The ciliated protozoa Tetrahymena thermophila and T. pyriformis have long been popular among molecular biologists as model organisms in which to elucidate the function of telomeres and have been instrumental in the study of ribosomal catalytic activity and the development of ribozymes (1, 2). In a smaller way they have captured the imagination of cell biologists for the remarkable way in which they embody the attributes of multicellular organisms in a single primitive cell (Fig. 1). They move, they have the equivalent of mouth parts to ingest bacteria that they deliver to digestive vacuoles and excrete the residues at their rear ends. They also have an osmoregulatory mechanism incorporated in a tubular-vesicular system that functions not unlike a kidney to permit them to live in saline or hypoosmotic environments (3). What recently has emerged is that they may provide a very powerful genetic tool for elucidation of the molecular mechanisms involved in the regulated secretion of proteins, polypeptide hormones, and neurotransmitters. Regulated exocytosis in metazoans takes a number of distinct forms exemplified by release of the amine neurotransmitters of synaptic vesicles mediated by changes in membrane potential, the fusion of glucose transporter containing vesicles in muscle and fat in response to insulin stimulation, the release of pancreatic digestive enzymes by gut peptides and neuronal stimulation and insulin secretion in response metabolic signals. The SNARE hypothesis (4) points to a common molecular mechanism of exocytosis; nevertheless, fundamental differences remain in the biogenesis of dense core vesicles (DCV) containing polypeptide hormones or digestive enzymes, which form directly from the trans–Golgi network, and the synaptic vesicles and glucose transporter vesicles, which appear to be derived from an early endosome compartment (5). The DCVs cannot be recycled in the same way as synaptic or transporter vesicles because their cargo is derived from the secretory pathway itself. Much has been learned from the analysis of secretory mutants in yeast concerning sorting from the Golgi to the digestive vacuole and the mechanism of exocytosis by either the classical pathway or alternative pathways via the cytoplasm. However, neither Saccharomyces cerevisiae nor the fission yeasts display intracellular storage of peptides for regulated secretion. Although they actively secrete peptide pheromones the analysis of these pathways is more relevant to the understanding of constitutive secretion of proteins such as albumin (alpha factor pathway) or fibroblast growth factor (A-factor pathway). Biochemical analyses of dense core secretory granules from a number of tissue sources in higher eukaryotes has been hampered by the heterogeneity of proteins associated with these structures (most of which perform cell-specific functions) and from the difficulty of obtaining enough starting material. For the most part, the available cell lines perform badly in comparison to the parental tissue in that they store relatively small amounts of product, sort inefficiently, and have a high rate of constitutive or basal secretion.
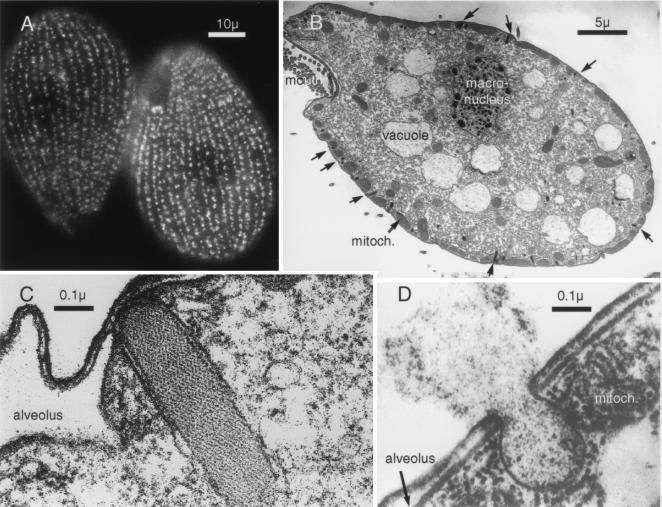
Figure 1.
Morphological features of Tetrahymena. (A) Immunofluorescence staining of mucocyst p80 demonstrates the presence of around 4,000 organelles arrayed as meridians covering the entire surface of the organism apart from the mouth structure. The cilia that provide locomotion lie between the meridians. (Scale bar = 10 μ.) (B) Electron micrograph of longitudinal section shows the mouth parts, digestive vacuoles and the macronucleus containing 45 copies of the five chromosomes. The mucocysts appear on the cell surface at docking sites interposed between mitochondria. (Scale bar = 5 μ.) (C) A docked mucocyst. The secretory material forms a compact paracrystalline core enveloped in a bilayer, which at the tip is apposed to the plasma membrane. On either side of the mucocyst are the flattened sacs of an alveolus, membrane-enveloped organelles that in ciliated protozoa act as dynamic Ca stores (30) (Scale bar = 0.1 μ.) (D) Exocytosis of a mucocyst shows fusion of the plasma and mucocyst membranes and expansion and expulsion of the mucocyst contents. The juxtaposition of the mitochondria and the mucocyst is also evident. (Scale bar = 0.1 μ.)
