Abstract
Human prostate cancer frequently metastasizes to bone marrow. What defines the cellular and molecular predilection for prostate cancer to metastasize to bone marrow is not well understood. CD4+CD25+ regulatory T (Treg) cells contribute to self-tolerance and tumor immune pathology. We now show that functional Treg cells are increased in the bone marrow microenvironment in prostate cancer patients with bone metastasis, and that CXCR4/CXCL12 signaling pathway contributes to Treg cell bone marrow trafficking. Treg cells exhibit active cell cycling in the bone marrow, and bone marrow dendritic cells express high levels of receptor activator of NFκB (RANK), and promote Treg cell expansion through RANK and its ligand (RANKL) signals. Furthermore, Treg cells suppress osteoclast differentiation induced by activated T cells and M-CSF, adoptive transferred Treg cells migrate to bone marrow, and increase bone mineral intensity in the xenograft mouse models with human prostate cancer bone marrow inoculation. In vivo Treg cell depletion results in reduced bone density in tumor bearing mice. The data indicates that bone marrow Treg cells may form an immunosuppressive niche to facilitate cancer bone metastasis and contribute to bone deposition, the major bone pathology in prostate cancer patients with bone metastasis. These findings mechanistically explain why Treg cells accumulate in the bone marrow, and demonstrate a previously unappreciated role for Treg cells in patients with prostate cancer. Thus, targeting Treg cells may not only improve anti-tumor immunity, but also ameliorate bone pathology in prostate cancer patients with bone metastasis.
Keywords: bone marrow, CXCL12, CXCR4, dendritic cell, prostate cancer, RANK, RANKL, regulatory T cell
Introduction
Bone marrow has long been known to be a primarily hematopoietic organ. However, there has been a growing realization regarding the importance of the bone marrow in immunity.1 For example, long-lived, antibody-secreting plasma cells reside in bone marrow.2 Further, a number of reports have shown that functional memory T cells exist in bone marrow.3,4 Bone marrow can serve as a site for naïve tumor associated antigen (TAA)-specific T cell priming.3-7 Interestingly, TAA-specific T cells isolated from bone marrow in tumor bearing mice and cancer patients are functional in vitro and are able to prevent tumor growth upon being transferred to another host. These observations indicate that the TAA-specific T cells are functionally suppressed in the bone marrow.4-10 In line with this, we and others have previously shown that in the homeostatic situation, mouse bone marrow harbors high levels of functional CD4+Foxp3+ regulatory T (Treg) cells.9,10 This suggests that Treg cells may form an immune suppressive niche in bone marrow, and this niche is physiologically important to keep potential inflammation at bay in this important and unique hematopoietic organ. However, it is unknown if bone marrow Treg cell compartment is altered in cancer patients with bone metastasis, and if so, what are the underlying cellular and molecular mechanisms?
In keeping these questions in mind, we further raise the point, in addition to immunosuppression, do bone marrow Treg cells affect bone pathology mediated by tumor bone metastasis in humans? It is well known that many human cancers including prostate cancer frequently metastasize to the bone marrow. However, the mechanisms that account for the cellular and molecular predilection for tumors to metastasize to bone marrow are not well defined. It is generally thought that tumor cells play major roles in bone pathology induced by tumor bone metastasis. It is also unknown whether immune cells including Treg cells have an impact on bone immunopathology in prostate cancer patients with bone metastasis. In order to preliminarily address these questions, we hypothesized that tumor bone marrow environmental cells provide cellular and molecular signals for Treg cell accumulation, and that high levels of Treg cells contribute to bone immunopathology in tumor bone marrow metastasis. We tested these hypotheses in patients with prostate cancer and in animal models. Our results demonstrate that high levels of Treg cells accumulate in the bone marrow in prostate cancer patients with bone metastasis, and the interaction between dendritic cells (DCs) and Treg cells promote Treg expansion, and in turn Treg cells suppress osteoclast differentiation and function, contribute to bone deposition, the predominant pathology of cancer bone metastasis.
Results
High levels of functional Treg cells in prostate cancer associated bone marrow
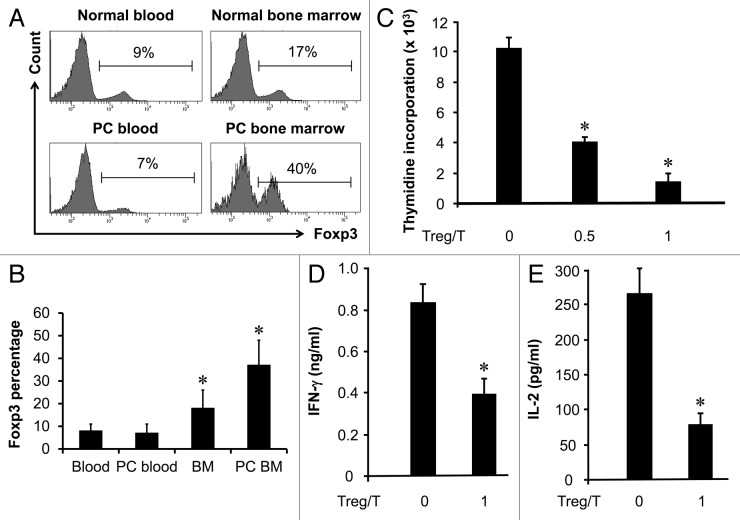
Prostate cancer frequently metastasizes to bone marrow. Recent reports suggest that bone marrow is a site for important T cell events.6,7,11 We previously observed high levels of Treg cells in normal bone marrow.9 We now examined the Treg compartment in patients with prostate cancer. We first showed that the fraction of Treg cells in CD4+ T cells was significantly higher in bone marrow in patients with prostate cancer without bone metastasis than that in normal blood and blood from patients with prostate cancer (n = 8, p < 0.01 for each). Interestingly, the levels of Treg cells were significantly higher in bone marrow from patients with prostate cancer bone marrow metastasis (37 ± 11%, n = 6, p < 0.001) than that in the bone marrow in prostate cancer patients without bone marrow metastasis (18 ± 8%, n = 6) (Fig. 1A and B). Thus, high levels of Treg cells are found in prostate cancer bone marrow metastasis.
