Abstract
Various Invariant NKT (iNKT) cell ligands have been shown as potent adjuvants in boosting T cell reactivates to antigens on professional APC. Non-professional APC, such as T cells, also co-expressing MHC class I and CD1d, have been unattractive cell vaccine carriers due to their poor immunogenicity. Here, we report that T cells as well as T cell lymphoma can efficiently generate antigen-specific cytotoxic T lymphocytes (CTL) responses in mice in vivo, when formulated to present iNKT ligand α-galactosylceramide (αGC) on their surface CD1d. Vaccination with αGC-pulsed EG-7 T-cell lymphoma induced tumor-specific CTL response and suppressed the growth of EG-7 in a CD8 T cell-dependent manner. Injection of αGC-loaded CD4 T cells in mice efficiently activated iNKT cells in vivo. While T cells loaded with a class I-restricted peptide induced proliferation but not effector differentiation of antigen-specific CD8 T cells, injection of T cells co-pulsed with αGC strongly induced IFNγ and Granzyme B expression in T cells and complete lysis of target cells in vivo. Presentation of αGC and peptide on the same cells was required for optimal CTL response and vaccinating T cells appeared to directly stimulate both iNKT and cytotoxic CD8 T cells. Of note, the generation of this cytotoxic T cell response was independent of IL-4, IFNγ, IL-12, IL-21 and costimulation. Our data indicate that iNKT cell can license a non-professional APC to directly trigger antigen-specific cytotoxic T cell responses, which provides an alternative cellular vaccine strategy against tumors.
Keywords: CD1d, cellular vaccine, cytotoxic T cell, iNKT cell, tumor immunity, α-galactosylceramide
Introduction
CD1d-restricted invariant NKT cells (iNKT) recognize glycolipid antigens presented on non-classical MHC class I-like molecule, CD1d. Recent studies revealed a crucial role of iNKT cells in the host defense against pathogens and tumors through direct interaction with microbial antigens or indirect activation.1 Moreover, iNKT cell acts as a bridge between innate and acquired immune system by regulating T cell activation and function. For instance, iNKT ligands have been shown with strong potency in enhancing T cell responses to soluble protein antigens or tumor antigens presented by professional antigen-presenting cells (APC). Co-administration of α-galactosylceramide (αGC) and protein antigens generates the antigen-specific CD4 T and CD8 T cell immunity in vivo.2,3 The generation of antigen-specific adaptive immunity depends on the activation and maturation of dendritic cells (DC) after αGC injection which triggers IFNγ and TNFα expression from iNKT cells.2,4 Costimulation such as CD40 and CD80/CD86 is required for the T cell immunity in this case.4 Furthermore, injection of DC-associated αGC induces the activation and expansion of iNKT cells and NK cells in vivo in tumor patients.5,6
Non-professional APC in immune system, such as T cells, express both MHC class I and CD1d. The expression of MHC class I on these cells may allow them to be protected from NK cell-mediated cytotoxic attack through interaction with inhibitory receptors.7 The role of CD1d expression on peripheral T cells in iNKT cell biology is not yet clear, although it is well established that CD1d expression on thymic T cells is essential for positive selection of iNKT cell.8,9 DC has been shown to be the most efficient APC for iNKT cell priming when αGC was injected as a soluble form, while other types of cell expressing CDld suppress iNKT cell activation in this condition.10 In addition to DC, vaccination with B cells copulsed with αGC and tumor-derived peptide generates an efficient anti-tumor CD8 T cell response in several murine tumor models.11 Furthermore, our recent study showed that autologous B lymphoma vaccine, manipulated to present αGC, can establish memory anti-tumor T cell immunity.12 Of note, Shimizu et al. recently showed that non-professional APCs, such as T and NK cells, can stimulate iNKT cell activation when they presented αGC in a cell-associated form.11,13 They also showed that tumors lacking costimulatory molecules, such as B16 melanoma and EL4 thymoma, can induce the activation of iNKT cells and NK cells in vivo when formulated to present αGC. These observations together raise an interesting question if iNKT ligand-loaded non-professional APC could act as an efficient APC in generating CD8 T cell response against the peptides loaded on their surface MHC class I. If this is true, it might be possible to use tumor cell itself or pathogen-infected cells to generate tumor- or pathogen-specific cytotoxic T cell immunity, which would be much easier, cost-effective and practical in clinical settings.
Therefore in the present study, we tested whether conventional T cells- as a surrogate CD1d-expressing non-professional APC- can actively interact with iNKT cells and CD8 T cells in the presence of an iNKT cell ligand and MHC class I-restricted peptide. Our results showed an efficient activation of iNKT cells and antigen-specific CD8 T cells in mice vaccinated with T cells presenting both αGC and peptide on the same cells, which induced an effective immunity against microbial infection and tumor. Moreover, vaccination with αGC-pulsed thymoma generated a tumor-specific cytotoxic T cell response in vivo. Thus, in the presence of iNKT cell help, non-professional APC, such as T cells, can stimulate cytotoxic T cell function, providing an alternative means of cell vaccination method.
Results
Vaccination with αGC-loaded EG-7 generates CD8+ T cell-dependent antitumor activity
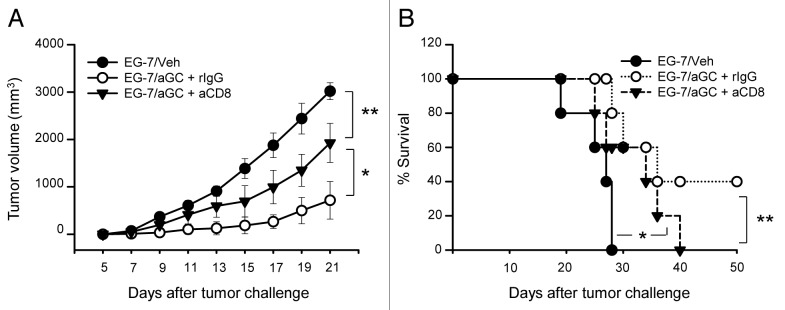
We first asked whether vaccination with tumor cells of T cell origin can trigger anti-tumor activity, when manipulated to present αGC. To track tumor-specific T cell responses more efficiently in vivo, we employed ovalbumin-expressing EL4 thymoma (EG-7) as our tumor model. To address protective antitumor activity by vaccination with αGC-loaded EG-7, we vaccinated mice with irradiated EG-7 co-cultured with 1 μg/mL of αGC overnight. EG-7 cells incubated with the same volume of solvent (5 μl/mL of 0.5% polysorbate) were used as control (EG-7/veh). After extensive washing, these cells were i.v. injected into syngenic naïve mice. These mice were subcutaneously implanted with live EG-7 and the tumor volume and survival were monitored daily. Mice were sacrificed when tumor diameters reached 20 mm. As depicted in Figure 1A and B, the vaccination with EG-7/αGC significantly reduced the growth of solid tumors and induced prolonged survival in comparison to those of EG-7/veh group. Tumors affect myelopoiesis and induce the expansion of CD11b+Gr-1+ myeloid-derived suppressor cell (MDSC) in the bone marrow, blood, spleen.14 Of note, we also observed a great decrease of CD11b+Gr-1+ population in the spleen of EG-7/αGC vaccinated mice (< 4%) compared with that of EG-7/veh group (> 20%) (Fig. 1C), another indicative of antitumor activity induced by the vaccination. Importantly, mice vaccinated with EG-7/αGC showed higher numbers of CD8+ T cells producing IFNγ upon SIINFEKL stimulation (Fig. 1D). These results overall indicate the induction of anti-tumor activity as well as tumor-specific CD8+ T cell responses upon the vaccination with EG-7/αGC in vivo.
