Abstract
The production of graceful skeletal movements requires coordinated activation of multiple muscles that produce torques around multiple joints. The work described herein is focused on one such movement, stance, that requires coordinated activation of extensor muscles acting around the hip, knee and ankle joints. The forces evoked in these muscles by external stimulation all have a complex dependence on muscle length and shortening velocities, and some of these muscles are bi-articular. In order to recreate sit-to-stand maneuvers in the anesthetized feline, we excited the hind limb musculature using intrafascicular multielectrode stimulation (IFMS) of the muscular branch of the sciatic nerve, the femoral nerve, and the main branch of the sciatic nerve. Stimulation was achieved with either acutely or chronically implanted Utah Slanted Electrode Arrays (USEAs) via subsets of electrodes 1) that activated motor units in the extensor muscles of the hip, knee, and ankle joints, 2) that were able to evoke large extension forces, and 3) that manifested minimal coactivation of the targeted motor units. Three hind limb force-generation strategies were investigated, including sequential activation of independent motor units to increase force, and interleaved or simultaneous IFMS of three sets of six or more USEA electrodes that excited the hip, knee, and ankle extensors. All force-generation strategies evoked stance, but the interleaved IFMS strategy also reduced muscle fatigue produced by repeated sit-to-stand maneuvers compared with fatigue produced by simultaneous activation of different motor neuron pools. These results demonstrate the use of interleaved IFMS as a means to recreate coordinated, fatigue-resistant multi-joint muscle forces in the unilateral hind limb. This muscle activation paradigm could provide a promising neuroprosthetic approach for the restoration of sit-to-stand transitions in individuals who are paralyzed by spinal cord injury, stroke, or disease.
Keywords: Neural prosthetics, intrafascicular stimulation, motor system, stance
1. Introduction
The reanimation of paralyzed muscles has been achieved using a number of electrode architectures that vary in invasiveness and complexity. These include surface electrodes [1, 2], epimysial electrodes [3], electrodes that are implanted within the central nervous systems [4, 5], peripheral nerve stimulating electrodes [6–8], and intrafascicular electrodes [9–13]. A number of these neuromuscular interfaces have been used in limited clinical applications to reanimate the muscles of human stance [14, 15], but their widespread use in producing stance in spinal-cord-injured subjects has been complicated by numerous challenges. These challenges include 1) the requirement for multiple interfaces to different motor nerves and/or extensor muscles, and the relatively complex surgeries and various problems associated with lead wire routing and connectors; 2) the inability to recruit muscle force physiologically, resulting in ungraceful and unnatural skeletal motions; 3) muscle fatigue that results from the over-stimulation of muscles required to ensure that the joints remain extended so the subject will not fall; and 4) the need to coordinate torques around multiple joints (hip, knee, and ankle), particularly when the biarticular muscles recruited can produce antagonistic actions around multiple joints.
The use of penetrating microelectrodes that can be inserted into the fascicles of the peripheral nerves [9–12] offer a new approach to the restoration of lost motor function resulting from nervous system damage or disease. The Utah Slanted Electrode Array (USEA) is an example of one such peripheral nerve interface [10]. Single USEAs that have been implanted in either the sciatic nerve, the femoral nerve, or the muscular branch of the sciatic nerve can be used to selectively activate many extensors and flexors around the ankle, knee, or hip joints, respectively [10, 16–19]. When implanted in the sciatic nerve of an anesthetized cat, the 100 microelectrodes of a single USEA can selectively activate up to nine of the extensor and flexor muscles of the ankle [10]. Further, a number of the implanted electrodes selectively activate independent groups of motor units in a targeted muscle, allowing recruitment of muscle forces by more biomimetic, physiologically based mechanisms that can generate controllable muscle forces [20, 21]. Finally, if the independent motor units in a targeted muscle are stimulated in an interleaved manner, muscle fatigue can be reduced, compared with that resulting from stimulation with conventional surface, cuff or epimysial electrodes [22] [19, 23, 24]. This relative fatigue resistance is achieved by sequentially stimulating each of a number of independent motor units, n, at a low, fatigue-resistant frequency, f. This interleaved stimulation evokes a summated muscle force as though it were being stimulated at a single high, non-tremor evoking frequency, n × f. Thus, interleaved intrafascicular microelectrode stimulation (IFMS) can achieve a physiological form of force production that is both fatigue-resistant and tremor-free [19]. Other approaches to minimize muscle fatigue due to prolonged stimulation have also been reported. Lower rates of muscle fatigue have been achieved with intramuscular stimulation as opposed to stimulation via nerve cuff electrodes, an effect attributed to an intramuscular electrode's selective access to more fatigue-resistant slow motor units [25]. Fatigue resistance can also be facilitated because skeletal muscle remodels as a function of its level of activation. Exercise programs or electrical stimulation protocols can be designed to promote the transformation of rapidly fatiguing motor units into slow, more fatigue-resistant motor units [26].