Figure 1. High levels of functional Treg cells in prostate cancer associated bone marrow. (A and B) High levels of CD4+Foxp3+ cells in bone marrow. Bone marrow and blood single cell suspensions were made from normal age-matched male donors (n = 8) and patients with prostate cancer (PC) (n = 6). The cells were subject to staining with anti-CD3, anti-CD4 and anti-FOXP3. Cells were analyzed with multiple color staining. The histogram showed the percentage of FOXP3+ cells in CD3+CD4+ (A). One of 6–8 donors is shown. Results are expressed as the mean values ± SD (B) *p < 0.01. PC, prostate cancer. (C–E) Bone marrow Treg cells are functional. CD3+CD4+CD25high cells were sorted with FACSAria, to high purity (> 97%), from bone marrow in patients with prostate cancer. Autologous CD3+CD25- blood T cells (5 × 104/ml) were stimulated with anti-CD3 (5 μg/ml) for 3 d with or without different numbers of Treg cells. T cell proliferation was detected by [3H] thymidine incorporation (C) and the production of interferon-γ (D) and IL-2 (E) were detected by ELISA. (n = 5; *p < 0.01). Three patients in Gleason grade 4 and 3 in grade 5, and 2 patients in stage T3a, 2 in T3b and 2 in M1.
We further tested if Treg cells associated with prostate cancer bone metastasis were functional. To this end, we employed a well-established assay that assesses the ability of Treg cells to mediate T cell suppression.12-15 We showed that bone marrow Treg cells from patients with prostate cancer inhibited T cell proliferation in a dose dependent manner (Fig. 1C). Bone marrow Treg cells also suppressed IFNγ and IL-2 production of T cells (Fig. 1D and E) (n = 5, *p < 0.01). Thus, Treg cells in bone marrow in patients with prostate cancer are functional regulatory T cells.
Treg cells migrate toward bone marrow via CXCR4/CXCL12 signaling pathway
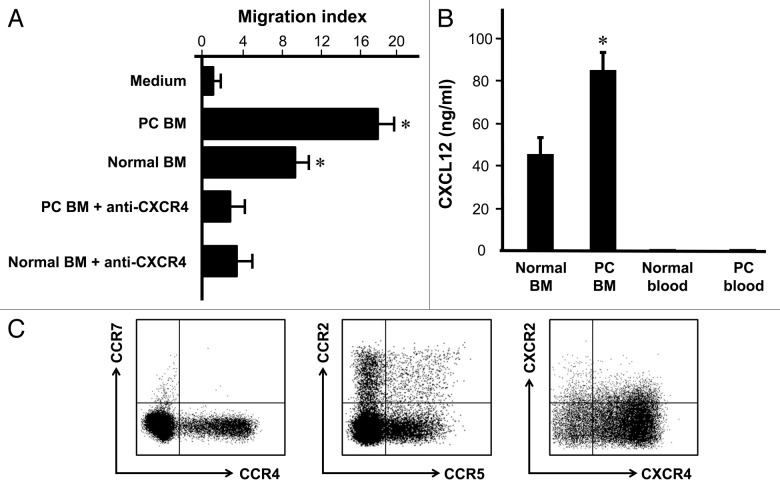
We next examined why Treg cells were accumulated in bone marrow associated with prostate cancer. The first possibility is that Treg cells efficiently traffic to bone marrow in patients with prostate cancer. We showed that Treg cells isolated from bone marrow associated with prostate cancer migrated toward human bone marrow fluid (Fig. 2A), and anti-human CXCR4 significantly decreased this migration (Fig. 2A). Further, we showed that human bone marrow produced a high level of CXCL12, the ligand for CXCR4. The levels of CXCL12 were higher in prostate cancer patients with bone marrow metastasis than normal donors (Fig. 2B). In further support, variable expression levels of several common chemokine receptors including CCR2, CCR4, CCR5, CCR7 and CXCR2 were detected in the bone marrow Treg cells, however, these Treg cells expressed high levels of CXCR4 (Fig. 2C). Therefore, the data suggests that Treg cells migrate to prostate cancer associated bone marrow by CXCR4/CXCL12 signaling pathway.