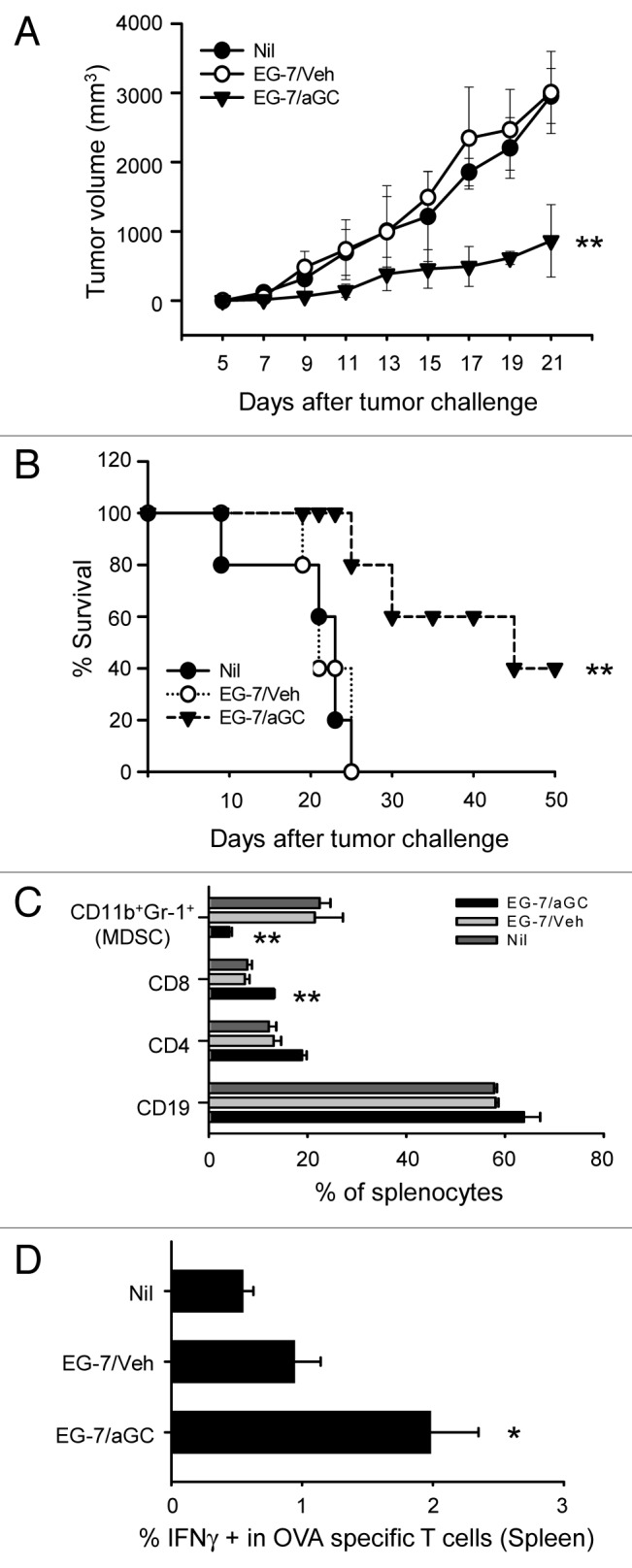
Figure 1. Vaccination with αGC-loaded EG-7 generated preventive antitumor activity. (A–D) EG-7 cells were co-cultured overnight with 1 μg/ml of αGC (EG-7/αGC) or vehicle followed by irradiation (50 Gy). C57BL/6 mice (n = 7 per group) were vaccinated with EG-7/veh, EG-7/αGC or untreated (Nil). One week later, all mice were subcutaneously injected with 1 × 106 live EG-7 cells and the survival (A) and tumor size (B) were checked. (C) At the 3 weeks later, splenocytes were isolated from tumor bearing mice and analyzed by flow cytometer after staining with anti-CD11b, anti-Gr1, anti-CD19, anti-CD4 and anti-CD8 antibodies. (D) Splenocytes were restimulated with SIINFEKL in the presence of Golgi-Plug for 5 h before intracellular staining of IFNγ on OVA specific T cells. *p < 0.05, **p < 0.005, p values were calculated with 2-way ANOVA (A), Kaplan-Meier method (B) or Student's t-tests (C and D) in comparison with the EG-7/veh group.
Therefore, we next examined if the observed anti-tumor activity by vaccination with EG-7/αGC depends on CD8+ T cells. We injected anti-CD8 depleting antibody into the vaccinated mice before we transplanted live EG-7 cells into them. Depletion of CD8+ cells greatly decreased the protective anti-tumor activity by vaccination with EG-7/αGC (Fig. 2A) and the survival of vaccinated mice (Fig. 2B). Taken together, these results indicate that antitumor immunity generated by αGC-loaded EG-7 is, at least in part, mediated by CD8 T cells.
Figure 2. CD8 T cells play a critical role in mediating antitumor activity. (A and B) C57BL/6 mice were vaccinated with EG-7/veh or EG-7/αGC on day -7 (n = 5 per group). EG-7/αGC vaccinated mice were intraperitoneally injected with CD8 depleting Ab (563.8) on day-3 and -1. On day 0, all mice were subcutaneously injected with 1 × 106 live EG-7 cells and the tumor volume (A) and the survival (B) were checked. *p < 0.01, **p < 0.005, p values were calculated with 2-way ANOVA (A) or Kaplan-Meier method (B) in comparison with EG-7/veh and EG-7/αGC+rIgG or EG-7/αGC+rIgG and EG-7/αGC+anti-CD8 group.