To date, the advantages of interleaved IFMS delivered via high electrode-count penetrating microelectrode arrays have mainly been demonstrated in experiments conducted in isometric feline gastrocnemius muscle [22] [13, 19, 20]. In this paper, we describe experiments that extend our findings to the whole feline hind limb as it undergoes a behaviorally relevant musculoskeletal maneuver: stance. The production of stance requires coordinated activation of multiple extensor muscles that produce torques around multiple joints. The production of stance is complicated by the multiple bi-articular muscles that produce antagonistic torques around the hip and knee joints and by the non-linear relationship between forces generated in a muscle and muscle length and shortening speed. In spite of these complications, we report herein the following results. 1) Activation of most of the extensors of the hip, knee, and the plantar flexors of the ankle joint can be achieved with surgical implantations of two or three USEAs in three nerves: the muscular branch of the sciatic nerve, the femoral nerve, and the main branch of the sciatic nerve. 2) Semi-automated algorithms can be used to characterize efficiently forces evoked by the 96 to 288 electrodes of the two to three implanted USEAs, and for the selection of an appropriate subset of electrodes for functional experimentation. 3) The generation of muscle force can be achieved via stimulation strategies that mimic those producing natural skeletal movements [21]. 4) Behaviorally relevant, three-joint torques can be produced in both acutely and chronically implanted animals. 5) Interleaved IFMS produces sit-to-stand maneuvers but with less fatigue than is produced with whole-nerve stimulation techniques achieved with more conventional electrode architectures. Taken together, these experiments illustrate the potential value of intrafascicular multielectrode stimulation as a promising approach that could be used to restore motor function in paralyzed individuals.
2. Material and Methods
2.1 Measurement of stance kinematics
The goal of the work described herein was to use interleaved IFMS delivered via subsets of USEA electrodes implanted in the nerves of the feline hind limb to produce fatigue-resistant three-joint stance behaviors with kinematics similar to those evoked by stroking the animal's back. The literature reports quantitative studies of a number of feline motor behaviors such as walking [27–29], but the kinematics of the sit-to-stand maneuver have not been reported. Thus, we needed kinematic measurements of the ankle, knee, and hip angles as a normal cat transitions from a sitting to a standing posture. These measurements were made at the University of Alberta in three adult cats, and the experiments are summarized here [30]. We used a high speed video camera (120 Hz, JVC9800, JVC Corporation) and 7 mm circular white reflective markers glued over the metatarsophalangeal (MTP), ankle, and hip joints and the iliac crest, pelvis, and spine to quantify the kinematics of the joint angles during sit-to-stand transitions. The animals were trained to sit with their paws positioned over four small custom-made force plates, and a reflexive stance behavior was initiated by stroking the backs of the animals. Data were obtained from 27 successful stance maneuvers. Custom MATLAB (The Mathworks, Natick, MA) routines were used to extract the marker locations from the video frames, to construct vectors connecting the markers, and to compute the time courses of hip, knee, and ankle joint angles from these vectors as the animal performed the sit-to-stand maneuvers.
2.2 Surgical approaches
Stimulation experiments were performed at the University of Utah on six adult cats (3.2 to 6.6 kg). Four cats were acutely implanted with USEAs and stimulation experiments were conducted immediately post implantation. In the other two animals, USEAs were implanted chronically, and stimulation experiments were conducted at various times after array implantations. Chronic implantation was used to examine capabilities under conditions that more closely approximate those relevant for clinical applications, and to investigate if the stability of evoked muscle forces would be improved by allowing the animal to recover from the effects of long-term surgical anesthesia and USEA implantation. The USEA implantation procedures were different for acute and chronic preparations. In three of the acute implantations (cats 1–3) and in one chronically implanted animal (cat 6), three 100-electrode USEAs were implanted; one each in the muscular branch of the sciatic nerve (to activate the extensor muscles of the hip), in the femoral nerve (to activate the extensor muscles of the knee), and in the main branch of the sciatic nerve (to activate plantar flexors). We will term these three muscle groups as “extensors” in this paper for convenience in generically describing the coordinated actions desired at the three joints. In one of the acutely implanted animals (cat 4) and in one of the chronically implanted animals (cat 5), two 100-electrode USEAs were used to obtain access to all three nerves. In these animals, one USEA was implanted in the femoral nerve, and the second was implanted just proximal to the bifurcation of the muscular branch and the main branch of the sciatic nerve. Implantation of a single USEA at this bifurcation provided access to nerve fibers in both the main branch of the sciatic nerve and to nerve fibers in the muscular branch. In all acutely implanted animals, the USEAs were connected to a custom connector consisting of three 36-pin Samtec connectors mounted on a small printed circuit board. In these acutely implanted animals, the printed circuit board was sutured to the animal's skin near the implant site. In (chronic) cat 5, 48 electrodes from each array (uniformly distributed acress each array) were connected to a single Tucker-Davis Technologies (TDT) 96-pin ZIF-Clip connector (Alachua, FL). In (chronic) cat 6, two ZIF-Clip connectors were used to connect to all three implanted USEAs: one connector accessed all 96 electrodes in the sciatic nerve implant, and the other connector accessed subsets of 48 electrodes from the other two USEAs, one each implanted in either the muscular branch of the sciatic nerve or the femoral nerve. The 48 wired electrodes were uniformly distributed across each of these two arrays. The TDT ZIF-Clip connectors were mounted in titanium shells to protect the lead wires and to minimize fluid access to the connector. The titanium shells were attached to the animal either with bone screws to the femur, or with sutures to the iliac crest and adjacent connective tissue. A thin, grounded gold screen filled with with Kwik-Cast (World Precision Instruments, Inc., Sarasota, Fla), a two-component silicone elastomer, was placed around the implanted USEAs in order to better immobilize the USEA in the nerve and to protect it from motion produced by the surrounding musculature. In acutely implanted animals, a thin silicone cuff served this role (Figure 1).