The potential of using Tetrahymena to investigate the molecular components of the regulated pathway of protein secretion and its regulation is illustrated by Haddad and Turkewitz (6) in this edition of the Proceedings. The question being addressed here is one that has intrigued endocrinologists and gastroenterologists alike, namely how does a secretory cell “know” that it has released its cargo and what signals the replenishment of the stored material. The raison d’etre of regulated protein secretion is, after all, to accommodate demands for very rapid and sometimes sustained release of hormones or enzymes that cannot be met by up-regulation of rates of transcription or translation. Like a rapid deployment force, such systems have an additional component in their regulation in that they respond to anticipated needs of the organism and not simply the intensity of the current stimulus. Haddad and Turkewitz (6) show that replenishment of granule contents occurs through up-regulation of transcription of genes specifically encoding secretory granule proteins even under conditions where the cell is deprived of nutrients. Similar responses are seen in endocrine cells where secretion evoked by specific secretagogues is accompanied by stimulation of the biosynthesis of the granule cargo often by translational control in the short term or by specific activation of gene transcription. The response of the pancreatic β cell to glucose is representative in that respect and is one of the best characterized (7, 8). The surprising answer from the present study (6) is that it is the exocytotic event per se that initiates the signal to the nucleus. Thus mutants without granules or mutants in which granules fail to dock or where docking occurs but exocytosis fails do not show activation of the transcription of the granule matrix proteins Grl 1 and Grl 4. In cells from multicellular organisms, conventional wisdom has it that the stimulus for secretion, a derived intracellular messenger, or the secreted product provides the signal for activation of transcription. Some evidence exists in favor of both mechanisms though none of it is entirely convincing. In the pancreatic β cell the pathways that lead to the activation of exocytosis, translation, and transcriptional activation share in common the requirement for glucose metabolism but diverge before the effects of the secretagogue on intracellular Ca or cyclic nucleotides. The transcriptional, translation, and secretory responses can be dissociated, for example, by blockage of L-type Ca channels or phosphodiesterase inhibitors. Whether secreted insulin activates insulin biosynthesis is unresolved. Although the β cell is equipped with classical insulin receptors, it is difficult to perceive how these could convey a graded response given that the concentration of the hormone in the interstitial space is likely to be saturating. The response might, of course, be mediated by low-affinity insulin-like growth factor 1 receptors or via receptors to the other minor bioactive constituents, which are cosecreted with insulin. In the case of Tetrahymena where the extracellular environment is variable and cannot be regulated by the organism and where exocytosis appears to be activated by nonspecific mechanical or chemical irritants it makes better sense for the organism to monitor the exocytotic event itself. The data presented by Haddad and Turkewitz (6) does not formally exclude an effect mediated by secreted peptides working on cell surface receptors or by more general mechanisms such as ingestion of the secreted material and a metabolic effect as is implicated in the response of CyP, a starvation-induced cysteine protease in these organisms. A signal originating at the exocytotic site also might explain the remarkable fact that when regranulation is complete the granule number approximates that present originally and also that no evidence is found for overshoot in granule biogenesis, which would be manifest, for example, in additional population of undocked granules.
The paper (6) itself is testimony to the recent development of techniques that have converted Tetrahymena from a genetically intractable organism to one in which one now can envisage performance of saturation mutagenesis and the delineation of a whole range of genes involved in the biogenesis and function of the DCV. In the broader context, it needs to be asked how convenient is the model to use and to what extent can the results be extrapolated to higher organisms? On the negative side is the fact that Tetrahymena uses a different genetic code to mammalian cells and has two nuclei, a transcriptionally inactive micronucleus that functions during meiosis and cell division and a transcriptionally active macronucleus that contains up to 40 copies of the genome (9, 10). Until recently the lack of vector systems and methods for bulk transformation of cells seemed to preclude manipulation of their genetic material by transient expression, stable transformation, or homologous recombination strategies. However, the development of strains permitting rapid generation of homozygous recessive mutants through parental cytogamy (11) and the progressive and complete inactivation or replacement of macronuclear genes by selection of neomycin-encoding transgenes using graded antibiotic treatment (12, 13) has changed the scenario dramatically. Further developments are likely with the introduction of ribosome-based vectors, which will permit use of antisense strategies that are at present unavailable in any other organism (14). As far as a model for the regulated pathway of secretion is concerned Tetrahymena has the advantage in that it can be grown in limitless quantities in simple defined media in solution culture with a generation time of 2.5 hr. Basal secretion is negligible; however, complete degranulation of the cell can be induced with a time course probably in milliseconds (15). It is clear from existing mutants that the regulated secretory pathway is not essential for survival and that deletion or mutagenesis of the structural genes associated with the pathway are unlikely to result in lethal phenotypes. Another ciliated protozoa, Paramecium, provides an alternative model for genetic studies of the regulated pathway; however, its genetic material seems to have undergone many more duplications, and thus the granule contents appear to be the product of possibly 100 genes (16) as opposed to the 7–10 proposed in Tetrahymena (J. W. Verbsky and A. P. Turkewitz, personal communication). Although similar to Tetrahymena in many respects Paramecium is actually a evolutionary distant organism separated by around 1.5 billion years, a similar distance from Tetrahymena to yeast (17). Both organisms are large in comparison to yeast and mammalian cells, which allows direct visualization and selection of individual secretory mutants and introduction of proteins, antibodies, and other material by microinjection. An additional feature described in the paper (6) is the ability of conjugating Tetrahymena to transfer cytosolic constituents before the exchange of genetic material, thus allowing performance of somatic complementation analysis.