Vaccination with αGC-loaded EG-7 induces ova-specific cytotoxic responses
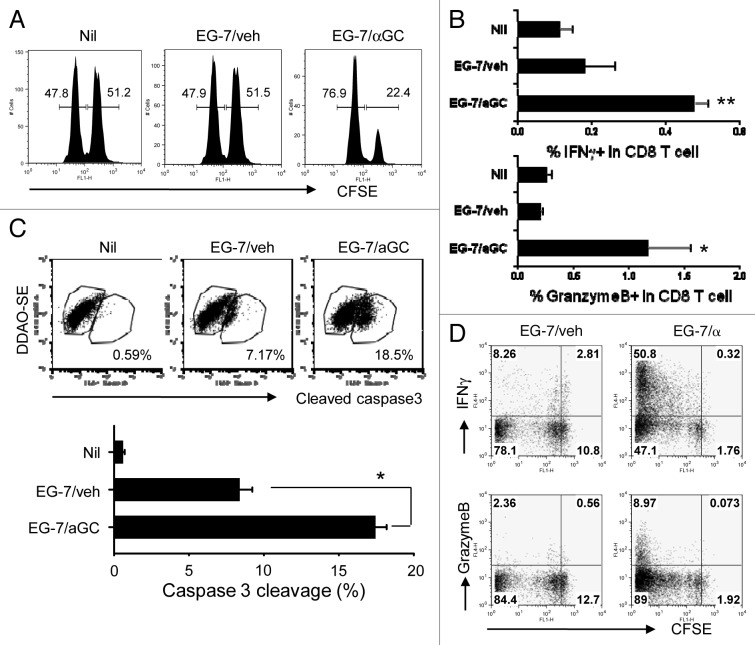
Our EG-7 tumor models (Fig. 1) and CD8 depletion experiment (Fig. 2) raised a hypothesis that tumor cells of T cell origin, when they present iNKT ligand, are able to induce cytotoxic T cell response specific for endogenous tumor antigens. To test our hypothesis, we co-cultured EG-7 cells with αGC or vehicle before being irradiated (EG-7/αGC and EG-7/veh, respectively). To increase the expression of peptide/MHC-I complex, EG-7 cells were first pre-treated with IFNγ. One week after vaccination, we performed an in vivo CTL assay to determine the antigen-specific cytotoxicity against SIINFEKL-loaded syngenic splenocytes11. When injected into naïve syngenic mice, EG-7/αGC resulted in an evident peptide-loaded target cell lysis whereas EG-7/veh did not (Fig. 3A; -0.37% and 72.8% lysis, respectively). To examine if CD8+ T cells in the vaccinated mice directly kill target cells in an antigen-specific way, we performed an in vitro CTL assay with purified CD8+ T cells from the vaccinated mice by analyzing caspase-3 activation in the target cells. As depicted in Figure 3B, the CD8+ T cells from the EG-7/αGC vaccinated mice induced a significantly higher percentage of caspase-3 cleavage in the target cells than those from the EG-7/veh vaccinated mice. In addition, the effective target lysis in EG-7/αGC-vaccinated mice was associated with increased antigen-specific IFNγ+ granzyme B+ CD8 T cells in the spleen (Fig. 3C). To further characterize the antigen-specific CD8 T cells, we adoptively transferred CFSE-labeled OT-I T cells (CD45.2) into congenic mice (CD45.1). The recipients were vaccinated with either EG-7/veh or EG-7/αGC and CD45.2+ cells were analyzed five days later. OT-I T cells in mice vaccinated with EG-7/veh underwent an extensive proliferation, but expressed little IFNγ (< 10%) or granzyme B (< 3%). By contrast, significantly higher population of OT-I T cells in EG-7/αGC-vaccinated mice expressed IFNγ (> 50%) and granzyme B (> 8%) (Fig. 3D). Taken together, these data demonstrate that αGC-loaded EG-7 thymoma induced tumor-specific CD8+ T cells to differentiate into cytotoxic effector T cells expressing high levels of IFNγ and granzyme B in vivo.
Figure 3. Vaccination with αGC-loaded EG-7 generates OVA-specific cytotoxic T cell response in vivo. (A and B) EG-7 cells were cocultured overnight with 1 μg/ml of αGC (EG-7/αGC) or vehicle (0.5% polysorbate, EG-7/veh) followed by irradiation (50 Gy). C57BL/6 mice (n = 3 per group) were vaccinated with EG-7/veh, EG-7/αGC or left untreated (Nil). (A) One week later, an in vivo CTL assay for SIINFEKL was performed. CFSEhigh, peptide-pulsed target; CFSElow, peptide-unpulsed control. (B) One week after vaccination, CD8+ T cells were isolated and incubated with 1 µg/mL peptide-pulsed splenocytes that had been labeled with DDAO-SE for 2 h. The cells were fixed, permeabilized and stained with anti–cleaved caspase-3 mAb. The levels of cleaved caspase-3 in the DDAO-SE-positive cells were analyzed by flow cytometry. Data shown are representative FACS plots (upper panels) and mean ± SE (lower panel). *p < 0.05, in comparison with EG-7/veh group. (C) One week after the vaccination, splenocytes were isolated and restimulated with SIINFEKL for 5 h in the precence of Golgi-Plug before intracellular staining of granzyme B and IFNγ. (D) Ovalbumin-specific CD8 T cells were isolated, labeled with 10 µmol/L CFSE and i.v. transferred into their syngenic mice as described in Figure 1. On the following day, mice were i.v. injected with irradiated EG-7 cells manipulated in vitro with indicated conditions. Five days later, lymphoid cells from the spleen of the recipient mice were restimulated with SIINFEKL for 5 h before intracellular staining of granzyme B and IFNγ. Data are representative of at least two separate experiments. *p < 0.05, **p < 0.01, in comparison with non-treated (Nil) group.
αGC induces cytotoxic T cell response to T cell-associated antigens
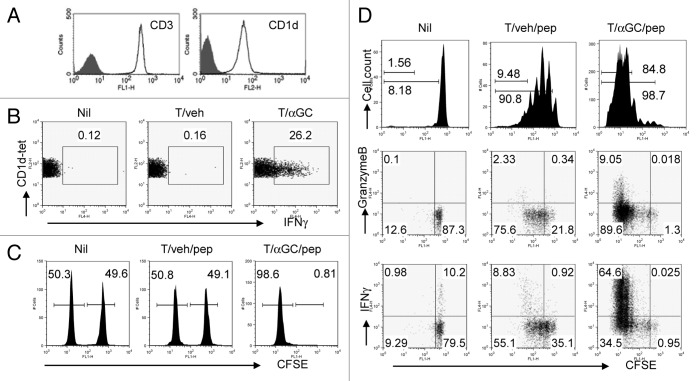
Our study thus far showed that, with help of αGC, tumors of T cell origin can trigger tumor-specific cytotoxic T cell responses. Therefore we next asked if conventional T cells can also act as antigen presenting cells and trigger cytotoxic T cell responses in vivo, when formulated to present αGC. A previous study by Shimizu et al. showed that T cells, purified by negative selection using magnetic beads and when loaded with αGC, stimulate iNKT cells in vivo.13 To further assess iNKT cell activation by T cells, we first sorted NK1.1−CD8−CD19−CD11b−CD11c−Gr-1−I-Ab−CD4+ cells from the lymphoid cells of C57BL/6 mice. These cells were virtually all CD3+CD1d+, indicating no contamination of professional APC (Fig. 4A). The sorted cells were loaded with αGC (T/αGC) or with vehicle (T/veh) and were i.v. injected into syngenic naïve mice. When we analyzed splenocytes six hours after the injection, we observed that CD1d-tetramer+ cells in mice receiving T/αGC produced IFNγ+ whereas the same population in mice receiving T/veh did not produce IFNγ (Fig. 4B). Therefore CD4+ T cells efficiently triggered iNKT cell activation in vivo when they were manipulated to present αGC.
