lytic potential but neither the tumor compartment, nor the previous therapies of patients were considered.
The recent findings on NK cell activation in cancer patients indicate that several important parameters require consideration for the choice of a NK-based therapy: immunogenicity and tumor capacity to modulate NK function, phenotype of circulating/tumor-infiltrating NK cells and the effect of the treatment on NK function.
Additional studies are necessary to characterize NK-TILs in patients with solid tumors of different origins, tumor stages and evolution in response to treatments. In solid tumors, the study of NK cells from normal vs. tumor tissue is fundamental to understand the modulation exerted by the tumor on NK functional status. Moreover, some tumors like melanoma or breast cancers mainly metastasize trough lymphatic vessels thus affecting LNs, important sites of NK cell development and maturation. All these parameters have to be considered for the development of new more effective NK-based therapies.
All in all, these recent findings indicate that it is justified to reconsider immunotherapy not as a competitive treatment but as a complementary approach that can be integrated in therapeutic strategies at different stages of the disease. In particular, NK-based therapies would be probably more effective for the treatment of minimal residual disease when associated to therapy inducing NK ligands on tumor cells and enhancing the NK recruitment to tumor sites.
Acknowledgments
This work was supported by grants from INSERM, la Fondation pour la Recherche Medicale (FRM), la Société Française de Dermatologie (SFD) and la Ligue Nationale contre le Cancer (Comité Ile de France). GF had a grant from Canceropole IdF and Ligue Nationale contre le Cancer, AP from FRM. We thank Dr S. Mazouz Dorval for analyses of stage III melanoma patients. We thank Drs E. Maubec and V. Fourchotte for providing samples of lymph node metastases and Dr D. Mitilian for samples of normal lymph nodes. We thank Drs N. Freiss-Rouass for measure of seric HLA-G and Dr S. Caillat-Zucmann for seric MICA molecules, Pr N. Gervois for seric HLA-E.
Glossary
Abbreviations:
- ADCC
antibody dependent cell cytotoxicity
- AML
acute myeloid leukemia
- CML
Chronic Myeloid Leukemia
- CRC
colorectal cancers
- CTC
Circulating tumor cells
- DC
Dendritic cell
- EMT
epithelial-mesenchymal transition
- GIST
gastrointestinal tumor
- HIF
Hypoxia-inducible factor
- HLA
human leukocyte antigen
- HSCT
hematopoietic stem cell transplantation
- ICAM-1
intracellular adhesion molecule-1
- IFN
interferon
- IM
Imatinib Mesylate
- IRF
Interferon regulatory factor
- IS
Immunological synapse
- KIR
Killer Immunoglobulin-like receptor
- LFA-1
lymphocyte function-associated antigen-1
- LMP2
EBV-latent membrane protein 2
- MICA/B
MHC-class-I-related Chain A/B
- NK
natural killer cell
- RCC
Renal cell carcinoma
- STAT-1
Signal Transducer and Activator of Transcription factor-1
- STRA-13
Stimulated by retinoic acid-13
- VHL
Von Hippel Lindau
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18312
References
- 1.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16:230–9. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 2.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–7. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 3.Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol. 2009;21:525–30. doi: 10.1016/j.coi.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Velardi A, Ruggeri L, Mancusi A, Burchielli E, Perruccio K, Aversa F, et al. Clinical impact of natural killer cell reconstitution after allogeneic hematopoietic transplantation. Semin Immunopathol. 2008;30:489–503. doi: 10.1007/s00281-008-0136-1. [DOI] [PubMed] [Google Scholar]
- 5.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 6.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 7.Levy EM, Roberti MP, Mordoh J. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011;2011:676198. doi: 10.1155/2011/676198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–39. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 9.Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008;20:545–57. doi: 10.1016/j.coi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 11.George DJ, Kaelin WG., Jr. The von Hippel-Lindau protein, vascular endothelial growth factor, and kidney cancer. N Engl J Med. 2003;349:419–21. doi: 10.1056/NEJMp030061. [DOI] [PubMed] [Google Scholar]
- 12.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 13.Gati A, Da Rocha S, Guerra N, Escudier B, Moretta A, Chouaib S, et al. Analysis of the natural killer mediated immune response in metastatic renal cell carcinoma patients. Int J Cancer. 2004;109:393–401. doi: 10.1002/ijc.11730. [DOI] [PubMed] [Google Scholar]