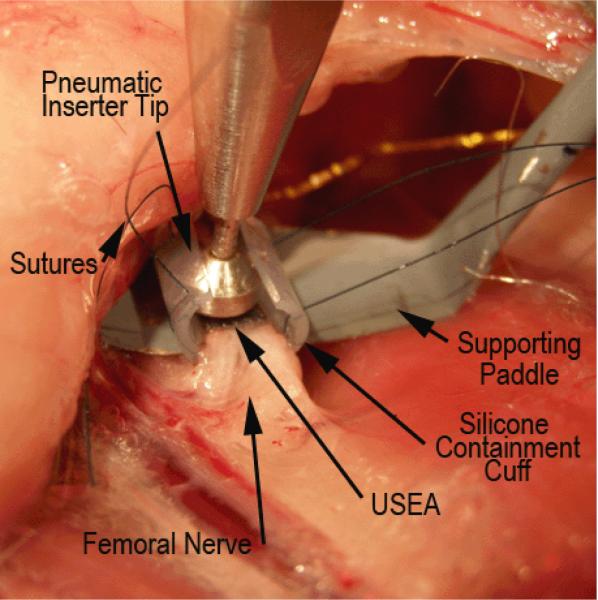
Figure 1.
Photograph of a USEA about to be implanted into feline femoral nerve. The photo shows the nerve which is being elevated and supported by a small paddle, a silicone containment cuff with prepositioned sutures for closing the cuff, a USEA, and the tip of the high-velocity pneumatic insertion tool,
The USEAs have been described elsewhere [10] and consisted of a square 10 × 10 grid of 100 microelectrodes. The implanted USEAs using the 96-pin TDT connector had only 96 functional stimulating electrodes. For simplicity in the remainder of this manuscript, we will refer to both the 96 and 100 electrode arrays as `100-electrode arrays'. Each electrode was separated from its neighboring electrodes by 0.4 mm. Electrode lengths were graded from 0.5 mm to 1.5 mm. The tip of each electrode was metalized with sputtered iridium oxide, and the rest of the array was insulated with parylene. The USEAs used in the four acute experiments (cats 1–4) had impedances of 125.2 +/− 48.2 kOhms (mean +/− SD, here and elsewhere in the manuscript) measured in saline with a 10mV, 1 kHz sine wave. The USEAs as measured in the chronically implanted cat 5 had impedances of 190.7+/− 23.2 kOhms. As reported elsewhere, the impledances of chronically implanted electrodes change with time [31–34]. The impedances in cat 6 were measured on two days post implant. On post-implant day 24, the electrodes in the sciatic implant and those on the femoral and muscular branch of the sciatic nerves were 159 +/− 26 kOhms and 206 +/− 39 kOhms, respectively. On post-implant day 71, the electrodes in the sciatic implant and those on the femoral and muscular branch of the sciatic nerves were 154 +/− 25 kOhms and 232 +/− 21 kOhms, respectively.
All surgical procedures were performed under protocols approved by the University of Utah Institutional Animal Care and Use Committee. Animals were induced with an IM injection of Telazol (10 mg/kg), intubated, mechanically ventilated, and anesthetized with Isoflurane (1.25–2.5%). The animals were hydrated with a lactated Ringer's solution (10ml/kg/hr). Anesthetic plane and animal status were monitored by recording expired CO2, core temperature, oxygen saturation, and heart rate at 15-min intervals. USEAs were implanted in the nerves of the left hind leg using high-velocity implantation [35]. A photograph of a USEA, about to be implanted into the femoral nerve is shown in Figure 1. The 1cm distal end of a 2 mil, tefon-insulated platinum-iridium wire was deinsulated and placed near to the implanted electrode array in order to provide a stimulus return. Lead wires for the USEA, chronically implanted in the femoral nerve were routed from the connector proximally towards the hip, where they exited between the biceps femoris and vastus lateralis muscles at the location of caudofemoralis. The wires were then routed under the skin to the femoral nerve via the anterior crease of the hip joint at the most proximal portion of the upper leg. Lead wires for the USEA implanted in the sciatic nerve were routed directly to the implant site from the connector on the femur.