Figure 4. Injection of conventional CD4 T cells coated with SIINFEKL and αGC induces a functional cytotoxic T cell response. (A) Lymphoid cells from spleen and lymph nodes of C57BL/6 mice were stained with FITC-conjugated anti-CD19, anti-NK1.1, anti-Gr-1, anti-CD11b, anti-CD11c, anti-CD8a, anti-I-Ab antibodies together with APC-conjugated CD4 Ab. Lineage negative and CD4+ cells were sorted by FACSAria. The sorted cells were stained with PE-conjugated anti-CD3 or anti-CD1d Ab. Filled histogram is isotype control. (B) Sorted CD4+ T cells were co-cultured with αGC (1 μg/ml). Cells were washed and intravenously injected into syngenic mice. Six hours later, lymphoid cells from spleen were stained with CD1d-tetramer and CD19 before intracellular IFNγ staining. CD1d-tetramer+CD19- cells were gated and analyzed. (C) The sorted CD4+ T cells were co-cultured with αGC (1 μg/ml) or vehicle (0.5% polysorbate) for 16 h including 1 h pulse with SIINFEKL. Cells were washed and intravenously injected into syngenic mice. One week later, syngenic lymphocytes were either loaded with 1 µmol/L peptides or left untouched before being labeled with CFSE at different concentrations (10 and 1 µmol/L, respectively). Equal numbers of the two populations were mixed and injected i.v. into mice. Eighteen to 24 h later, lymphoid cells from spleen and lymph nodes were analyzed to assess peptide-specific killing. (D) OT-I T cells were isolated using anti-CD8 microbeads and AutoMacs. These cells were labeled with 10 µmol/L CFSE and i.v. transferred into their syngenic mice. On the following day, mice were i.v. injected with T cells manipulated in vitro with indicated conditions. Forty-eight hours later, lymphoid cells from the spleen of the recipient mice were stained with phycoerythrin (PE)-conjugated anti-Vα2 antibody and then analyzed by flow cytometry. For intracellular Granzyme B and IFNγ staining, cells were restimulated with SIINFEKL for 5 h before intracellular staining of these molecules according to manufacturer’s instruction. Data are representative of at least two independent experiments.
We next asked whether T/αGC can induce antigen-specific cytotoxicity when they were additionally pulsed with MHC class I-restricted peptide. SIINFEKL peptide (1ng/mL) was added to T cells in the last one hour when they were incubated with αGC or vehicle (T/αGC/pep and T/veh/pep, respectively). After extensive washing, C57BL/6 mice were immunized with T/veh/pep or T/αGC/pep. One week later, we performed an in vivo CTL assay to determine the antigen-specific cytotoxicity against SIINFEKL-loaded syngenic splenocytes. Mice vaccinated with T/αGC/pep showed complete lysis of target cells while mice vaccinated with T/veh/pep did not (Fig. 4C).
To analyze whether T/veh/pep vaccination results in cytotoxic function of antigen-specific CD8 T cells, we isolated CD8+ T cells from OT-I mice, labeled them with 10 μM CFSE before transferring into C57BL/6 mice, which were then vaccinated with T/veh/pep or T/αGC/pep. When OT-I cells in the spleen of recipients were analyzed 48 hours after vaccination, both types of T cell vaccination induced the proliferation of OT-I T cells in vivo, although the proportion of T cells that had undergone over five times of division was higher in mice receiving T/αGC/pep vaccination than those with T/veh/pep (Fig. 4D, upper panels). More importantly, OT-I T cells in mice vaccinated with T/αGC/pep displayed remarkably higher expression of granzyme B and IFNγ compared with those in T/veh/pep-vaccinated mice (Fig. 4D, middle and lower panels). These data demonstrate that in response to an antigen on CD4 T cells as a non-professional APC, although antigen-specific CD8 T cells could proliferate, their functional differentiation into cytotoxic cells could only occurr in the presence of iNKT cell ‘help’.
T/αGC/Pep vaccination confers protection against Listeria monocytogenes and melanoma
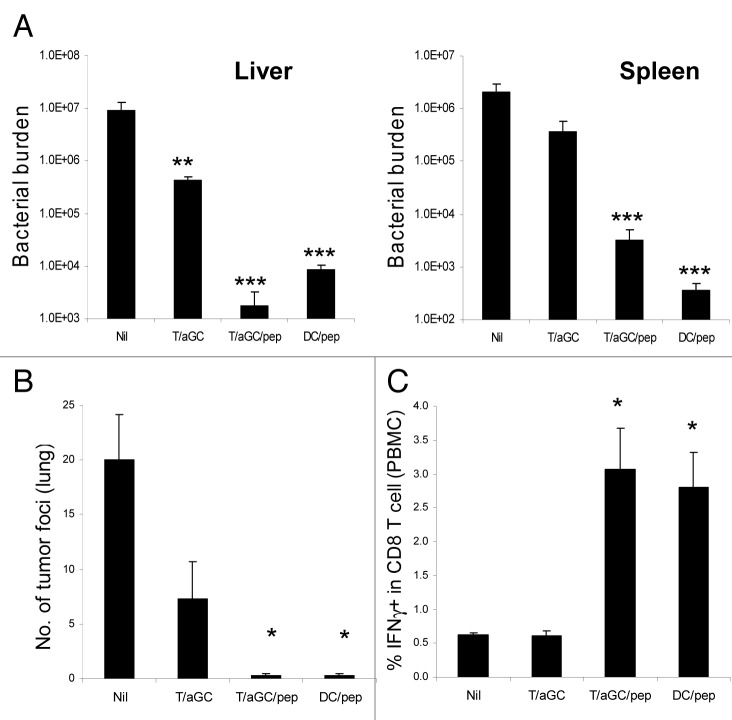
We next tested whether the cytotoxic T cell response generated by T/αGC/pep vaccination is effective enough in suppressing the growth of intracellular bacteria and tumor in an antigen-specific manner. We first employed L. monocytogenes infection model since clearance of this bacterium is largely dependent on CD8 T cell response. Mice were vaccinated with T/αGC, T/αGC/pep or peptide-pulsed dendritic cells (DC/pep) as a control. Ten days later, the vaccinated mice were i.v. injected with L. monocytogenes expressing OVA and the bacterial burden in the spleen and liver was measured. As expected, mice vaccinated with DC/pep showed significantly lower bacterial burden in both spleen and liver compared with non-vaccinated mice (Fig. 5A). Compared with non-vaccinated group, mice vaccinated with T/αGC showed slightly lower bacterial burden, especially in the liver. In contrast, mice vaccinated with T/αGC/pep also showed significantly lower bacterial burden in both organs, which is comparable to those of DC/pep-vaccinated mice (Fig. 5A).