Figure 2. Treg migrate toward bone marrow through CXCR4/CXCL12. (A) Bone marrow Treg were subject to migrating with normal human bone marrow fluid or human bone marrow fluid from patients with bone marrow metastasis. Anti-CXCR4 and isotype were added in the migration assay (n = 6; *p < 0.01; compared with medium or anti-CXCR4). (B) Human bone marrow expressed high level of CXCL12. CXCL12 was measured by ELISA in bone marrow fluid and blood from normal donors (n = 6) and patients with prostate cancer (n = 5). Results are expressed as mean ± SD *p < 0.01. (C) Bone marrow Treg cells expressed high levels of CXCR4. Bone marrow cells were stained with anti-CCR2, CCR4, CCR5, CCR7, CXCR2 and CXCR4 and Treg cell markers, and analyzed with LSR II. The chemokine receptor expression was determined by gating on CD3+CD4+Foxp3+ cells. One of 6 representatives is shown.
Treg cells actively expand in tumor associated bone marrow
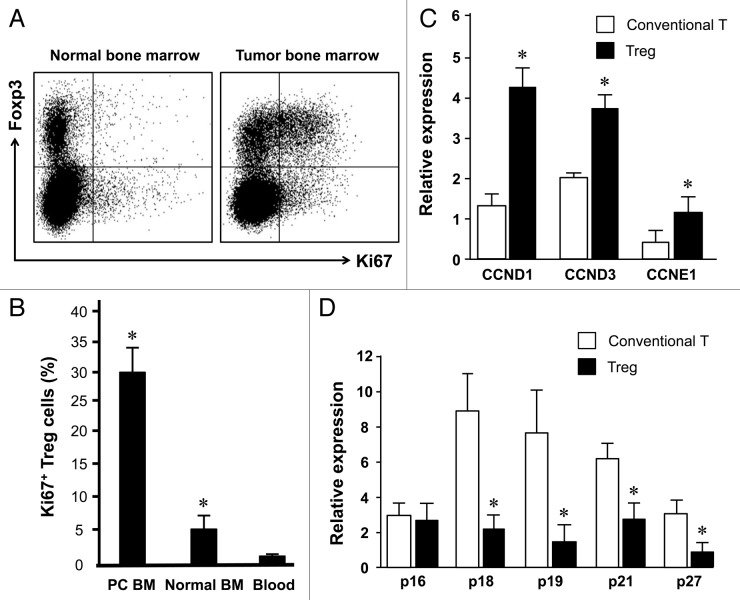
After examining the possibility of Treg cell bone marrow trafficking, we further analyzed whether Treg cells were actively expanded in the tumor associated bone marrow in patients with prostate cancer. We showed that normal bone marrow Treg cells contained up to 7% Ki67 expressing cells whereas there were 1% Ki67+ Treg cells in peripheral blood. Interestingly, there were 32% Ki67+ bone marrow Treg cells in patients with prostate cancers (Fig. 3A and B). In accord with this, the expression of multiple cyclin genes was higher in bone marrow Treg cells than conventional T cells (Fig. 3C). On the contrary, the expression of multiple CDK inhibitors was lower in bone marrow Treg cells than conventional T cells (Fig. 3D). The data suggests that Treg cells in the tumor associated bone marrow selectively experience a pathological expansion.
Figure 3. Treg cells actively expand in tumor associated bone marrow. Blood and bone marrow were immediately stained with anti-CD3, anti-CD4, anti-FOXP3 and anti-Ki67 and were analyzed with LSR II. (A) Representative dot plots showed Ki67 expression in normal vs. tumor bone marrow Foxp3+ Treg cells. (B) Results are expressed as the percentage of Ki67 expressing cells in CD4+FOXP3+CD3+ cells (Treg cells) (mean ± SEM) (n = 6–8, *, Tumor bone marrow compared with normal bone marrow, and other compartments, p < 0.001). (C and D) Bone marrow Treg cells expressed different levels of cell cycling genes. Expression of multiple cyclin genes (C) and CDK inhibitors (D) was quantified in bone Treg cells and conventional T cells by real-time PCR. Results are expressed as mean ± SD n = 5, *p < 0.01.
RANK+ DCs induce Treg cell expansion in tumor associated bone marrow
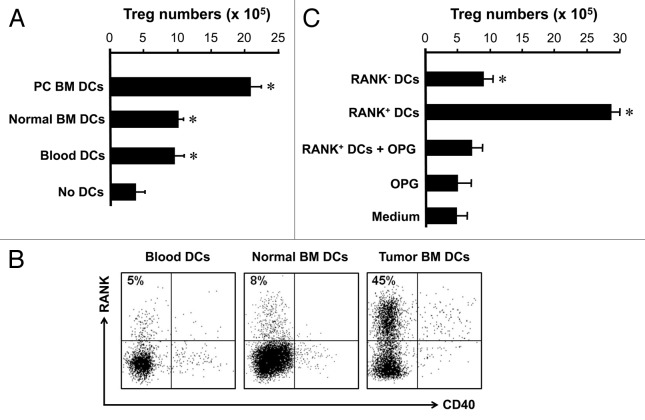
We further investigated the cellular mechanism by which Treg cells were expanding in the tumor associated bone marrow. We hypothesized that tumor associated DCs might induce Treg cells expansion. We sorted blood Treg cells and cocultured with DCs from different sources. We showed that regardless of their source, DCs efficiently induced Treg cell expansion. However, tumor associated bone marrow DCs were superior to inducing Treg cell expansion as compared with DCs from normal bone marrow and blood (Fig. 4A). Interestingly, tumor bone marrow-associated DCs highly expressed RANK. DCs from normal blood or bone marrow without tumor in the bone marrow expressed little RANK (Fig. 4B). These DCs expressed similar levels of CD40 (Fig. 4B). To study the effects of RANK+ DCs on Treg cell expansion, we sorted RANK+ and RANK- DCs from tumor associated bone marrow, and cultured with Treg cells. We observed that RANK+ and RANK- DCs induced Treg cell expansion, however, RANK+ DCs were more efficient than RANK- DCs to induce Treg cell expansion (Fig. 4C). Furthermore, blocking RANK/RANKL signaling pathway with recombinant osteoprotegerin (OPG) ablated the stimulatory effects of RANK+ DCs on Treg cell expansion (Fig. 4C). These observations suggest that RANK+ DCs may be responsible for Treg cell expansion in patients with prostate cancer.