The implanted animals were positioned in one end of a cantilevered trough from which its hind legs were suspended (Figure 2). Approximately ¾ of the animal's weight was balanced with a constant force spring (A3×50 Neg'Ator Spring, Stock Drive Products, New Hyde Park, NY) located on the opposite end of the trough. Thus, when the trough stop was removed, the weight of the animal caused the trough to rotate downward and the animal assumed a sitting posture. A mechanical `sitting' stop on the trough limited the downward rotation of the trough in order to keep the hind limb extensors from excessive elongation. When the extensor muscles of the implanted limb were activated, the animal produced a stance maneuver and the trough rotated upward. The amplitude of each sit-to-stand/stance/stand-to-sit maneuver was reflected in the angular rotation of the trough which was measured with a potentiometer attached to the axis of rotation of the trough. Stimulation that evoked a maximal stance produced a maximal trough rotation of from 11 to 15.5 degrees. The animal's MTP joint on its left foot was attached to a six degree-of-freedom load cell (Gamma-US-15, ATI, Apex, NC, USA) with plastic ties. The load cell had resonant frequencies of 1.4 kHz in the X and Y directions and 2 kHz in the Z direction and was used to monitor force es evoked by stimulation of individual USEA electrodes. Monitored forces were sampled at 10 kHz and stored in a Cerebus data acquisition sysstem (Blackrock Microsystems, Salt Lake City, UT).
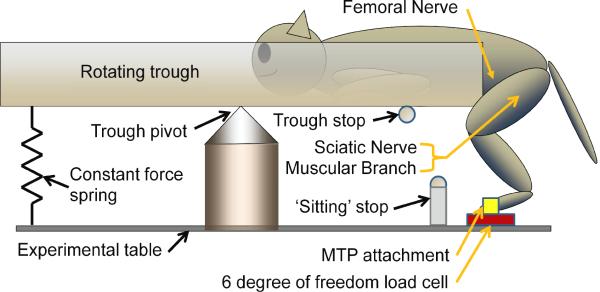
Figure 2.
Experimental set-up (drawing not to scale). The animal was posiitioned on one end of a cantilevered trough from which its hind limbs were suspended. The MTP joint was attached to a 6 degree of freedom load cell with plastic ties. Approximately ¾ of the cat's weight was counterbalanced with a constaant force spring. USEAs were implanted in thhe sciatic, femoral and muscular branch nerves. A meechanical `sitting' stop limited the extent of thhe sitting posture to keep the hind limb extensors from excessive elongation. The animal was keept in an approximate standing posture for mapping of USEA electrodes by a trough stop that was removed for all stance experiments.
2.3. Multichannel stimulator
All stimulation was produced with a custom-built, 1100-channel, constant-voltage stimulation unit [36], and stimulation strength was controlled by varying the monopolar, monophasic cathodic stimulus pulse width (from 0.2 to 1024 μs, with 0.2 μs resolution). Monophasic stimulation was selected to facilitate control programming, and charge balance was achieved with capacitor coupled outputs. The constant-stimulus voltage level used in each experiment was set beetween −2.4 V and −4.0 V, depending upon the condition of the extensor muscles and the quality of the USEA implantation. The pulse duration, frequency, and phasing of stimulation delivered to each of the selected electrodes was under the control of custom C++ software [37]. Any number of electrodes could be stimulated simultaneously or in a sequential fashion for interleaved stimulation, with individually addressable pulse widths.
2.4. Response characterization using load cell data, mapping, and overlap algorithms
Three USEAs implanted in the nerves of one hind limb contained as many as 2888 functional electrodes (or as many as 600 electrodes for arrays implanted in both hind limbs). The characterization of the responses evoked by up to 288 individual USEA electrodes represents a formidable practical challenge. The procedures were performed isometrically, and consisted of 1) mapping of force vectors and measurement of twitch-force recruitment-curves for all electrodes where stimulation evoked a force of at least 0.5 N in response to a 256 μs single stimulus, 2) selecting a subset of electrodes that activated large extension torques around each targeted joint, and 3) measuring the overlap of muscle excitation by pairs of the selected electrodes (i.e., the degree of independence of stimulated motor units). In each animal studied, these procedures were performed prior to evoking stance maneuvers and fatiguing sequences. When stance maneuvers were evoked, forces were monitored by the load cell attached to the animal's MTP joint. Detailed descriptions of the use of evoked force vectors to characterize the electrodes have been reported elsewhere [16, 18], but are summarized here. Identification of electrodes that activated extensor muscles was determined by the production of downward forces measured with the load cell. Twitch force recruitment curves were generated via each of the electrodes with a minimum of five approximately equally spaced stimulus strengths from threshold to maximum twitch force. Excitation overlap between pairs of electrodes was quantified using a refractory technique [18, 38] where two 0.5 s stimulus trains were delivered via two of the selected electrodes, with the phasing of the stimuli in the second train adjusted such that the individual stimuli in the second train were delivered during the refractory period of fibers excited by the individual stimuli in the first stimulus train (a 0.75 ms delay). If the stimulus train delivered via each electrode activated two totally independent sets of motor units, then the force generated by the paired stimulus trains would be approximately equal to the sum of the forces evoked by each stimulus train delivered in isolation. In contrast, if the two trains activated some shared motor units, then the force produced by paired stimulation would be reduced by the degree of overlap, thereby allowing quantification of the amount of excitation overlap [18]. As a result of these characterization procedures, three final subsets of electrodes were selected for activation of extensor muscles for the hip, knee, and ankle joints, and these electrodes were used in subsequent experimentation. Six electrodes were selected to activate each joint in the acute experiments. In cat 5, 18 electrodes were used to evoke hip and ankle extension, and 6 electrodes were used for knee extension. In cat 6 on day 24 we used 9, 10, and 12 electrodes, and on day 71 we used 12, 17, and 16 electrodes for hip, knee, and ankle extension, respectively.