Figure 5. Vaccination with T cell-based vaccine generates protective immunity against L. monocytogenes infection and tumor challenge. C57BL/6 mice (n = 3 mice per group) were vaccinated with the indicated cellular vaccine (day 0) before they were challenged with 5 × 104 live L. monocytogenes expressing OVA (day 10). Three days after the bacterial challenge, bacterial burden in spleen and liver was measured (A). Data are a representative of three separate experiments. (B) C57BL/6 mice (n = 3 mice per group) were vaccinated with the indicated cellular vaccine (day 0). Ten days later, recipients were intravenously challenged with live 2 × 105 B16-OVA. Two weeks after the tumor challenge, tumor foci in the lung were measured. (C) PBMC was isolated in mice vaccinated with the indicated cellular vaccine and restimulated with SIINFEKL in the presence of Golgi-Plug for 5 h before intracellular staining of IFNγ. Data are mean ± SE *p < 0.05, **p < 0.01, ***p < 0.001 in comparison with non-treated (Nil) group. Data are representative of two separate experiments.
To assess if the vaccinated mice were also resistant to tumor growth, we i.v. injected OVA-expressing B16 melanoma cells into the vaccinated mice. Fourteen days later, we counted tumor foci in the lung of recipients. Compared with non-vaccinated mice, mice vaccinated with T/αGC had less tumor foci (Fig. 5B). On the other hand, fewer tumor foci were found in mice vaccinated with T/αGC/pep or DC/pep (Fig. 5B). Intracellular staining of peripheral blood mononuclear cells after peptide restimulation revealed that both DC/pep and T/αGC/pep vaccinations efficiently induced peptide-specific IFNγ-producing CD8 T cells (Fig. 5C), which correlated well with anti-Listeria and anti-metastatic activity in the vaccinated mice. Collectively, these data demonstrate that vaccination with T/αGC/pep established protective immunity against intracellular bacteria and tumor in a peptide-specific manner.
T cells simultaneously presenting iNKT and class I-restricted ligands directly induce antigen-specific cytotoxicity
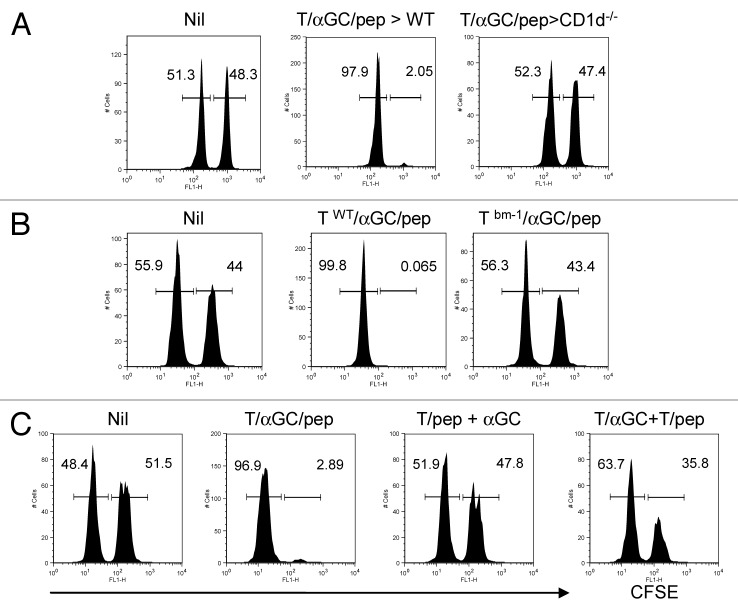
We next sought to elucidate the mode of action in the efficient induction of peptide-specific cytotoxicity during T/αGC/pep vaccination. When we vaccinated CD1d-deficient mice with T/αGC/pep, we did not observe peptide-specific cytotoxicity in our in vivo CTL assay (Fig. 6A). Therefore, the antigen-specific cytotoxicity elicited by T/αGC/pep requires iNKT cells in vivo.
Figure 6. Peptide and αGC on the same T cells are required for the optimal priming of CTL by iNKT-mediated T cell vaccine. (A) C57BL/6 (WT) or CD1d−/− mice were vaccinated with T cells co-pulsed with αGC and SIINFEKL (1 × 106 per mouse). (B) T cells from WT or bm-1 mice were co-pulsed with αGC and SIINFEKL before being i.v. injected into WT mice. (C) C57BL/6 mice were vaccinated with T cells co-pulsed with αGC and SIINFEKL (1 × 106 per mouse) or ‘a combination of T cells pulsed with SIINFEKL and of T cells pulsed with αGC’ (1 × 106 each) or T cells pulsed with SIINFEKL plus free form of αGC (i.p.). CFSEhigh, peptide-pulsed target; CFSElow, peptide-unpulsed control. Data are a representative of at least two separate experiments.
Next we asked if vaccinated T cells directly stimulate CD8 T cells or require host APC. We utilized bm-1 mouse whose cells are able to load SIINFEKL onto their MHC I, but the resulting complex cannot be recognized by OT-I TCR due to a mutation in the H-2K region.15 In this experiment, vaccination with T/αGC/pep using T cells from bm-1 mice failed in induction of antigen-specific target cell lysis (Fig. 6B), indicating that appropriate MHC/peptide complex on the vaccinating T cells is essential and that they probably directly primed OT-I cells.
Since αGC and class I peptide are both presented by the same T cells, this suggests that iNKT ligand may provide a “dangerous” signal to the immune system. To test this hypothesis, C57BL/6 mice were vaccinated with T/pep in combination with either soluble αGC (2μg, i.p.) or T/αGC. In mice vaccinated with the former combination, little peptide-specific CTL was generated (Fig. 6C, 13.4% vs. 97.2% lysis in T/αGC/pep-vaccinated mice). Moreover, mice vaccinated with T/αGC plus T/pep only showed a moderate peptide-specific CTL, significantly less efficient than that of T/αGC/pep (Fig. 6C, 47.2% lysis vs 97.2% in T/αGC/pep-vaccinated mice). Thus, these data demonstrate that the vaccinating T cells act as direct stimulators for both iNKT and CD8 T cells in vivo and that presentation of αGC and peptide by the same T cell is the most efficient regimen in generating antigen-specific CTL.
