Abstract
Effective treatment of solid cancers by tumor-directed DC-vaccines still remains a challenge in clinical oncology. For therapeutic success, knock-down of tumor-specific tolerance appears mandatory before a potent tumor-specific cytotoxic T-cell response can be triggered by DC-vaccinations. Evidence is emerging that lytic virus infection in tumors can provide valuable help.
Keywords: DC-vaccination, immunotherapy, oncolysis, tumor, virotherapy
Tumor-responsive cytotoxic T-cells play an important role in tumorimmunosurveillance and have great potential for curative tumor therapy. When adoptively transferred, T-cells are able to eradicate established tumors, and their intrinsic ‘seek and destroy’ function makes them a unique tool to address metastatic diseases. Since priming and expansion of cytotoxic T-cells is governed by dendritic cells (DC), the development of antitumoral DC-vaccines has been expedited during the past decade. However, despite recent progress, such as Sipuleucel-T (Provenge™) for treatment of metastatic prostate tumors, DC-based immunotherapies against established solid tumors did not meet the high expectations.
If applied against solid tumors, DC-vaccines are facing strong tumor-specific tolerogenic conditions. Tumor-specific tolerance is the result of complex regulatory events and adaptation processes which allow the tumor to be accepted by the host’s organism even though a significant spectrum of immunogenic alterations is present. The tumor microenvironment decisively contributes to the development of tumor-specific tolerance by specifically recruiting suppressor cell types like tumor-associated macrophages (TAMs), in particular those of the M2 phenotype, regulatory T-cells (Treg) and myeloid derived suppressor cells (MDSC). These cells provide an immunosuppressive, antiapoptotic milieu by release of cytokines such as TGFβ, IL-10 and IL-6 thus converting tumor tissue into an immunoprivileged site. Immunosuppressive functions do not only effectively choke down priming and expansion of T-cells, but also promote their functional anergy or may cause selective deletion. Breaking tumor-specific tolerance is therefore regarded as a major goal of cancer immunotherapy. Tumor-targeted, oncolytic virus infections could play a pivotal role in this regard. Many virus species such as adenovirus, poxvirus, measles, HSV, VSV or Reovirus have been used as oncolytic agents or were genetically reengineered for this purpose.1 Recently, a GM-CSF armed poxvirus (JX-594) has successfully entered clinical development.2 Besides their function to selectively infect and kill tumor cells, oncolytic viruses also represent a promising strategy to induce tumor-directed immunity by induction of local inflammation and cross-presentation of cellular antigens. It is now accepted that both oncolysis and immune response contribute to the therapeutic benefit of virotherapy.3
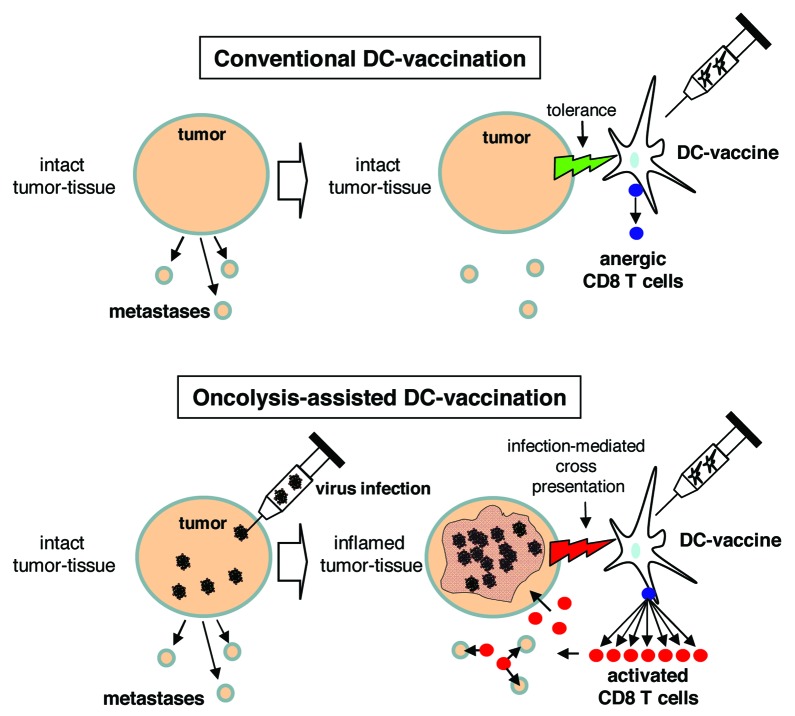
In a recent study,4 we investigated whether a tumor-directed DC-vaccination in tumor-bearing mice could provoke an effective antitumoral immune response, if the tumor is affected by a virus infection (Fig. 1). Injection of the telomerase-selectively replicating adenovirus hTert-Ad in subcutaneous tumors led to a fulminant but transient tumor inflammation characterized by lytic destruction of large tumor areas and massive lymphocyte infiltrations. Histologically, tumor inflammation was apparent at day 3 following virus injection. Virus clearance was then initiated and tumor recovery began. According to our hypothesis, a success of tumor-directed DC-vaccination, indicated by increasing numbers of tumor-directed CD8 T-cells, was only observable when the vaccine was given during apparent tumor inflammation. More important, the raised T-cell response led to a dramatically improved therapeutic outcome and even facilitated elimination of prestablished lung colonies, an important requirement for the treatment of disseminated diseases. Due to the correlation between oncolytic tumor inflammation and the success of vaccination we named this therapeutic regimen ‘oncolysis-assisted DC-vaccination’ (ODC). Observations that increased T-cell responses could be successfully raised against endogenous tumor-associated antigens such as telomerase and that antitumoral response increased at the expense of the virus-directed humoral response corroborated the physiologic relevance of ODC.
