Abstract
In this overview, we discuss the role of class II-associated invariant chain peptide (CLIP) in acute myeloid leukemia (AML), one of the few tumors expressing HLA class II. The clinical impact, function and regulation of CLIP expression on leukemic cells is addressed, indicating its potential as immunotherapeutic target in AML.
Keywords: human leukocyte antigen, immune surveillance, leukemic cell, major histocompatibility complex, T cell immunity, tumor immunogenicity
Several immunotherapeutic strategies are currently available or tested to trigger the immune system against tumor cells in cancer patients. In this respect, the capacity of tumor cells to affect or escape from T cell immunity should be taken into consideration for the success of immunotherapy. Until now, many immune escape mechanisms have been demonstrated in tumor cells that interfere with T cell function, such as the production of immunosuppressive cytokines and the inhibition of T cell-induced apoptosis. The first requirement for interaction between tumor cells and T cells is efficient antigen presentation. Defects in the HLA class I (HLA-I) antigen presentation pathway are frequently seen in tumor cells, but less is reported on HLA class II (HLA-II) antigen presentation as most tumors lack expression of HLA-II and costimulatory molecules. However, leukemic cells of patients with acute myeloid leukemia (AML) do express these molecules, suggesting that HLA-II antigen presentation plays a role in T cell immunity for this type of tumor.
We recently provided evidence that the presentation of the class II-associated invariant chain peptide (CLIP) on leukemic cells can serve as an immune escape mechanism in AML by disturbing the activation of tumor-reactive CD4+ T cells.1 Separation of both CLIP- and CLIP+ leukemic cells from the same untreated AML patients made it possible to examine the effect of CLIP expression on the function of autologous CD4+ T cells. CD4+ T cells cultured with CLIP- leukemic cells showed stronger activation, increased polarization toward Th1 and effector memory cells, and higher antigen-specificity as compared with CD4+ T cells from CLIP+ cocultures.
The detrimental impact of CLIP with regard to CD4+ T cell function fits with previous observations that high expression of CLIP on leukemic cells at diagnosis is associated with a high relapse risk and poor survival in AML.2 To explain the underlying mechanism of this effect, one could refer to studies using HLA-II-transfected tumor cells. In the absence of the precursor of CLIP, the invariant chain (Ii), these tumor cells have increased ability to present endogenous antigens and activate tumor-specific CD4+ T cells.3 Because Ii classically binds to HLA-II molecules in the endoplasmic reticulum to block premature binding of endogenous antigens and mediate their transport to endosomal compartments,4 a possible explanation may be that CLIP on leukemic cells indicates reduced presentation of potentially leukemia-associated endogenous antigens on HLA-II molecules. In one AML patient, it was shown that antigen-specific CD4+ T cells from CLIP- cocultures responded to CLIP- leukemic cells, but not to CLIP+ leukemic cells and monocytes.1 Moreover, in CLIP- leukemic cell lines, HLA-II processing was independent of Ii and relied on the proteasome and transporter associated with antigen processing (TAP),5 two mediators of endogenous antigen processing for HLA-I molecules. Most interestingly, abundance of CLIP on primary leukemic cells was also found for a specific subtype of HLA-II- AML, acute promyelocytic leukemia.6 Further analysis of this HLA-II-unrelated association of CLIP in leukemic cells revealed that it promiscuously bound to HLA-I molecules as well (submitted for publication), predominantly in TAP-deficient cells. This suggests additional involvement of CLIP in aberrant HLA-I antigen presentation and escape from CD8+ cytotoxic T cell (CTL)-mediated eradication.
In either way, CLIP on leukemic cells probably contributes to a leukemia-protective T cell environment leading to outgrowth of AML. Since the presence of CLIP on residual leukemic cells at follow-up is associated with increased relapse risk in AML (submitted for publication), CLIP also seems to be involved in T cell immunity following first induction chemotherapy and is thus a promising target for immunotherapeutic intervention to prevent disease recurrence.