Cytokine and costimulation requirements in the CTL response elicited by T/αGC/Pep
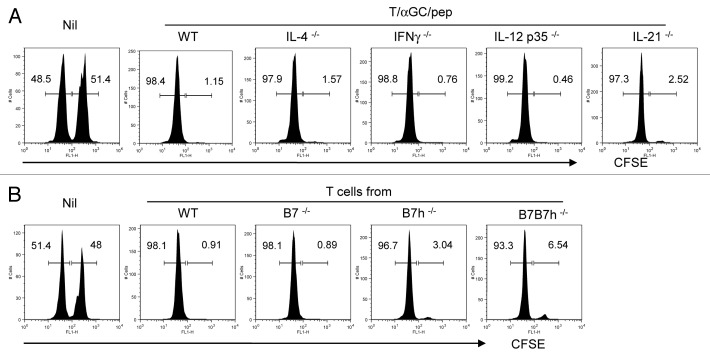
Upon activation, iNKT cells promptly produce a wide range of cytokines including IL-4 and IFNγ. IL-4 produced by activated iNKT cells was shown to promote CD8 T cell proliferation.16 IFNγ and IL-12 produced upon iNKT and DC interaction mediate anti-tumor activity.17,18 Recent studies showed that IL-21 is produced by iNKT cells upon TcR stimulation and regulates the activation and expansion of iNKT cells.19,20 Therefore, we asked whether these cytokines produced after iNKT cell activation had any role in the generation of CTL in our T cell vaccine model. We vaccinated IL-4−/−, IFNγ−/−, IL-12 p35−/− or IL-21−/− mice with T/αGC/pep and performed in vivo CTL assay. As depicted in Figure 7A, we observed a complete peptide-specific CTL activity in all of cytokine-deficient mice we tested. Thus, none of these cytokines is necessary in the generation of CTL activity by the T cell vaccine.
Figure 7. Molecular requirements for efficient indication of CTL by iNKT-mediated T cell vaccine. (A) C57BL/6 mice (WT) or various cyokine-deficient mice with C57BL/6 background were vaccinated with T cells copulsed with αGC and SIINFEKL. (B) Pure CD4+ T cells were isolated from C57BL/6 (WT), B7−/−, B7h−/− or B7B7h−/− as described in Figure 1. Isolated T cells were co-pulsed with αGC and SIINFEKL ex vivo before injected into WT recipient. A week later, an in vivo CTL assay was performed. CFSEhigh, peptide-pulsed target; CFSElow, peptide-unpulsed control. Data are representative of three separate experiments.
Costimulatory signals are essential in regulating the activation and expansion of iNKT cells. Moreover, CD28 and ICOS signals are required for the anti-tumor activity of activated iNKT cells such as cytotoxicity and anti-metastatic activity.2,21-23 Activated T cells express CD80 and CD86 and may regulate immune response through T:T interaction.24,25 Therefore we asked whether these costimulatory factors were necessary for the induction of CTL in our model. T cells were isolated from C57BL/6(WT), B7−/− (B7.1−/−B7.2−/−), B7h−/− or B7B7h−/− and loaded with αGC and SIINFEKL ex vivo. The T/αGC/pep were injected into WT mice and in vivo CTL assay was performed. As depicted in Figure 7B, we observed a normal target cell lysis in mice vaccinated with costimulation-deficient T cells. Thus, the induction of peptide-specific cytotoxicity by the T cell vaccine does not require B7 and B7h costimulation.
Discussion
In the present study, we showed that vaccination with conventional CD4 T cells presenting both CD1d-αGC and MHC class I-restricted peptide triggered iNKT cell activation and generated an antigen-specific cytotoxic T lymphocyte response in vivo. Mice receiving this novel T cell vaccine were resistant to L. monocytogenes infection as well as to tumor challenge in an antigen-dependent manner. Moreover, vaccination with αGC-pulsed T cell lymphoma also induced CTL response specific for an endogenous tumor antigen. Mechanistic analyses indicate that the vaccinated T cells directly stimulated peptide-specific cytotoxic T cells and that the CTL responses induced by these ‘T cell vaccines’ were independent of cytokines such as IFNγ, IL-12 and IL-21. Therefore T cells as well as tumors of T cell origin can act as antigen-presenting cells that efficiently trigger cytotoxic T cell responses, when they are formulated to stimulate iNKT cells in vivo.
Dendritic cells loaded with various iNKT ligands are potent inducer of T cell immunity. We recently showed that B cells co-pulsed with αGC and class I peptide also induce antigen-specific CTL response in vivo as efficiently as well.11 Moreover, our recent study showed that B lymphoma cells loaded with αGC elicited a strong protective immunity that is dependent on CD4 T cells.12 However, it has been unclear whether non-professional APC in the presence of iNKT cell help could induce T cell effector function. In the present study, we found that CD8 T cells were activated by antigens presented by CD4 T cells and proliferated extensively. However, they did not produce significant amounts of IFNγ or Granzyme B that are associated with cytotoxic effector function, indicating that CD4 T cells are not an efficient APC. It is possible that T cells, like other non-professional APC, do not express high levels of costimulatory molecules, which is required for CD8 T cell effector function.26 We have also previously observed this type of non-productive activation in CD8 T cells reactive to a tissue antigen, which is mediated by PD-1-PDL1 interaction.27 In the presence of αGC and hence iNKT “help,” antigen-specific CD8 T cells were able to undergo effector differentiation by the CD4 T cell vaccine that results in protection against infection and tumor. Since iNKT ligands are either derived from infectious agents or induced during infection, their presence on non-professional APC may provide a “dangerous” signal to the immune system and, through iNKT cells, “license” a productive T cell activation and their effector differentiation.
How iNKT cell help CD8 cell effector differentiation remains unclear at this stage. After activation, iNKT cells promptly produce a wide range of cytokines which can further activate themselves and other immune cells including NK, DC, T and B cells. IFNγ and IL-12 are critical mediators for T cell immunity. IFNγ produced by iNKT cells and NK cells mediates anti-angiogenic activity of αGC.18 IL-12 produced by DC after interaction with iNKT cells is critical for inducing IFNγ in iNKT cells.17 However, in our T cell vaccine model, neither IFNγ nor IL-12 was required for CTL generation. Recent studies suggest that IL-21 is a crucial cytokine for iNKT cell activation and function.19,20 However, an efficient induction of CTL response by our T cell vaccine in IL-21-deficient mice excludes the role of this cytokine in the generation of CTL response in our model. Moreover, although vaccinated T cells were found to directly stimulate iNKT and CD8 T cells, this action does not appear to require costimulation. We recently found that dendritic cells loaded with αGC induced iNKT cell activation in the absence of B7 and B7h (data not shown). It is unclear at this stage whether there is any signal in addition to TcR that is required for iNKT and CD8 T cell activation by our T cell vaccine.