Figure 1. Schematic comparison of conventional and oncolysis-assisted DC-vaccination. In a conventional DC-vaccination (upper panel), a tumor-directed DC-vaccine is applied though a solid tumor mass is present in the patient. Consequently, tolerogenic properties of the tumor restrict efficient T cell priming. For oncolysis-assisted DC-vaccination (lower panel), a lytic virus infection is initiated in the tumor tissue to break tumor integrity and tolerance, and to provide tumor-associated antigens for cross presentation. Thus, onset of virus-mediated oncolysis and inflammation allows for efficient DC-vaccination.
Viral vectors are complex ‘biologics’ and are therefore suspected being troublesome in therapeutic applications. We therefore tried to mimic viral inflammation by use of TLR-ligands that are currently under clinical investigation as vaccination adjuvants. Interestingly, none of the used TLR ligands could successfully replace a ‘true’ virus infection in ODC. Only viral replication and oncolysis allowed for significant cross-presentation of tumor-associated antigens by tumor-resident DCs. Attempts to maximize ODC-mediated therapeutic efficacy by depletion of Tregs, a classical intervention to enhance T-cell-dependent immune responses, evened out all benefits that were characteristic for ODC. These findings are consistent with previous evidence that Tregs are not only suppressor cells but actively participate in orchestrating immune responses.5 We found that a compensatory induction of MDSC upon Treg depletion accounts for the failure of ODC. Furthermore, it has been reported that Tregs can undergo a rapid reprogramming into activated T helper cells under inflammatory conditions, a process that might play an important role in priming T-cell responses against new, cross-presented antigens.6 Therefore, it will be interesting to investigate a putative role of Treg reprogramming in ODC. Finally, it will be necessary to confirm the potential of ODC in the context of other virus types.
Taken together, our study supports the growing evidence that viruses are promising means for cancer immunotherapy. Correspondingly, it has been demonstrated that sequential delivery of a tumor-associated antigen by heterologous carrier viruses is able to significantly boost antitumoral immunity.7 Moreover, a large library of tumor-associated antigens has been applied via a virus-based platform to successfully cure tumors thus illustrating the great potential of viruses as targeted immune activators.8 It has been shown that conventional regimen such as radio- or chemotherapy or targeted molecular therapies are able to elicit detectable tumor-targeted immune responses that significantly determine the therapeutic outcome.9,10 In the future of tumor therapy, viruses could be ideally combined with such conventional or molecular therapies to pursue a multimodal strategy to break therapy resistance and boost tumor-directed immunity. In those complex regimens, intelligent adjustment of single treatments and their precise scheduling will be a challenge to fully exploit the potential of cancer immunotherapy.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/18099
References
- 1.Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther. 2009;9:1163–76. doi: 10.1517/14712590903170653. [DOI] [PubMed] [Google Scholar]
- 2.Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 3.Prestwich RJ, Errington F, Diaz RM, Pandha H, Harrington KJ, Melcher AA, et al. The Case of Oncolytic Viruses vs The Immune System: Waiting On The Judgment of Solomon. Hum Gene Ther. 2009;20:1119–32. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woller N, Knocke S, Mundt B, Gurlevik E, Struver N, Kloos A, et al. Virus-induced tumor inflammation facilitates effective DC cancer immunotherapy in a Treg-dependent manner in mice. J Clin Invest. 2011;121:2570–82. doi: 10.1172/JCI45585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–4. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, et al. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33:942–54. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridle BW, Stephenson KB, Boudreau JE, Koshy S, Kazdhan N, Pullenayegum E, et al. Potentiating cancer immunotherapy using an oncolytic virus. Mol Ther. 2010;18:1430–9. doi: 10.1038/mt.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kottke T, Errington F, Pulido J, Galivo F, Thompson J, Wongthida P, et al. Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors. Nat Med. 2011;17:854–9. doi: 10.1038/nm.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 10.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]