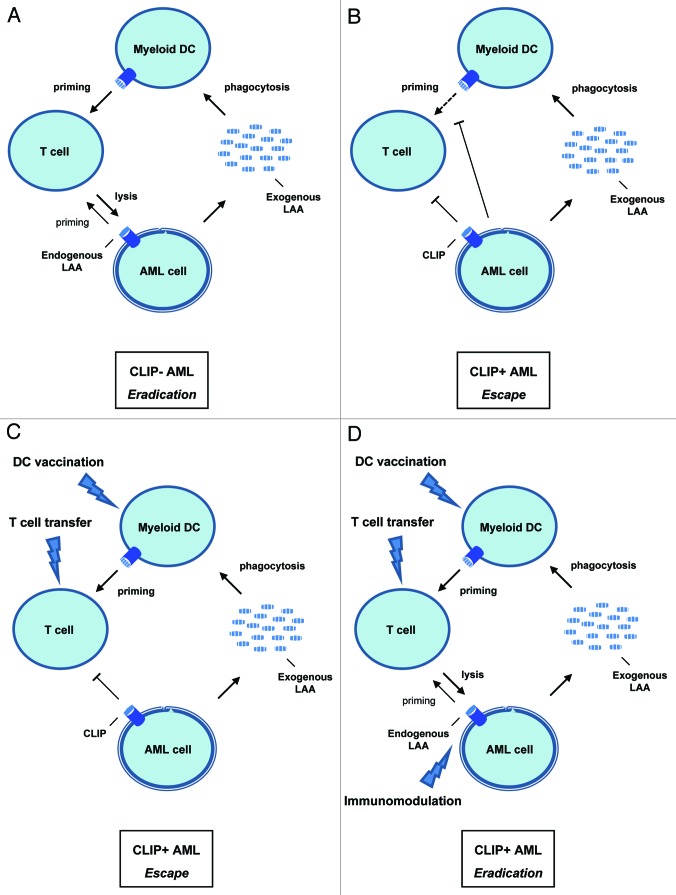
In Figure 1, a model is illustrated to show the potential influence of CLIP expression by leukemic cells on AML immunopathogenesis and on currently developed immunotherapeutic approaches to introduce anti-leukemic T cell immunity in AML, including dendritic cell (DC) vaccination and adoptive T cell transfer.7,8 In patients with CLIP- AML, leukemic cells should be well-recognized by presenting leukemia-associated antigens (LAAs) to CD4+ T cells or CTLs (Fig. 1A). This then initiates a potential feed-forward loop wherein exogenous antigens are internalized by DCs and presented for priming of leukemia-specific T cells. Only leukemia-specific priming of T cells might be suboptimal in these patients, which makes for example vaccination with LAA-loaded DCs a potential treatment option. However, in CLIP+ AML, CLIP on leukemic cells indicates deficient presentation of endogenous LAAs. This can put the feed-forward loop on hold, either directly by preventing their detection and eradication by T cells, or indirectly by creating an immunosuppressive environment that causes DC or T cell inactivation (Fig. 1B). Administration of ex vivo generated LAA-loaded DC vaccines or leukemia-specific T cells to these patients may establish LAA presentation to stimulate T cell priming.9 Still, it does not deal with CLIP expressed by leukemic cells that results in low immunogenicity and direct impairment of T cells (Fig. 1C). To circumvent this, additional immunotherapeutic strategies need to be included in current protocols that down-modulate CLIP in vivo (Fig. 1D). As a result, LAA presentation on HLA-I and HLA-II molecules might be simultaneously enhanced leading to effective T cell immunity against leukemic cells. One strategy may be to enhance the processing machinery of endogenous antigens, such as the stimulation of proteasomal and TAP function by histone deacetylase inhibitors (HDACi).10 The design of approaches that target the immune escape mechanisms used by leukemic cells to inhibit T cell activation could have great promise for future active immunotherapy in AML.
Figure 1.