Nonetheless, T cells loaded with an iNKT ligand serves as a potent cellular vaccination approach. A mild suppression of tumor growth and L. monocytogenes growth was observed in mice vaccinated with T/αGC, indicating that an effective innate immunity was induced. However, vaccination with T/αGC/pep was far more effective in eliciting CD8 T cell immunity and specifically suppressing tumor growth and L. monocytogenes growth, which was comparable to DC vaccination. Based on our results, we propose that T cells from individuals infected with T cell-tropic pathogens—such as HIV—could be used as a source of cellular vaccine for triggering pathogen-specific cytotoxic response. Notably, i.v. injection of irradiated EG-7 thymoma did not induce an OVA-specific CTL response; however, injection of αGC-loaded, irradiated EG-7 efficiently generated an OVA-specific CTL response. As a result, vaccination with αGC-loaded, irradiated EG-7 induced a potent anti-tumor activity against challenge with live EG-7, which was CD8+ T cell-dependent. Therefore we propose that vaccination of αGC-loaded T lymphoma may result in the generation of tumor-specific CTL response.
Our finding is novel in that non-professsional APCs are also able to trigger cytotoxic T cell immunity with the help of iNKT cell. T cell is a major population in blood and can be easily expanded and cultured in vitro. Although resting human T cells express little CD1d, activated T cells as well as thymocytes in humans express CD1d on their surface.28 Moreover, cutaneous T cell lymphomas in humans have been reported to express high levels of CD1D.29 These observations suggest that our findings can be directly applicable in clinical settings. Therefore, tumors that are not professional APC in origin, especially thymoma and T lymphoma, could be used as efficient APC for cytotoxic T cell anti-tumor immunity when they are formulated to stimulate iNKT cells. Our T cell vaccine strategy will provide an alternative cellular vaccine regimen that can induce the efficient activation of both innate and adaptive immunity in vivo.
Materials and Methods
Mice
C57BL/6, OT-I, C57BL/6bm1 (bm-1), IL-4−/−, IFNγ−/−, IL-12p35−/−, CD80−/−CD86−/− (B7−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6.SJL mice (Ly5.1) were purchased from Taconic Farm. B7h−/− and B7B7h−/− mice were generated and backcrossed in our laboratory.26,30 CD1d−/− mice were bred in our animal facility. IL-21−/− mice were previously described31 and bred in our animal facility. All mice were kept under specific pathogen-free conditions in our animal facility (MD Anderson Cancer Center). All animal studies were approved by the Institutional Animal Care and Use Committee of MD Anderson Cancer Center.
Preparation of T and dendritic cells
Conventional CD4 T cells were purified from lymphoid cells of C57BL/6 mice by sorting CD8− NK1.1− CD19− CD11b− CD11c− Gr-1− I-Ab− but CD4+ cells with a FACS Aria®. These cells were > 99.5% CD3+CD4+ and CD1d+. In some experiments, CD4 T cells were isolated from CD1d−/− or bm-1 mice. Bone marrow-derived DCs were generated by culturing bone-marrow cells in the presence of GM-CSF and IL-4 for 6 d. Sorted T cells were co-cultured with αGC (1 µg/mL) or the same volume of solvent vehicle (5 µl/mL of 0.5% polysorbate) overnight. In some experiments, these cells were further incubated with SIINFEKL peptide for 1 h.
EG-7 cells were cultured in the presence of IFNγ (100 ng/mL) for 48 h including overnight culture with αGC or vehicle as described above. These EG-7 cells were washed and irradiated with Cs source (50 Gy) before i.v. injected into syngenic mice.
CFSE-labeled OT-I adoptive transfer
Ovalbumin-specific CD8 T cells were isolated from the lymphoid cells of OT-I mice using anti-CD8 magnetic beads and AutoMacs (> 92% are Vα2+). These cells were labeled with 10 µM of CFSE and i.v. transferred into C57BL/6 mice. In some experiment, Ly5.1 congenic mice were used as recipients. Recipient mice were i.v. injected with CD4 T cells or irradiated EG-7 cells formulated ex vivo with indicated conditions. Two to five days later, lymphoid cells from the spleen of recipient mice were isolated and cultured with 0.1 μg/mL SIINFEKL in the presence of GolgiPlug (1 μL/mL) for 5 h. These cells were stained with phycoerythrin (PE)-conjugated anti-Vα2 Ab or PE-conjugated anti-Ly5.2 Ab before permeabilized and further stained with APC-conjugated anti-IFNγ Ab or Alexa 647-conjugated anti-granzyme B Ab. Total 1 × 106 events were collected and CFSE+ PE+ cells were gated and analyzed by FACS Calibur.
iNKT cell staining
For intracellular staining of IFNγ in iNKT cells, splenocytes and liver mononuclear cells from mice injected with vehicle-pulsed T cells, αGC-loaded T cells were stained with PE-conjugated αGC-loaded/CD1d-tetramer together with FITC-conjugated anti-CD19 Ab (for splenocytes) or FITC-conjugated anti-TCRβ Ab. These cells were permeabilized and stained with APC-conjugated anti-IFNγ Ab. CD19−CD1d-tetramer+ or TCRβ+CD1d-tetramer+ cells were defined as iNKT cells and analyzed.
In vitro and vivo cytotoxic T lymphocytes response assay
The in vitro SIINFEKL- specific cytotoxic T lymphocytes response was measured using a caspase-3 cleavage assay.32 In brief, CD8 T cells were isolated from the vaccinated mice by using anti-CD8 microbeads and magnetic activated cell sorting. Splenocytes from syngenic mice were used as target cells after labeled with DDAO-SE and 1 µg/mL peptide. The sorted CD8 T cells were co-cultured with the target splenocytes at a 1:1 ratio for 2 h at 37°C. The cells were permeabilized and stained with PE-conjugated anti-cleaved caspase-3 antibody. The percentage of cleaved caspase-3-positive cells among DDAO-SE-labeled target cells were analyzed by flow cytometry. The in vivo SIINFEKL-specific cytolytic activity of CD8 T-cell responses was measured as described previously.33 Briefly, splenocytes were either loaded with 1 µg/mL peptide or left untouched before being labeled with CFSE at different concentrations (10 and 1 µmol/L, respectively). Equal numbers of the two populations were mixed and injected i.v. into mice. 18–48 h later, splenocytes from the recipients were analyzed by flow cytometer to assess the peptide-specific killing. The ovalbumin-specific lysis was calculated as follows: r = % CFSElow / % CFSEhigh and % lysis = [1 – (runprimed / rprimed)] × 100, where r is the ratio.