- 14.Igarashi T, Wynberg J, Srinivasan R, Becknell B, McCoy JP, Jr., Takahashi Y, et al. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 2004;104:170–7. doi: 10.1182/blood-2003-12-4438. [DOI] [PubMed] [Google Scholar]
- 15.Schleypen JS, Von Geldern M, Weiss EH, Kotzias N, Rohrmann K, Schendel DJ, et al. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int J Cancer. 2003;106:905–12. doi: 10.1002/ijc.11321. [DOI] [PubMed] [Google Scholar]
- 16.Perier A, Fregni G, Wittnebel S, Gad S, Allard M, Gervois N, et al. Mutations of the von Hippel-Lindau gene confer increased susceptibility to natural killer cells of clear-cell renal cell carcinoma. Oncogene. 2011;30:2622–32. doi: 10.1038/onc.2010.638. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov SV, Salnikow K, Ivanova AV, Bai L, Lerman MI. Hypoxic repression of STAT1 and its downstream genes by a pVHL/HIF-1 target DEC1/STRA13. Oncogene. 2007;26:802–12. doi: 10.1038/sj.onc.1209842. [DOI] [PubMed] [Google Scholar]
- 18.Gobin SJ, van den Elsen PJ. Transcriptional regulation of the MHC class Ib genes HLA-E, HLA-F, and HLA-G. Hum Immunol. 2000;61:1102–7. doi: 10.1016/S0198-8859(00)00198-1. [DOI] [PubMed] [Google Scholar]
- 19.Aptsiauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol. 2007;601:123–31. doi: 10.1007/978-0-387-72005-0_13. [DOI] [PubMed] [Google Scholar]
- 20.Luo L, Lu J, Wei L, Long D, Guo JY, Shan J, et al. The role of HIF-1 in up-regulating MICA expression on human renal proximal tubular epithelial cells during hypoxia/reoxygenation. BMC Cell Biol. 2010;11:91. doi: 10.1186/1471-2121-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei L, Lu J, Feng L, Long D, Shan J, Li S, et al. HIF-1alpha accumulation upregulates MICA and MICB expression on human cardiomyocytes and enhances NK cell cytotoxicity during hypoxia-reoxygenation. Life Sci. 2010;87:111–9. doi: 10.1016/j.lfs.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Baron F, Turhan AG, Giron-Michel J, Azzarone B, Bentires-Alj M, Bours V, et al. Leukemic target susceptibility to natural killer cytotoxicity: relationship with BCR-ABL expression. Blood. 2002;99:2107–13. doi: 10.1182/blood.V99.6.2107. [DOI] [PubMed] [Google Scholar]
- 23.Cebo C, Voutsadakis IA, Da Rocha S, Bourhis JH, Jalil A, Azzarone B, Turhan AG, Chelbi-Alix M, Chouaib S, Caignard A. Altered IFNgamma signaling and preserved susceptibility to activated natural killer cell-mediated lysis of BCR/ABL targets. Cancer Res. 2005;65:2914–20. doi: 10.1158/0008-5472.CAN-04-1932. [DOI] [PubMed] [Google Scholar]
- 24.Cebo C, Da Rocha S, Wittnebel S, Turhan AG, Abdelali J, Caillat-Zucman S, et al. The decreased susceptibility of Bcr/Abl targets to NK cell-mediated lysis in response to imatinib mesylate involves modulation of NKG2D ligands, GM1 expression, and synapse formation. J Immunol. 2006;176:864–72. doi: 10.4049/jimmunol.176.2.864. [DOI] [PubMed] [Google Scholar]
- 25.Terme M, Borg C, Guilhot F, Masurier C, Flament C, Wagner EF, et al. BCR/ABL promotes dendritic cell-mediated natural killer cell activation. Cancer Res. 2005;65:6409–17. doi: 10.1158/0008-5472.CAN-04-2675. [DOI] [PubMed] [Google Scholar]
- 26.Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–82. doi: 10.1101/gad.1437206. [DOI] [PubMed] [Google Scholar]
- 27.Pierson BA, Miller JS. The role of autologous natural killer cells in chronic myelogenous leukemia. Leuk Lymphoma. 1997;27:387–99. doi: 10.3109/10428199709058306. [DOI] [PubMed] [Google Scholar]
- 28.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99:3661–7. doi: 10.1182/blood.V99.10.3661. [DOI] [PubMed] [Google Scholar]
- 29.Kiladjian JJ, Bourgeois E, Lobe I, Braun T, Visentin G, Bourhis JH, et al. Cytolytic function and survival of natural killer cells are severely altered in myelodysplastic syndromes. Leukemia. 2006;20:463–70. doi: 10.1038/sj.leu.2404080. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Iglesias T, Del Toro-Arreola A, Albarran-Somoza B, Del Toro-Arreola S, Sanchez-Hernandez PE, Ramirez-Duenas MG, et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9:186. doi: 10.1186/1471-2407-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fregni G, Perier A, Pittari G, Jacobelli S, Sastre X, Gervois N, et al. Unique Functional Status of Natural Killer Cells in Metastatic Stage IV Melanoma Patients and Its Modulation by Chemotherapy. Clin Cancer Res. 2011;17:2628–37. doi: 10.1158/1078-0432.CCR-10-2084. [DOI] [PubMed] [Google Scholar]