The question of whether the granules of Tetrahymena (mucocysts) or Paramecium (trichocysts) are truly analogous to, say, the zymogen granules of the exocrine pancreas or the various neurosecretory granule types is not fully resolved but appears to be answered in the affirmative. Tetrahymena, in fact, has two other secretory pathways that can be defined kinetically, morphologically, or genetically: a constitutive release mechanism whereby proteins appear in the media within half an hour of their synthesis, and another responsible for the release of hydrolyic enzymes contained in cytoplasmic “secretory lysosomes” (t1/2 = 4 hr). The mucocyst-mediated pathway in which a DCV is stably docked at the site of exocytosis (18–20) has its parallel in higher organisms in cells with rapid secretory responses as typified by cortical granules of oocyte. Ellipsoidal granules like the mucocyst (0.2 × 1 μ) are unusual in mammals but encountered in granules like the Weibel–Palade bodies of endothelial cells, whose shape is dictated by the formation of parallel linear paracrystalline arrays of von Willebrand factor.
The matrix of the Tetrahymena mucocyst is derived from seven or eight major gene products that share little structural similarity with other secreted products of cells with a regulated pathway apart from the general property of a largely acidic character. The mucocyst and trichocyst cores, in common with many other DCVs, form compacted paracrystalline arrays, which upon exocytosis and exposure to extracellular calcium undergo an explosive expansion. This results in the encapsulation of the organism with the secreted product, a response that is thought to protect the organism from predation by other protists (21). Whether mucocysts and trichocysts contain protective bioactive molecules analogous to the secretions of the skin of many amphibia has not been explored. It is, however, indicated by the fact that the stored proteins are generated as proproteins, which are processed to mature forms by limited proteolysis upon segregation to the immature granules (22, 23). That said, the processing sites, although defined by primary structure, differ from the polybasic motif found in yeast and higher organisms that are cleaved by the family of subtilisin-related endopeptidases (24). Although the process of cataloguing the sequences of the secreted proteins of Tetrahymena is well underway, little is presently known concerning the granule membrane components and regulatory machinery. It will be intriguing to learn whether homologues exist in these organisms of the syntaxin, SNAPs, synaptobrevin, synaptotagmin, or other molecules, which have been implicated in synaptic vesicle docking and exocytosis, and whether granule formation is dependent on Golgi-specific clathrin adaptors, ADP ribosylation factors, and lipid-modifying enzymes. GTP-binding proteins of the Rab family are present in ciliated protozoa (25), and they also may have signal transduction pathways dependent upon tyrosine phosphorylation and dephosphorylation (26, 27), which possibly play a role in dense core granule biogenesis in higher organisms (28, 29).
The next step in elucidation of the mechanism by which transcription is coupled to exocytosis in Tetrahymena probably will come from further screening and examination of mutants that uncouple the process. At present it is conjectured that the signal from the exocytotic event to the nucleus may be mediated either by the cytoskeleton or associated with the phosphorylation/dephosphorylation of soluble factors. Recessive mutants here, however, may be lethal, and conditional mutants may need to be developed. In a very different context, the results of the present study (6) suggest an immediate approach to the cloning of minor mucocyst constituents through the application of subtractive hybridization or differential display techniques, because as is seen in other systems, the biosynthesis of secretory granule proteins appears to be coordinately regulated. If the impact of yeast genetics on elucidation of the molecular mechanisms involved in membrane traffic is any indication, one can foresee a very rapid exploitation of this model to unravel the molecular mechanisms involved in the biogenesis and exocytosis of dense-cored secretory granules, particularly in the area of the granule membrane components, which play a fundamental role in the mechanism of biogenesis, movement, and exocytosis, and hitherto have escaped direct isolation and identification by biochemical procedures.