Figure 4. RANK+ DCs induce Treg cell expansion in tumor associated bone marrow. (A) Prostate cancer associated bone marrow DCs stimulated Treg cell expansion. Bone marrow lin-CD11c+ DCs were sorted from human bone marrow, and cultured with blood Treg cells for 4 d as described. The resultant cells were stained with anti-Foxp3. Results are expressed the absolute numbers of Treg cells ± SD n = 5, p < 0.01. (B) Prostate cancer BM DCs expressed RANK. BM cells were stained for DC phenotype and RANK. Results are expressed the percent of RANK+ cells in lin-CD11c+ DCs. One of 5 is shown. (C) Prostate cancer BM DCs mediated Treg cell expansion through RANK. RANK+ and RANK- DCs were sorted and cultured with Treg cells as described (A) in the absence or presence of OPG. Cells were collected for detecting Foxp3 with LSR by gating on CD3+CD4+ viable cells. Results are expressed as the absolute numbers of Foxp3+ cells ± SD n = 5, *p < 0.01.
Treg cells suppress osteoclast differentiation and function
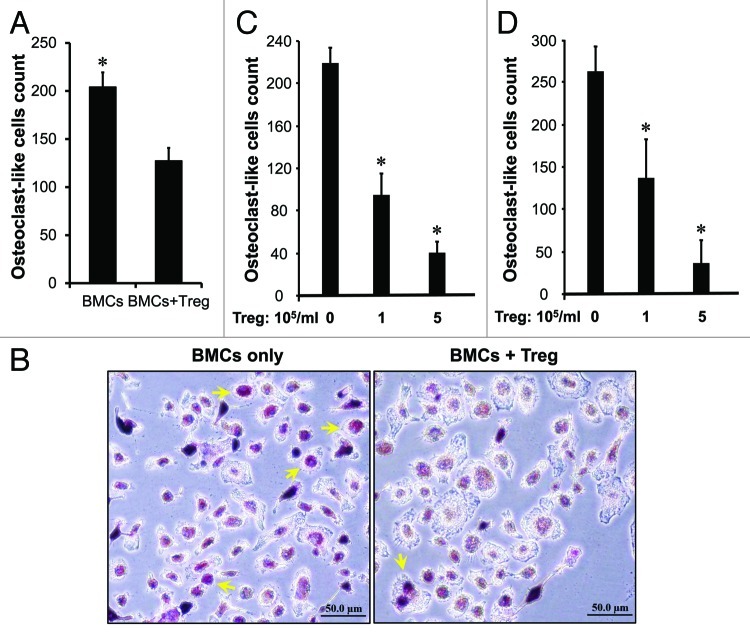
In addition to immune suppression (Fig. 1), we hypothesized that high levels of Treg cells in the tumor associated bone marrow might affect bone pathology in prostate cancer. To test this, we sorted Treg cells and tested their potential roles in osteoclast differentiation induced by recombinant RANKL and M-CSF. As expected, Treg cells reduced the numbers of tartrate-resistant acid phosphatase (TRAP)-positive cells induced by RANKL (Fig. 5A and B) and M-CSF in a dose dependent manner (Fig. 5C). As activated CD8+ T cells were observed in bone marrow in patients with cancer,3-6 we further tested if Treg cells affected T cell-mediated osteoclast differentiation. We observed that TRAP+ cells were increased in CD8+ T cell coculture as compared with no T cells. Treg cells reduced the numbers of TRAP+ cells induced by CD8+ T cells in a dose dependent manner (Fig. 5D). The data indicates that Treg cells suppress osteoclast differentiation.
Figure 5. Treg cells suppress osteoclast differentiation in vitro. (A–B) Treg cells suppressed osteoclast differentiation mediated by RANKL. Mouse bone marrow cells (BMCs) were cultured as described with RANKL in the absence or presence of Treg cells. The cultured cells were subject to TRAP staining. TRAP-positive multinucleated (> 3 nuclei) cells were counted. (A) Results are reported as the number of osteoclast (OC)-like cells per coverslip ± SD n = 5, *p < 0.01. (B) Representative images showed osteoclast (OC)-like cells. (C and D) Treg cells suppressed osteoclast differentiation mediated by M-CSF and CD8+ T cells. Mouse bone marrow cells were cultured as described with M-CSF (C) or activated CD8+ T cells (D) in the presence of different concentrations of Treg cells. Results are reported as the number of osteoclast (OC)-like cells per coverslip ± SD n = 6, *p < 0.01.