- 32.Konjević G, Mirjacic Martinovic K, Vuletic A, Jovic V, Jurisic V, Babovic N, et al. Low expression of CD161 and NKG2D activating NK receptor is associated with impaired NK cell cytotoxicity in metastatic melanoma patients. Clin Exp Metastasis. 2007;24:1–11. doi: 10.1007/s10585-006-9043-9. [DOI] [PubMed] [Google Scholar]
- 33.Markel G, Seidman R, Besser MJ, Zabari N, Ortenberg R, Shapira R, et al. Natural killer lysis receptor (NKLR)/NKLR-ligand matching as a novel approach for enhancing anti-tumor activity of allogeneic NK cells. PLoS ONE. 2009;4:e5597. doi: 10.1371/journal.pone.0005597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–22. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schleypen JS, Baur N, Kammerer R, Nelson PJ, Rohrmann K, Grone EF, et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin Cancer Res. 2006;12:718–25. doi: 10.1158/1078-0432.CCR-05-0857. [DOI] [PubMed] [Google Scholar]
- 36.Wittnebel S, Da Rocha S, Giron-Michel J, Jalil A, Opolon P, Escudier B, et al. Membrane-bound interleukin (IL)-15 on renal tumor cells rescues natural killer cells from IL-2 starvation-induced apoptosis. Cancer Res. 2007;67:5594–9. doi: 10.1158/0008-5472.CAN-06-4406. [DOI] [PubMed] [Google Scholar]
- 37.Kornstein MJ, Stewart R, Elder DE. Natural killer cells in the host response to melanoma. Cancer Res. 1987;47:1411–2. [PubMed] [Google Scholar]
- 38.Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863–75. doi: 10.1002/cncr.23239. [DOI] [PubMed] [Google Scholar]
- 39.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–22. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 40.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011;17:700–7. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 41.Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. 2011;17:678–89. doi: 10.1158/1078-0432.CCR-10-2173. [DOI] [PubMed] [Google Scholar]
- 42.Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2001;121:1058–63. doi: 10.1067/mtc.2001.113026. [DOI] [PubMed] [Google Scholar]
- 43.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–8. doi: 10.1016/S0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 44.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–83. doi: 10.1002/(SICI)1097-0142(20000201)88:3<577::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 45.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–8. doi: 10.1002/(SICI)1097-0142(19970615)79:12<2320::AID-CNCR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 46.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 47.McKay K, Moore PC, Smoller BR, Hiatt KM. Association between natural killer cells and regression in melanocytic lesions. Hum Pathol. 2011 doi: 10.1016/j.humpath.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 48.Esendagli G, Bruderek K, Goldmann T, Busche A, Branscheid D, Vollmer E, et al. Malignant and non-malignant lung tissue areas are differentially populated by natural killer cells and regulatory T cells in non-small cell lung cancer. Lung Cancer. 2008;59:32–40. doi: 10.1016/j.lungcan.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 49.Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 50.Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, et al. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol. 2009;183:4921–30. doi: 10.4049/jimmunol.0901226. [DOI] [PubMed] [Google Scholar]
- 51.Blümke K, Bilkenroth U, Schmidt U, Melchior A, Fussel S, Bartel F, et al. Detection of circulating tumor cells from renal carcinoma patients: experiences of a two-center study. Oncol Rep. 2005;14:895–9. [PubMed] [Google Scholar]
- 52.Rao C, Bui T, Connelly M, Doyle G, Karydis I, Middleton MR, et al. Circulating melanoma cells and survival in metastatic melanoma. Int J Oncol. 2011;38:755–60. doi: 10.3892/ijo.2011.896. [DOI] [PubMed] [Google Scholar]
- 53.Sanislo L, Vertakova-Krakovska B, Kuliffay P, Brtko J, Galbava A, Galbavy S. Detection of circulating tumor cells in metastatic breast cancer patients. Endocr Regul. 2011;45:113–24. doi: 10.4149/endo_2011_03_113. [DOI] [PubMed] [Google Scholar]
- 54.Sergeant G, Penninckx F, Topal B. Quantitative RT-PCR detection of colorectal tumor cells in peripheral blood–a systematic review. J Surg Res. 2008;150:144–52. doi: 10.1016/j.jss.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Grégoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, et al. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–82. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 57.Raffaghello L, Prigione I, Airoldi I, Camoriano M, Levreri I, Gambini C, et al. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia. 2004;6:558–68. doi: 10.1593/neo.04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunker K, Schlaf G, Bukur J, Altermann WW, Handke D, Seliger B. Expression and regulation of non-classical HLA-G in renal cell carcinoma. Tissue Antigens. 2008;72:137–48. doi: 10.1111/j.1399-0039.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- 59.Ibrahim EC, Guerra N, Lacombe MJ, Angevin E, Chouaib S, Carosella ED, et al. Tumor-specific up-regulation of the nonclassical class I HLA-G antigen expression in renal carcinoma. Cancer Res. 2001;61:6838–45. [PubMed] [Google Scholar]