2.5 Measurement of fatigue for interleaved IFMS and more conventional stimulation paradigms
We used fatiguing sequences of sit-to-stand maneuvers in order to quantify the differences in the rate of muscle fatigue associated with muscle activation evoked by interleaved IFMS and more conventional stimulation paradigms. We quantified the time course of muscle fatigue by monitoring the kinematics with a video camera, the rotation of the trough with a potentiometer, and the ground reaction forces with the 6 degree of freedom load cell. Of these indices, the rotation of the trough best reflected the ability of the animal to repeatedly stand, and provided the most quantifiable index of muscle fatigue. Each fatiguing sequence was continued until the hind limb muscles could no longer elevate the trough by an arbitrary criterion amount, two degrees, at which time the fatiguing sequence was terminated and the muscles were allowed to relax.
A number of challenges were associated with this experimental paradigm. First, we wanted to compare interleaved IFMS stimulation with the type of whole-nerve stimulation that would be achieved with a nerve cuff electrode, or with the whole muscle activation that would be produced with an epimysial electrode. Rather than adding nerve cuff electrodes to the nerves already implanted with USEAs or epimysial electrodes to the targeted muscles, we approximated whole-nerve stimulation by using simultaneous IFMS delivered via the same sets of electrodes that were used to produce stance with interleaved IFMS. In the remainder of this paper, we refer to this simultaneous form of IFMS as `synchronous IFMS'. Further, in order to eliminate tremor produced by synchronous stimulation via these electrodes, we stimulated the set of electrodes used in interleaved IFMS evoked maneuvers at the composite stimulation frequency used in the interleaved IFMS evoked maneuvers. For example, in the acute experiments described in this manuscript, six electrodes were used to deliver interleaved IFMS to the nerves innervating extensor muscles for each joint, with a stimulation rate on each of these electrodes of 8 Hz. This stimulation paradigm produced a composite muscle stimulation frequency of 48 Hz (6 electrodes * 8 Hz). Accordingly, to evoke a stance maneuver using synchronous IFMS we stimulated simultaneously via all six electrodes at 48 Hz. To produce a stance maneuver using synchronous IFMS with kinematics similar to those evoked with interleaved IFMS, the stimulus strengths used in this synchronous IFMS stimulation paradigm had to be greatly reduced. In order to make a comparison between the rates of fatigue associated with fatiguing sequences evoked by interleaved and synchronous IFMS, we had to ensure that the levels of muscle activation for both types of stimulation evoked stance maneuver kinematics that were as close as possible to each other. This was achieved by adjusting stimulus strengths (pulse widths) on each synchronous IFMS electrode so as to produce sit-to-stand kinematics that were close to those evoked with interleaved IFMS.
Because we were stimulating the extensors of the hip, knee, and ankle to fatigue, after each fatiguing sequence the animal was allowed to recover from the stimulation for 30 min before the next fatigue sequence was begun. However, we anticipated that the muscles might not fully recover to their pre-fatigue strengths with a 30 min rest period, so the order of fatiguing sequences evoked by interleaved and synchronous IFMS was varied among animals to compensate for a potential gradual degradation in muscle strength (which indeed occurred). In some experiments, we started the fatiguing sequences with interleaved IFMS, followed by synchronous IFMS, and in others, we inverted this order. In these experiments, we performed from two to four fatiguing sequences in each of seven experimental sessions across the six cats. The numbers of stance maneuvers that animals were able to perform until they could no longer achieve the two-degree criterion were averaged for both intereaved and synchronous IFMS for each of the seven experimental sessions. Because cut-off scores were used, and because data did not appear to be normally distributed across experimental sessions and animals, group data for these seven experimental sessions are presented as medians, and we used the non-parametric Wilcoxon matched-pairs signed-ranks test to test for significant differences in numbers of stance maneuvers evoked by interleaved and synchronous IFMS.
3. Results
3.1 Joint angle kinematics during feline stance
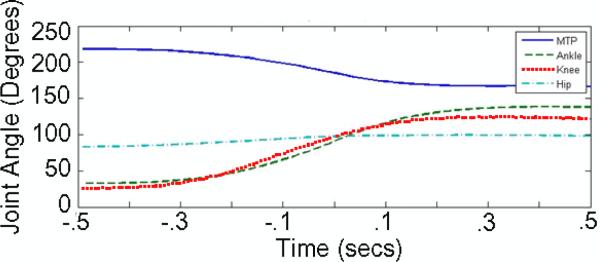
The kinematics of a sit-to-stand maneuver in a normally behaving cat manifest considerable variability that reflect issues such as motivation and alertness. As one goal of the work described herein was to evoke stance maneuvers in the anesthetized cat, we needed data on how fast a cat typically stands, and the hip, knee and ankle angle trajectories during the stance maneuver. Because of the variability of normal cat stance, we needed only approximate estimates of these parameters to recreate a stance maneuver in the anesthetized animal with kinematics similar to those evoked by stroking the non-anesthetized, sitting animal's back. Therefore, we recorded the sit-to-stand maneuvers initiated by stroking the back of a normally sitting cat. Figure 3 shows the kinematics of the hip, knee, ankle and MTP joint typically recorded during one such back-stroke-evoked stance maneuver. Two results from this kinematic analysis were subsequently used to guide us in setting IFMS parameters to evoke stance that had kinematics similar to those of Figure 3: first, the stance behavior occurred smoothly in about 0.5–0.7 s. Second, the kinematics for all three joints were very similar: rotations around each joint started at about the same times, the peak rotational velocity around each joint occurred at about the same time, and the rotation around each joint stopped at about the same time. In the remainder of this paper we use the operational definition that the kinematics of Figure 3 are representative of `normal stance'.