References
- 1.Blackburn E H. Nature (London) 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 2.Cech T R. Nobel Lect Biosci Rep. 1990;10:239–261. doi: 10.1007/BF01117241. [DOI] [PubMed] [Google Scholar]
- 3.Hausmann K, Bradbury P C. Ciliates, Cells as Organisms. Stuttgart: Gustav Fischer; 1996. p. 485. [Google Scholar]
- 4.Hay J C, Scheller R H. Curr Opin Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- 5.Herman G A, Bonzelius F, Cieutat A M, Kelly R B. Proc Natl Acad Sci USA. 1994;91:12750–12754. doi: 10.1073/pnas.91.26.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddad A, Turkewitz A P. Proc Natl Acad Sci USA. 1997;94:10675–10680. doi: 10.1073/pnas.94.20.10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guest P C, Bailyes E M, Rutherford N G, Hutton J C. Biochem J. 1991;274:73–78. doi: 10.1042/bj2740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odagiri H, Wang J, German M S. J Biol Chem. 1996;271:1909–1915. doi: 10.1074/jbc.271.4.1909. [DOI] [PubMed] [Google Scholar]
- 9.Bruns P J. In: The Molecular Biology of Ciliated Protozoa. Gall J G, editor. New York: Academic; 1986. pp. 27–44. [Google Scholar]
- 10.Prescott D M. Microbiol Rev. 1994;58:233–267. doi: 10.1128/mr.58.2.233-267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole E S, Bruns P J. Genetics. 1993;132:1017–1031. doi: 10.1093/genetics/132.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassidy-Hanley D, Bowen J, Lee J H, Cole E A L, VerPlank L A, Gaertig J, Gorovsky M A, Bruns P J. Genetics. 1997;146:135–147. doi: 10.1093/genetics/146.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaertig J, Gu L, Hai B, Gorovsky M A. Nucleic Acids Res. 1994;22:5391–5398. doi: 10.1093/nar/22.24.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweeney R, Fan Q, Yao M-C. Proc Natl Acad Sci USA. 1996;93:8518–8523. doi: 10.1073/pnas.93.16.8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoll G, Braun C, Plattner H. J Cell Biol. 1991;113:1295–1304. doi: 10.1083/jcb.113.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madeddu L, Gautier M-C, Vayssié L, Houari A, Sperling L. Mol Biol Cell. 1995;6:649–659. doi: 10.1091/mbc.6.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright, A.-D. & Lynn, D. H. (1997) Arch. Protistenkd., in press.
- 18.Allen R D. J Protozool. 1967;14:553–565. doi: 10.1111/j.1550-7408.1967.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 19.Beisson J, Lefort-Tran M, Pouphile M, Rossignol M, Satir B. J Cell Biol. 1976;69:126–143. doi: 10.1083/jcb.69.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pouphile M, Lefort-Tran M, Plattner H, Rossignol M, Beisson J. Biol Cell. 1986;56:151–162. [Google Scholar]
- 21.Harumoto T, Miyake A. J Exp Zool. 1991;260:84–92. [Google Scholar]
- 22.Adoutte A, Garreau de Loubresse N, Beisson J. J Mol Biol. 1984;180:1065–1081. doi: 10.1016/0022-2836(84)90271-7. [DOI] [PubMed] [Google Scholar]
- 23.Turkewitz A P, Madeddu L, Kelly R B. EMBO J. 1991;10:1979–1987. doi: 10.1002/j.1460-2075.1991.tb07727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chilcoat N D, Melia S M, Haddad A, Turkewitz A P. J Cell Biol. 1996;135:1775–1787. doi: 10.1083/jcb.135.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson J B. J Protozool. 1991;38:495–501. doi: 10.1111/j.1550-7408.1991.tb04823.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Himes R H, Dentler W L. J Biol Chem. 1994;269:21460–21466. [PubMed] [Google Scholar]
- 27.Kissmehl R, Treptau T, Hofer H W, Plattner H. Biochem J. 1996;317:65–76. doi: 10.1042/bj3170065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin C D, Shields D. J Cell Biol. 1996;135:1471–1483. doi: 10.1083/jcb.135.6.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasmeier C, Hutton J C. J Biol Chem. 1996;271:18161–18170. doi: 10.1074/jbc.271.30.18161. [DOI] [PubMed] [Google Scholar]
- 30.Stelly N, Mauger J-P, Claret M, Adoutte A. J Cell Biol. 1991;113:103–112. doi: 10.1083/jcb.113.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]