L. monocytegenes infection and tumor challenge
C57BL/6 mice were vaccinated with the indicated cellular vaccine (day 0) before they were i.v. challenged with 5 × 104 live L. monocytogenes expressing OVA (day 10). Three days after the bacterial challenge, bacterial burden in spleen and liver was measured by culturing serially diluted homogenized spleen and liver on brain-heart infusion agar plate. Peptide-specific IFNγ-secreting cells in PBMC were measured after restimulation with SIINFEKL in the presence of Golgi-Plug for 5 h followed by intracellular staining of IFNγ. In a tumor challenge model, mice vaccinated with indicated cellular vaccines were i.v. challenged with live 2 × 105 B16-OVA 10 d after vaccination. Two weeks after the tumor challenge, tumor foci in the lung were counted and OVA-specific CD8 T cells were measured after staining with PE-conjugated OT-I tetramer in combination with FITC-conjugated anti-CD8 Ab. To test protective antitumor effect by autologous vaccine, αGC loaded EG-7 cells were irradiated before i.v. vaccination (1x106 cells). At 7 d later, mice were s.c. challenged 1 × 106 live EG-7 cells and then the survival and tumor size of mice were monitored. To deplete CD8 T cells, 100 µg of anti-CD8 mAb (563.8) was i.p. injected twice (on day-3 and -1) into the mice vaccinated with EG-7/αGC on day-7. On day 0, mice were i.v. challenged with 1 × 106 live EG-7 cells and the survival and tumor volume were monitored. Tumor volume based on caliper measurements were calculated by the modified ellipsoidal formula, 1/2(Length × Width2).
Statistics
Statistical values were assessed by the Student's t-test. P values were expressed and error bars are SE. The correlation of tumor volume was analyzed for significance using 2-way analysis of variance (ANOVA).The Kaplan-Meier method was used to estimate the survival outcomes and groups were compared with log-rank statistic.
Acknowledgments
We thank Dr. Hao Shen for Listeria-Ova, the FACS Core Facility at the MD Anderson for assistance with cell sorting and the NIH tetramer core facility (Atlanta, GA) for providing PBS57-loaded CD1d-tetramers. This study was funded in part by grants from the National Institutes of Health (C.D.) and an MD Anderson Interdisciplinary Research Project (C.D.). C.D. is a Cancer Research Institute Investigator and a Research Trust Fellow of MD Anderson Cancer Center. D.Z. is supported by MD Anderson Cancer Center and a Developmental Award from NCI Joe Moakley Leukemia Spore grant. The Flow Cytometry Core Facility is supported by The University of Texas MD Anderson Cancer Center Support Grant CA16672 (NIH).
Disclosure of Potential Conflicts Of Interest Statement
No potential conflicts of interests were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18479
References
- 1.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–17. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 2.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung Y, Chang WS, Kim S, Kang CY. NKT cell ligand alpha-galactosylceramide blocks the induction of oral tolerance by triggering dendritic cell maturation. Eur J Immunol. 2004;34:2471–9. doi: 10.1002/eji.200425027. [DOI] [PubMed] [Google Scholar]
- 4.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–18. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–17. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–74. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–93. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 8.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–6. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–8. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 10.Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, et al. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J Immunol. 2005;174:4696–705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 11.Chung Y, Kim BS, Kim YJ, Ko HJ, Ko SY, Kim DH, et al. CD1d-restricted T cells license B cells to generate long-lasting cytotoxic antitumor immunity in vivo. Cancer Res. 2006;66:6843–50. doi: 10.1158/0008-5472.CAN-06-0889. [DOI] [PubMed] [Google Scholar]
- 12.Chung Y, Qin H, Kang CY, Kim S, Kwak LW, Dong C. An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent antilymphoma immunity. Blood. 2007;110:2013–9. doi: 10.1182/blood-2006-12-061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu K, Goto A, Fukui M, Taniguchi M, Fujii S. Tumor cells loaded with alpha-galactosylceramide induce innate NKT and NK cell-dependent resistance to tumor implantation in mice. J Immunol. 2007;178:2853–61. doi: 10.4049/jimmunol.178.5.2853. [DOI] [PubMed] [Google Scholar]
- 14.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norbury CC, Basta S, Donohue KB, Tscharke DC, Princiotta MF, Berglund P, et al. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304:1318–21. doi: 10.1126/science.1096378. [DOI] [PubMed] [Google Scholar]
- 16.Ueda N, Kuki H, Kamimura D, Sawa S, Seino K, Tashiro T, et al. CD1d-restricted NKT cell activation enhanced homeostatic proliferation of CD8+ T cells in a manner dependent on IL-4. Int Immunol. 2006;18:1397–404. doi: 10.1093/intimm/dxl073. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–8. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, et al. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood. 2002;100:1728–33. [PubMed] [Google Scholar]
- 19.Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med. 2005;201:1973–85. doi: 10.1084/jem.20042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–34. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 21.Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol. 2001;166:6012–8. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 22.Ikarashi Y, Mikami R, Bendelac A, Terme M, Chaput N, Terada M, et al. Dendritic cell maturation overrules H-2D-mediated natural killer T (NKT) cell inhibition: critical role for B7 in CD1d-dependent NKT cell interferon gamma production. J Exp Med. 2001;194:1179–86. doi: 10.1084/jem.194.8.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaneda H, Takeda K, Ota T, Kaduka Y, Akiba H, Ikarashi Y, et al. ICOS costimulates invariant NKT cell activation. Biochem Biophys Res Commun. 2005;327:201–7. doi: 10.1016/j.bbrc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Cross AH, Lyons JA, San M, Keeling RM, Ku G, Racke MK. T cells are the main cell type expressing B7-1 and B7-2 in the central nervous system during acute, relapsing and chronic experimental autoimmune encephalomyelitis. Eur J Immunol. 1999;29:3140–7. doi: 10.1002/(SICI)1521-4141(199910)29:10<3140::AID-IMMU3140>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 25.Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions. J Immunol. 2004;172:34–9. doi: 10.4049/jimmunol.172.1.34. [corrections] [DOI] [PubMed] [Google Scholar]
- 26.Nurieva R, Thomas S, Nguyen T, Martin-Orozco N, Wang Y, Kaja MK, et al. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25:2623–33. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting Edge: Programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol. 2006;177:8291–5. doi: 10.4049/jimmunol.177.12.8291. [DOI] [PubMed] [Google Scholar]
- 28.Exley M, Garcia J, Wilson SB, Spada F, Gerdes D, Tahir SM, et al. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 2000;100:37–47. doi: 10.1046/j.1365-2567.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nebozhyn M, Loboda A, Kari L, Rook AH, Vonderheid EC, Lessin S, et al. Quantitative PCR on 5 genes reliably identifies CTCL patients with 5% to 99% circulating tumor cells with 90% accuracy. Blood. 2006;107:3189–96. doi: 10.1182/blood-2005-07-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurieva RI, Mai XM, Forbush K, Bevan MJ, Dong C. B7h is required for T cell activation, differentiation, and effector function. Proc Natl Acad Sci USA. 2003;100:14163–8. doi: 10.1073/pnas.2335041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 32.He L, Hakimi J, Salha D, Miron I, Dunn P, Radvanyi L. A sensitive flow cytometry-based cytotoxic T-lymphocyte assay through detection of cleaved caspase 3 in target cells. J Immunol Methods. 2005;304:43–59. doi: 10.1016/j.jim.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Coles RM, Mueller SN, Heath WR, Carbone FR, Brooks AG. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J Immunol. 2002;168:834–8. doi: 10.4049/jimmunol.168.2.834. [DOI] [PubMed] [Google Scholar]