Figure 3.
Time course of hip, knee, ankle and MTP joint angles evoked by stroking the back of an implanted feline.
3.2. Access to hind limb muscles: mapping of USEAs
A single USEA implant provides access to many motoneurons that innervate a number of muscles. In the six cats reported herein, a total of 465 electrodes (out of 1344 wired electrodes; or 34.6% the of wired electrodes) were identified that activated extensors with a mean maximum twitch force of 2.2 +/− 2.3 N. Other electrodes activated flexors, or did not recruit responses possibly due to their tips bing located in non-neural interfascicular tissue.
However, one cannot predict a priori which specific USEA electrodes will activate which particular muscles. Thus, activation maps were first created for each implanted USEA to characterize this relationship, and this was done by recording evoked 3-D force vectors with the load cell that was attached to the animal's MTP joint. Although many electrodes target the hind limb extensors, some electrodes can evoke only small forces, and when stimulus strength is increased, some of these electrodes can begin to excite antagonist muscles. Also, some electrodes excite a subpopulation of motoneurons that are excited by other individual electrodes in the array and, therefore, provide redundant excitation. Other subsets of electrodes excite biarticular muscles, which can produce extension at one joint, but flexion at another. From the activation maps measured in the acute implantations, three subsets of approximately twelve electrodes were identified that could potentially be used for activation of each joint. Stimulation via each of these electrodes evoked downward forces of 2.6 +/− 0.8 N, and excited motoneurons with no more than 41+/− 20% overlap in excitation. From each of these three pools of electrodes, six electrodes were selected for activation of each joint for the fatigue experiments. These electrodes had the lowest overlap in excitation (22 +/− 16%) and produced acceptable maximal twitch forces (3.3 +/− 0.7 N).
In the chronically implanted animals, the maximal twitch forces evoked with stimuli delivered via selected electrodes were lower (1.1 +/− 0.1 N) than in the acutely implanted animals. Therefore, in order to recreate normal stance maneuvers all electrodes that evoked extension forces greater than approximately 0.5 N were used to generate stance during the chronic animal fatigue experiments.
3.3. Sit-to-stand transition with force recruitment using sequential motoneuron activation
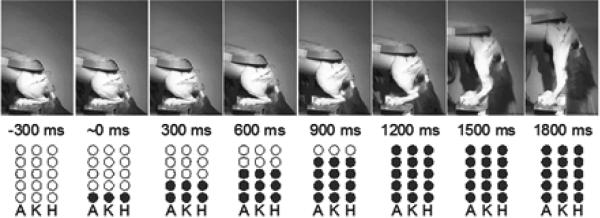
Graded increases in force in normal skeletal musculature are produced by two mechanisms: 1) increasing the firing rate of individual motoneurons; and 2) the sequential activation of successively larger numbers of motoneurons that innervate a target muscle [21]. As described above, the selected electrodes in the implanted USEAs were chosen because they activated relatively independent groups of motoneurons. Thus, we were able to use this physiological force recruitment strategy to generate smoothly graded forces in the hind limb extensors by sequential application of stimulation via successively more electrodes. An example of graded force recruitment in the three joints of stance that was produced by sequential activation of motoneuron pools for extensors of a given joint is illustrated in Figure 4. In this example, we initiated a slow sit-to-stand maneuver by applying stimuli to only one selected electrode in each of the USEAs implanted in the sciatic, femoral, and muscular branch nerves. Simultaneous stimulation on each of these three electrodes was delivered at 40 Hz. 300 ms after the start of stimulation on these three electrodes, we added stimulation via three additional electrodes, one per USEA, producing somewhat greater muscle forces. This incremental stimulation protocol was continued until stimuli were delivered via five electrodes on each USEA (in this experiment, the ordering of the added electrodes was not investigated). The simultaneous activation of the different motoneuron pools controlling all three joints mimicked the kinematics shown in Figure 3, where activation of all three joints occurs together (Results section 3.1). This stimulation strategy generated a slow, concurrent activation of extensor muscles of the three joints, resulting in a smooth sit-to-stand maneuver that achieved complete hind limb extension at 1800 ms after the start of stimulation (Figure 4). In contrast, sequential activation of the extensors for the different joints (first ankle, followed by knee, then hip) produced uncoordinated stance: for example, without activation of hip extensors, the hip everted during activation of ankle plantar flexors (data not shown).
Figure 4.
Production of smooth, slow stance by sequential addition of stimulation via triplets of electrodes. Filled circles indicate the sequence of stimulation delivered to fifteen electrodes across three USEAs: five electrodes targeting the ankle (A), five targeting the knee (K), and five targeting the hip (H).