The potential role of CLIP in AML immunopathogenesis and immunotherapy. Situations before and after immunotherapy in patients with AML are proposed: (A) Tumor immunity in untreated CLIP- AML; LAA-specific T cell priming and recognition are optimal due to enhanced endogenous LAA presentation by leukemic cells. (B) Tumor immune escape in untreated CLIP+ AML; leukemia-specific T cell priming as well as recognition are hampered because of inhibition of DC function and low immunogenicity by CLIP+ leukemic cells. (C) Tumor immune escape in treated CLIP+ AML; although priming of leukemia-specific T cells is resolved by DC vaccination or T cell transfer, leukemic cells still escape their recognition by expressing CLIP. (D) Tumor immunity in treated CLIP+ AML; by using DC vaccination or T cell transfer in combination with in vivo immunomodulatory drugs, both T cell priming and recognition are targeted, which might induce a potent immune response against leukemic cells.
Glossary
Abbreviations:
- AML
acute myeloid leukemia
- CLIP
class II-associated invariant chain peptide
- HDACi
histone deacetylase inhibitor
- HLA-I
HLA class I
- HLA-II
HLA class II
- Ii
invariant chain
- LAA
leukemia-associated antigen
- TAP
transporter associated with antigen processing
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18100
References
- 1.van Luijn MM, van den Ancker W, Chamuleau ME, Zevenbergen A, Westers TM, Ossenkoppele GJ, et al. Absence of class II-associated invariant chain peptide on leukemic blasts of patients promotes activation of autologous leukemia-reactive CD4+ T cells. Cancer Res. 2011;71:2507–17. doi: 10.1158/0008-5472.CAN-10-3689. [DOI] [PubMed] [Google Scholar]
- 2.Chamuleau ME, Souwer Y, van Ham SM, Zevenbergen A, Westers TM, Berkhof J, et al. Class II-associated invariant chain peptide expression on myeloid leukemic blasts predicts poor clinical outcome. Cancer Res. 2004;64:5546–50. doi: 10.1158/0008-5472.CAN-04-1350. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong TD, Clements VK, Martin BK, Ting JP, Ostrand-Rosenberg S. Major histocompatibility complex class II-transfected tumor cells present endogenous antigen and are potent inducers of tumor-specific immunity. Proc Natl Acad Sci USA. 1997;94:6886–91. doi: 10.1073/pnas.94.13.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha N, Neefjes J. MHC class II molecules on the move for successful antigen presentation. EMBO J. 2008;27:1–5. doi: 10.1038/sj.emboj.7601945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Luijn MM, Chamuleau ME, Ressing ME, Wiertz EJ, Ostrand-Rosenberg S, Souwer Y, et al. Alternative Ii-independent antigen-processing pathway in leukemic blasts involves TAP-dependent peptide loading of HLA class II complexes. Cancer Immunol Immunother. 2010;59:1825–38. doi: 10.1007/s00262-010-0908-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Luijn MM, Westers TM, Chamuleau ME, van Ham SM, Ossenkoppele GJ, van de Loosdrecht AA. Class II-associated invariant chain peptide expression represents a novel parameter for flow cytometric detection of acute promyelocytic leukemia. Am J Pathol. 2011;179:2157–61. doi: 10.1016/j.ajpath.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 8.Urba WJ, Longo DL. Redirecting T cells. N Engl J Med. 2011;365:754–7. doi: 10.1056/NEJMe1106965. [DOI] [PubMed] [Google Scholar]
- 9.van den Ancker W, van Luijn MM, Ruben JM, Westers TM, Bontkes HJ, Ossenkoppele GJ, et al. Targeting Toll-like receptor 7/8 enhances uptake of apoptotic leukemic cells by monocyte-derived dendritic cells but interferes with subsequent cytokine-induced maturation. Cancer Immunol Immunother. 2011;60:37–47. doi: 10.1007/s00262-010-0917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setiadi AF, Omilusik K, David MD, Seipp RP, Hartikainen J, Gopaul R, et al. Epigenetic enhancement of antigen processing and presentation promotes immune recognition of tumors. Cancer Res. 2008;68:9601–7. doi: 10.1158/0008-5472.CAN-07-5270. [DOI] [PubMed] [Google Scholar]