Treg cells increase bone formation in tumor bearing mice
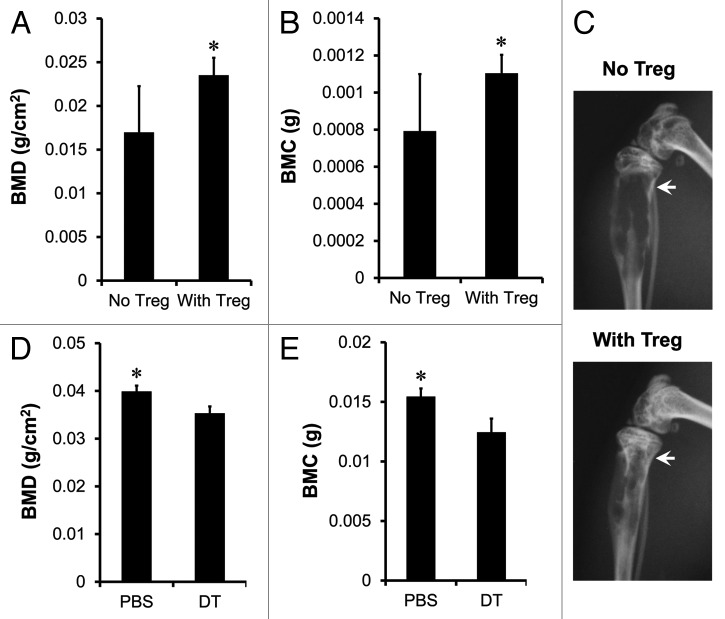
We tested the effects of Treg cells in vivo on bone pathology in our established human prostate cancer chimeric model. Human prostate cancer cells PC-3 were inoculated into tibia of NOD.SCID mice by intratibial injection.16-20 Tumor bone establishment was examined with both in vivo bioluminescence and radiographic imaging. Activated Treg cells were transfused into the mice by intravenous injection seven days after the establishment of bone metastasis. We showed that Treg cells increased bone mineral density (BMD) (Fig. 6A), bone mineral content (BMC) (Fig. 6B) and ameliorated bone destruction of tibial trabeculae (Fig. 6C) compared with control.
Figure 6. Treg cells suppress osteoclast differentiation and function in vivo. (A–C) Human Treg cells suppressed osteoclast differentiation. Prostate cancer bone metastasis was established in NOD.SCID mice with PC-3 intratibial injection. On day 7 after tumor inoculation, mice were intravenously transfused with activated Treg cells. On day 25, BMD (A) and BMC (B) of tibial trabeculae were measured. Representative radiographic images showed the bone destruction of tibial trabeculae (C) on day 25. Results are expressed as mean ± SD n = 6/group, *p < 0.05. (D and E) Mouse Treg cells suppressed osteoclast differentiation. RM1 tumor bone metastasis was established in DEREG mice with tumor intratibial injection. Treg cells were depleted with DT injection. On day 15, BMD (D) and BMC (E) of tibial trabeculae were measured. Results are expressed as mean ± SD n = 5/group, *p < 0.05. DT, diphtheria toxin.
We further investigated the role of Treg cells in bone pathology in immune competent mice. To this end, RM1 tumor was initially established in the Bacterial artificial chromosome (BAC)-transgenic depletion of regulatory T cell (DEREG) mouse model.21 Then, we injected diphtheria toxin to deplete Treg cells. Treg cells were efficiently depleted on day two, and were gradually recovered on day ten in different organs (Fig. S1). Interestingly, the levels of bone marrow Treg cells remained low on day 17 (Fig. S1). We measured BMD and BMC on day 15. We observed that Treg cell depletion resulted in partial but significant reduction of BMD (Fig. 6D) and BMC (Fig. 6E). Altogether, the data indicates that Treg cells may suppress osteoclast differentiation or function in vivo in immune competent mice.
Discussion
In this report, we observed active Treg cell recruitment and expansion in bone marrow of prostate cancer patients with bone metastasis. Furthermore, the bone marrow Treg cells tilt the balance between osteoclast and osteoblast activity, which potentially contributes to osteoblastic bone lesions that characterize prostate cancer. Finally, we have defined that CXCR4/CXCL12 and RANK/RANKL are crucial molecular signaling pathways for Treg cell bone marrow trafficking and expansion, respectively.
Several immune suppressive elements including Treg cells form immunosuppressive networks in the tumor microenvironment.22-26 We have previously demonstrated that under homeostatic situation, the levels of Treg cells in bone marrow are relatively high in healthy human beings as compared with peripheral blood and lymph nodes.9 This observation was confirmed in a FOXP3 bicistronic reporter knock-in mouse model.10 However, it was unknown if and how bone marrow Treg cells are altered in pathological scenarios. We have now observed higher levels of Treg cells in bone marrow in prostate cancer patients with bone metastasis as compared with healthy donors and patients without bone metastasis. Interestingly, prostate cancer cells and bone marrow stromal cells express high levels of CXCL1227-29 and bone marrow Treg cells express CXCR4, and efficiently migrate toward bone marrow through CXCR4/CXCL12 signaling pathway.9 That activated, but not resting Treg cells express high amount of CXCR4, indicates that Treg cells may be activated in the tumor and efficiently traffic to bone marrow, where they exert immune suppression and that immunologically facilitates tumor bone marrow metastasis.