3.4. Reduction of muscle fatigue with interleaved IFMS
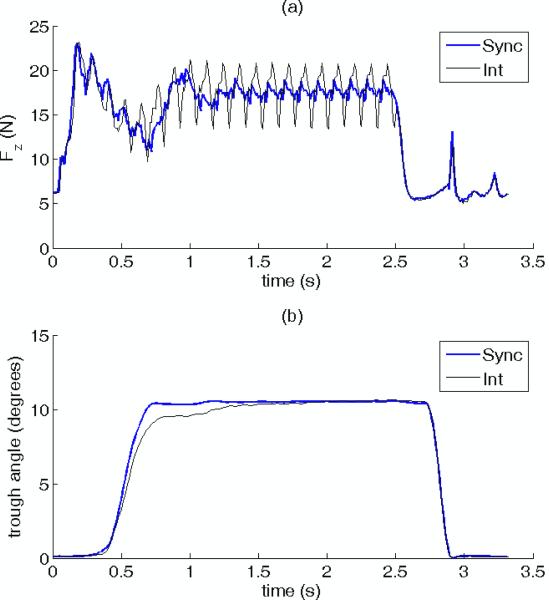
In order to produce the graceful skeletal motion shown in Figure 4 using the physiological force recruitment strategy of sequential activation of added motor units, we had to use a high stimulation frequency in order to minimize muscle tremor. However, this high frequency stimulation produces undesired muscle fatigue. A different muscle activation strategy that mitigates muscle fatigue is interleaved IFMS. To quantify the reduction in muscle fatigue achieved with interleaved IFMS compared with synchronous IFMS, two to four sequences of repeated sit-to-stand maneuvers were evoked with either interleaved or synchronous IFMS. The sequences consisted of consecutive episodes of a sit-to-stand/stance/stand-to-sit maneuver evoked by 2.5 s of stimulation, followed by two seconds of rest between episodes. In order to make a valid comparison between interleaved and synchronous IFMS, the stimulus strengths delivered on each selected electrode used for synchronous IFMS were adjusted in an ad hoc manner to make the kinetics and amplitudes of the sit-to-stand maneuver approximately equal to those that were evoked with interleaved IFMS stimulation. Further, the phasing of the interleaved stimuli were roughly adjusted to reduce tremor, and this phasing was used in the synchronous stimulation. Figure 5 illustrates two sit-to-stand/stance/stand-to-sit episodes preceding two fatiguing sequences that were evoked with interleaved and synchronous IFMS in one preparation. Figure 5a shows the vertical force kinetics and Figure 5b shows the trough angles associated with the sit-to-stand/stance/stand-to-sit maneuver. These figures illustrate that this ad hoc adjustment of stimulus strengths could be successfully achieved. Comparison of Figures 5a and 5b further illustrate that although there was considerable tremor in the force recorded under interleaved stimulation, the mass of the animal and trough damped out a majority of the tremor, resulting in a relatively smooth maneuver. Because the phasing of the synchronous stimuli was the same as the interleaved stimuli, there is also tremor seen in the synchronous record of Figure 5a. The figures also show approximately a 0.3 s latency between the start of stimulation (and the production of vertical force), and the start of trough rotation. At the end of stimulation (and muscle force production), there is another 0.3 s latency before the trough begins to rotate back towards the sitting posture. Table 1 presents the stimulus pulse widths that were used to produce approximately equivalent sit-to-stand kinematics for both interleaved and synchronous IFMS. In order to achieve equivalent stance kinematics, the mean pulse width for synchronous IFMS for all three joints had to be reduced by factors of from 9 to 28. Across all experiments, synchronous pulse widths had to be lowered by an average factor of 9.6 to achieve equivalent kinematics. Also, the pulse widths of the electrodes implanted in the muscular branch of the sciatic nerve were 6.7 and 5 times longer than the pulse widths for electrodes in the femoral and sciatic nerves. This suggests that the muscular branch electrodes may have had relatively poor access to the efferent fibers innervating the hip extensors. Conversely, the shorter pulse widths used for electrodes in the sciatic and femoral nerve implants suggest that the electrodes in these USEAs may have more closely abutted the efferent fibers innervating the ankle and knee extensors.
Figure 5.
a) Ground reaction forces and b) trough angles measured during two sit-to-stand episodes preceding fatiguing sequences evoked by interleaved (thin lines) and synchronous (thick lines) IFMS. The stimuli delivered via each USEA electrode used for synchronous IFMS were adjusted on an ad-hoc basis to produce a stance with amplitude and kinematics that were approximately equal to those produced by interleaved IFMS stimulation. The larger ripple seen in this figure reflects imperfect balancing of interleaved stimulation delivered via USEA electrodes.
Table 1.