- 60.Lesport E, Baudhuin J, LeMaoult J, Sousa S, Doliger C, Carosella ED, et al. Human melanoma cell secreting human leukocyte antigen-G5 inhibit natural killer cell cytotoxicity by impairing lytic granules polarization toward target cell. Hum Immunol. 2009;70:1000–5. doi: 10.1016/j.humimm.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 61.Paul P, Rouas-Freiss N, Khalil-Daher I, Moreau P, Riteau B, Le Gal FA, et al. HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci USA. 1998;95:4510–5. doi: 10.1073/pnas.95.8.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LeMaoult J, Zafaranloo K, Le Danff C, Carosella ED. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J. 2005;19:662–4. doi: 10.1096/fj.04-1617fje. [DOI] [PubMed] [Google Scholar]
- 63.Campoli M, Ferrone S. Tumor escape mechanisms: potential role of soluble HLA antigens and NK cells activating ligands. Tissue Antigens. 2008;72:321–34. doi: 10.1111/j.1399-0039.2008.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allard M, Oger R, Vignard V, Percier JM, Fregni G, Perier A, et al. Serum Soluble HLA-E in Melanoma: A New Potential Immune-Related Marker in Cancer. PLoS ONE. 2011;6:e21118. doi: 10.1371/journal.pone.0021118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–80. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 66.Budagian V, Bulanova E, Orinska Z, Pohl T, Borden EC, Silverman R, et al. Reverse signaling through membrane-bound interleukin-15. J Biol Chem. 2004;279:42192–201. doi: 10.1074/jbc.M403182200. [DOI] [PubMed] [Google Scholar]
- 67.Khawam K, Giron-Michel J, Gu Y, Perier A, Giuliani M, Caignard A, et al. Human renal cancer cells express a novel membrane-bound interleukin-15 that induces, in response to the soluble interleukin-15 receptor alpha chain, epithelial-to-mesenchymal transition. Cancer Res. 2009;69:1561–9. doi: 10.1158/0008-5472.CAN-08-3198. [DOI] [PubMed] [Google Scholar]
- 68.Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA. 2003;100:4120–5. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hagemann T, Bozanovic T, Hooper S, Ljubic A, Slettenaar VI, Wilson JL, et al. Molecular profiling of cervical cancer progression. Br J Cancer. 2007;96:321–8. doi: 10.1038/sj.bjc.6603543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mansfield AS, Holtan SG, Grotz TE, Allred JB, Jakub JW, Erickson LA, et al. Regional immunity in melanoma: immunosuppressive changes precede nodal metastasis. Mod Pathol. 2011;24:487–94. doi: 10.1038/modpathol.2010.227. [DOI] [PubMed] [Google Scholar]
- 71.Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA. 2009;106:20847–52. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nardin A, Wong WC, Tow C, Molina TJ, Tissier F, Audebourg A, et al. Dacarbazine promotes stromal remodeling and lymphocyte infiltration in cutaneous melanoma lesions. J Invest Dermatol. 2011;131:1896–905. doi: 10.1038/jid.2011.128. [DOI] [PubMed] [Google Scholar]
- 73.Krusch M, Salih J, Schlicke M, Baessler T, Kampa KM, Mayer F, et al. The kinase inhibitors sunitinib and sorafenib differentially affect NK cell antitumor reactivity in vitro. J Immunol. 2009;183:8286–94. doi: 10.4049/jimmunol.0902404. [DOI] [PubMed] [Google Scholar]
- 74.Borg C, Terme M, Taieb J, Menard C, Flament C, Robert C, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004;114:379–88. doi: 10.1172/JCI21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beano A, Signorino E, Evangelista A, Brusa D, Mistrangelo M, Polimeni MA, et al. Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med. 2008;6:25. doi: 10.1186/1479-5876-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonilla F, Alvarez-Mon M, Merino F, Giron JA, Menendez JL, Espana P, et al. Natural killer activity in patients with breast cancer. Eur J Gynaecol Oncol. 1990;11:103–9. [PubMed] [Google Scholar]
- 78.Mozaffari F, Lindemalm C, Choudhury A, Granstam-Bjorneklett H, Helander I, Lekander M, et al. NK-cell and T-cell functions in patients with breast cancer: effects of surgery and adjuvant chemo- and radiotherapy. Br J Cancer. 2007;97:105–11. doi: 10.1038/sj.bjc.6603840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]