Treg cells in bone marrow with prostate cancer metastasis express high levels of Ki67. This indicates that tumor-associated bone marrow is an organ where Treg cells actively expand. Although active bone marrow trafficking may be one of the reasons that Treg cells accumulate in bone marrow of patients with prostate cancer, extensive Treg cell expansion is an additional cause. We have determined that CXCL12/CXCR4 signaling pathway is responsible for Treg bone marrow migration and we have further explored cellular and molecular mechanisms governing Treg cell expansion in bone marrow. We demonstrated that tumor associated DCs, but not the control counterparts, induce Treg cell expansion. Interestingly, DCs from bone marrow with metastatic tumor selectively and highly expressed RANK and blockade of RANK/RANKL signaling pathway disables the effects of DC-mediated Treg cell expansion. Although IL-2 induces Treg cell expansion30-33 and TGFβ promotes Treg cell conversion from naïve T cells,34 our data indicates that the interaction between DCs and Treg cells through RANK/RANKL signaling pathway is an additional signal crucial for Treg cell expansion in specific pathological environments including prostate cancer. In support of our human studies in cancer, it has been reported that RANKL and RANK signals are implicated in Treg cell expansion in mouse models with diabetes35 and UV-induced immune suppression.36 Taken together, to ensure Treg cell bone marrow accumulation and function, bone marrow environmental cells, such as RANK+ DCs provide specific molecular signals for Treg cell expansion and in turn Treg cells mediate immune suppression, and contribute to tumor bone metastasis. Our studies focused on prostate cancer, however, it would also be interesting to examine the relationship between tumor bone metastasis and Treg cells in other human cancer settings including breast cancer.
The formation and remodeling of bone is a complex physiological process relying on a strict balance between the resorptive activity of osteoclasts and the synthesis of bone matrix by osteoblasts. RANKL/RANK is an important molecular signal pathway controlling bone remodeling and is essential for the development and activation of bone-resorbing osteoclasts. Normal bone homeostasis is achieved by a balance between the bone resorbing effects of RANKL and its natural decoy receptor OPG.37 Inhibition of RANKL activity with either OPG or a soluble RANK can inhibit prostate cancer growth including in bone.19 RANKL antibody (Denosumb) was recently approved to inhibit skeletal-related events in prostate cancer.38,39 The function of RANKL/RANK interactions outside the bone cells and tumor cells is not well defined in the context of prostate cancer. Activated T cells produce RANKL and can directly trigger osteoclastogenesis in vitro through RANK, while systemic activation of T cells in vivo results in a RANKL-mediated increase in osteoclastogenesis and bone loss.40 Although RANKL/RANK pathway is involved in Treg cell expansion in the cancer metastasis to bone marrow, we show that Treg cells inhibit osteoclast differentiation mediated by activated T cells or M-CSF and RANKL, and directly increase bone mineral density in prostate cancer. This is consistent with the observation in mouse autoimmune disease models.41,42 It remains to be defined how Treg cells do so in vivo in patients with prostate cancer. It is generally thought that prostate cancer cells play a major role in bone pathology in prostate cancer patients with bone metastasis. Our results indicate that Treg cells directly and indirectly suppress osteoclast differentiation and function, contribute to bone deposition, the important pathology of cancer bone metastasis. Given the high amount of Treg cells in prostate cancer associated bone marrow, our data provides important novel insight into osteoimmunology. As CXCR4/CXCL12 and RANKL/RANK signaling pathways are involved in immune and tumor pathology in prostate cancer bone metastasis, it is suggested that the combinatorial blockade of these two signaling pathways would be a valid option to treat patients with prostate cancer, and to ameliorate prostate cancer bone metastasis.
In summary, bone marrow in patients with prostate cancer harbors a high prevalence of functional Treg cells due to active recruitment and expansion. Treg cells interact with bone environmental cells, and this interaction provides an immune and biological environment that could favor tumor retention, growth and invasion. Thus, bone marrow Treg cells contribute to tumor bone metastatic pathology. Targeting Treg cell bone marrow recruitment and expansion may be therapeutically meaningful to treat cancer patients with bone metastasis.
Patients and Materials and Methods
Human subjects
Human bone marrow aspiration was obtained from the posterior iliac crest based on standard clinical procedure (http://www.mayoclinic.com/health/bone-marrow), and was placed in an anticoagulant tube. Certain amounts of bone marrow aspirates were used to make the appropriate slides for pathology. Most of the bone marrow aspirates were frozen in -80°C for further functional experiments as we described previously.9 The cellular particles were diluted into single cell suspension with flow cytometry buffer for phenotyping and functional experiments as we described.9 Peripheral blood was from commercial Buffy coat cells. Donors gave written, informed consent. The study was approved by the Local Institutional Review Boards.
Mice
Bacterial artificial chromosome (BAC)-transgenic depletion of regulatory T cell (DEREG) mice on the C57BL/6 background were described previously21 and kept in specific pathogen-free conditions. NOD.SCID mice and wild type C57BL/6 mice were purchased from The Jackson Laboratory. Animal protocol was approved by the Unit for Laboratory Animal Medicine of University of Michigan and was conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Cell lines
Human prostate cancer cell line PC-3 and murine prostate cancer cell line RM143 were used in this study. PC-3 cells were stably transfected with a lux reporter vector which contains a constitutively active promoter driving luciferase expression.19,44 PC-3 and RM1 cells were cultured in vitro in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 100U/ml penicillin, 100μg/ml streptomycin and regularly passaged by trypsinization.
Flow cytometry analysis
T cells were first stained extracellularly with specific antibodies against human CD3 and CD4 (BD Biosciences), then were fixed and permeabilized with Perm/Fix solution (eBioscience) and finally were stained intracellularly with anti-Ki67, anti-IL-2, anti-IFNγ (BD Biosciences) and anti-Foxp3 antibodies (eBioscience). Human dendritic cells were stained with lineage markers, anti-CD11c and anti-RANK antibodies (R&D System). Samples were acquired on a LSR II (BD Biosciences) and data were analyzed with DIVA software (BD Biosciences).
T cell proliferation and cytokine expression
Primary T cells were stimulated with 2.5 µg/mL anti-CD3 and 1.25 µg/mL anti-CD28 monoclonal antibody (BD Biosciences) for 4 d in the presence of different concentrations of Treg cells. T cell proliferation was defined by thymidine incorporation on day 3. T cell cytokines were determined by LSR II.
DC and Treg cell coculture
Lin-CD11c+ DCs were cultured with sorted CD4+CD25high T cells45,46 in the presence of 2.5 µg/mL anti-CD3 (BD Biosciences) and 5 ng/ml IL-2 (R&D System) for 4 d. T cell phenotype was determined by LSR II. The absolute numbers of Foxp3+ cells were recorded.
Migration assay
Migration was assessed as we described using human CD4+CD25high Treg cells (5–20 × 104).14,45 Treg cells were induced to migrate with 100 ng/ml recombinant human CXCL12 (R&D System), or human bone marrow fluid. Treg cells were incubated with 500 ng/ml mouse anti-human-CXCR4 (R&D System) for 2 h as indicated. Identity of migrating Treg cells was further confirmed by flow cytometry analysis for CD3, CD4 and Foxp3 expression. Migration was expressed as a percentage of migrated cells after subtracting the spontaneous migration (Migration index).14,45
Quantitative real-time PCR and ELISA
General quantitative real-time PCR was done as described.45,47 The expression of detected genes was calculated as the relative expression to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The information of the primers was listed in Table S1. CXCL12 protein in the bone marrow fluids was detected by ELISA (R&D System).
Osteoclast differentiation assay
Osteoclast differentiation assay was performed as we described.19 Briefly, bone marrow cells (BMCs) were obtained from C57BL/6 mice by flushing the medullary cavities of tibia and femur of the bilateral hind limbs with Dulbecco modified Eagle's medium (DMEM). These cells were cultured with 10 ng/ml M-CSF (R&D System), 50 ng/ml recombinant soluble RANKL (PeproTech) for 3 d in the presence of 20 ng/ml recombinant mouse IL-2 (R&D System). Different concentrations of CD8+ T cells or Treg cells (1 × 106/ml) were pre-activated with 2.5 μg/ml anti-CD3 and 1.25 μg/ml anti-CD28 monoclonal mouse antibodies (BD Biosciences) for 12 h as we described,48 and then added into the BMCs culture for 3 d. The resultant cells were evaluated in quadruplicates. Osteoclast-like cells were identified as tartrate-resistant acid phosphatase (TRAP)-positive multinucleated (> 3 nuclei) cells with leukocyte acid phosphatase kit (Sigma) as described.19,49
In vivo bioluminescent imaging and radiography
In vivo Bioluminescent Imaging was performed with an IVIS Spectrum imaging system (Xenogen Corporation, Alameda, CA) and high-resolution radiographic images of mice were obtained using a Faxitron laboratory radiography system LX-60 (Faxitron X-ray Corporation, Wheeling, IL) at 30 KVp for 10 sec.19,44,49
Human chimeric model
The stable transfectant PC-3 cells used in in vivo study were first tested in vitro by adding 20 μl luciferin (40 mg/ml) into the 96-well plate. These PC-3 cells (5 × 105) were intratibially injected into 4–5-week-old, male NOD.SCID mice.16-20 Tumor bone establishment was examined with both the in vivo bioluminescence intensity on a cryogenically cooled imaging system (Xenogen Corporation, Alameda, CA) coupled to a data acquisition computer and radiographic imaging. Once tumors were well established in the bone, activated Treg cells (6 × 106)45,47 were injected intravenously into mice on day 7 after human prostate cancer inoculation. On day 25, all the mice were sacrificed and tibiae were harvested and fixed in 10% formalin. BMD and BMC of tibial trabeculae were measured on an Eclipse Peripheral Dexa Scanner (Norland Medical Systems).44
In vivo treg depletion
DEREG mice were injected intratibially with RM1 cells into the right tibia as described.43 Mice were given 1 μg diphtheria toxin (DT) (Merck) diluted in 100μl endotoxin-free PBS or 100 μl endotoxin-free PBS alone by intraperitoneal injection on day 1, 3 and 5. DT treatment efficiently reduced Treg cells in bone marrow (Fig. S1).50 All the mice were sacrificed on day 15 and tibiae were fixed in 10% formalin. BMD and BMC were measured.
Supplementary Material
Supplementary PDF file supplied by authors.
Acknowledgments
This research is supported in part by research grants from the National Institutes of Health CA133620 (W.Z.), CA93900 (E.T.K.) and the NIH through the University of Michigan’s Cancer Center Support Grant (P30CA46592). We thank to Deborah Postiff in the tissue procurement core for her technical assistance.
Glossary
Abbreviations:
- regulatory T cell
Treg cell
- dendritic cell
DC
- receptor activator of NFκB
RANK
- receptor activator of NFκB ligand
RANKL
Disclosure opf Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18480
References
- 1.Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, et al. Bone marrow and the control of immunity. Cell Mol Immunol. 2011 doi: 10.1038/cmi.2011.47. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–72. doi: 10.1016/S1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 3.Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174:1269–73. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- 4.Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–70. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Tripp RA, Topham DJ, Watson SR, Doherty PC. Bone marrow can function as a lymphoid organ during a primary immune response under conditions of disrupted lymphocyte trafficking. J Immunol. 1997;158:3716–20. [PubMed] [Google Scholar]
- 6.Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–7. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 7.Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, et al. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–8. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 8.Feuerer M, Rocha M, Bai L, Umansky V, Solomayer EF, Bastert G, et al. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer. 2001;92:96–105. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1152>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–5. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 10.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–31. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhodapkar MV, Krasovsky J, Osman K, Geller MD. Vigorous Premalignancy-specific Effector T Cell Response in the Bone Marrow of Patients with Monoclonal Gammopathy. J Exp Med 2003;198:1753-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 13.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 14.Zou W, Machelon V, Coulomb-L'Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–46. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 15.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 16.Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, et al. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146:1727–36. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 17.Cooper CR, Chay CH, Gendernalik JD, Lee HL, Bhatia J, Taichman RS, et al. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer. 2003;97:739–47. doi: 10.1002/cncr.11181. [DOI] [PubMed] [Google Scholar]
- 18.Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2007;13:62–9. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, et al. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107:1235–44. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Zhang J, Dai J, Dehne LA, Mizokami A, Yao Z, et al. Osteoblasts induce prostate cancer proliferation and PSA expression through interleukin-6-mediated activation of the androgen receptor. Clin Exp Metastasis. 2004;21:399–408. doi: 10.1007/s10585-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 21.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, et al. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–47. doi: 10.1016/S1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber H, Wu TH, Nachman J, Kast WM. Immunodominance and tumor escape. Semin Cancer Biol. 2002;12:25–31. doi: 10.1006/scbi.2001.0401. [DOI] [PubMed] [Google Scholar]
- 24.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 25.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 26.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–27. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 27.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- 28.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Cook K, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 29.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 30.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–7. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–74. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 32.Wei S, Kryczek I, Edwards RP, Zou L, Szeliga W, Banerjee M, et al. Interleukin-2 Administration Alters the CD4+FOXP3+ T-Cell Pool and Tumor Trafficking in Patients with Ovarian Carcinoma. Cancer Res. 2007;67:7487–94. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- 33.Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller ET, et al. Cutting Edge: Opposite Effects of IL-1 and IL-2 on the Regulation of IL-17+ T Cell Pool IL-1 Subverts IL-2-Mediated Suppression. J Immunol. 2007;179:1423–6. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green EA, Choi Y, Flavell RA. Pancreatic lymph node-derived CD4(+)CD25(+) Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity. 2002;16:183–91. doi: 10.1016/S1074-7613(02)00279-0. [DOI] [PubMed] [Google Scholar]
- 36.Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, et al. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372–9. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 37.Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 38.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 39.McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–31. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 40.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 41.Zaiss MM, Axmann R, Zwerina J, Polzer K, Guckel E, Skapenko A, et al. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007;56:4104–12. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]
- 42.Zaiss MM, Frey B, Hess A, Zwerina J, Luther J, Nimmerjahn F, et al. Regulatory T cells protect from local and systemic bone destruction in arthritis. J Immunol. 2010;184:7238–46. doi: 10.4049/jimmunol.0903841. [DOI] [PubMed] [Google Scholar]
- 43.McCabe NP, Madajka M, Vasanji A, Byzova TV. Intraosseous injection of RM1 murine prostate cancer cells promotes rapid osteolysis and periosteal bone deposition. Clin Exp Metastasis. 2008;25:581–90. doi: 10.1007/s10585-008-9175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai J, Lu Y, Yu C, Keller JM, Mizokami A, Zhang J, et al. Reversal of chemotherapy-induced leukopenia using granulocyte macrophage colony-stimulating factor promotes bone metastasis that can be blocked with osteoclast inhibitors. Cancer Res. 2010;70:5014–23. doi: 10.1158/0008-5472.CAN-10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 46.Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69:3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- 47.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–81. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, et al. Cutting Edge: Induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177:40–4. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 49.Park JW, Hong K, Carter P, Asgari H, Guo LY, Keller GA, et al. Development of anti-p185HER2 immunoliposomes for cancer therapy. Proc Natl Acad Sci USA. 1995;92:1327–31. doi: 10.1073/pnas.92.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, et al. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70:7788–99. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PDF file supplied by authors.