The implant site, and the pulse widths of six electrodes per targeted joint selected to produce sit-to-stand maneuvers with approximately equivalent kinematics using interleaved and synchronous IFMS in one preparation. Individual electrode stimulation frequencies for interleaved and synchronous IFMS stimulation were 8 Hz and 48 Hz, respectively. −3.0 V stimuli were used for both interleaved and synchronous stimulation.
| Implant Site | Array # | Interleaved Pulse Width (μs) | Synchronous Pulse Width (μs) |
|---|---|---|---|
| Muscular Branch | 2 | 216 +/− 136 | 7.7 +/− 4.9 |
| Femoral | 1 | 32.1 +/− 13.7 | 3.5 +/− 1.5 |
| Sciatic | 2 | 43.2 +/− 35.3 | 4.8 +/− 3.8 |
Sample video frames of four sit-to-stand episodes from two fatiguing sequences are shown in Figure 6 from one preparation. These frames further illustrate that the kinematics and amplitudes of the initial behaviors for interleaved and synchronous IFMS were very similar, indicating that functional stimulus strengths were well matched at the outset of each fatiguing sequence. Despite these similar starting conditions, by the fifteenth episode, the amplitude of the stance behavior evoked by synchronous IFMS was greatly reduced, whereas the stance behavior produced by interleaved IFMS was maintained. These results indicate that, as hypothesized, interleaved stimulation mitigated fatigue compared with synchronous IFMS.
Figure 6.
Interleaved IFMS produced sit-to-stand transitions that were slower to fatigue than behaviors produced by synchronous IFMS. Frames (a) and (c): the first episodes in two fatiguing sequences evoked with interleaved and synchronous IFMS, respectively. Frames (b) and (d): the fifteenth episode in these sequences evoked with interleaved and synchronous IFMS. Stimuli for initial episodes were successfully adjusted to produce stances with approximately equal amplitudes and kinematics. By the fifteenth episode, interleaved IFMS manifested little fatigue, but synchronous IFMS manifested substantial fatigue. Frames show the left (activated) leg in the foreground, and the right leg and tail in the background. Also shown are the connectors and lead-wire ribbon cables on the animal's back, and the end of the rotating trough from which the animal's hind limbs were suspended.
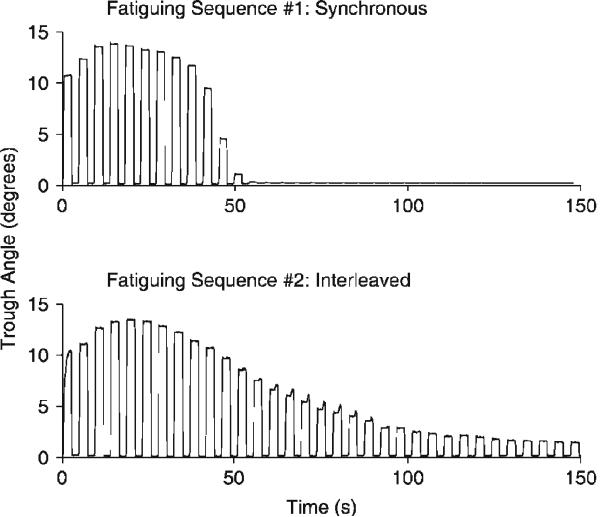
An example of the complete time courses for the generation of muscle fatigue for both interleaved and synchronous IFMS (e.g., Figure 6) can be seen by plotting the trough angle of each stance behavior as a function of time (Figure 7). As illustrated, interleaved IFMS conferred greater fatigue resistance. For both stimulation paradigms there was a brief period of muscle force potentiation in the early episodes. However, for synchronous IFMS the stance behavior failed to meet the two-degree criterion after only 11 episodes. In contrast, interleaved IFMS continued for 24 episodes before the two-degree criterion could no longer be achieved.
Figure 7.
Interleaved IFMS confers fatigue resistance. The amplitude of stance responses (trough angles) produced for each sit-to-stand/stance/stand-to-sit episode are depicted for fatiguing sequences of synchronous (top) and interleaved (bottom) IFMS. In this experiment, the fatiguing sequence produced by synchronous IFMS was delivered before the sequence of interleaved IFMS.
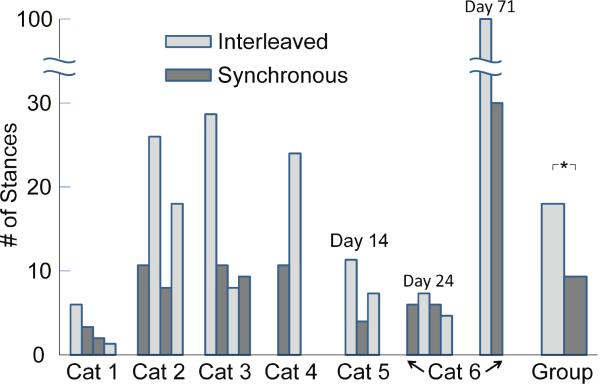
With the single exception of the final fatiguing episode delivered to cat 1, the faster rate of fatigue produced with synchronous IFMS compared with interleaved IFMS was seen in all animals studied (Figure 8). This figure plots the numbers of episodes in the fatiguing sequences performed in each of the six animals before the stimulation could no longer evoke a sit-to-stand amplitude greater than the two-degree fatigue criterion. In most cases, the rate of fatigue was considerably greater for synchronous IFMS than for interleaved IFMS. That is, the mean number of episodes to the two-degree fatigue criterion for interleaved IFMS was larger than the mean number of episodes to fatigue for synchronous IFMS. Also shown in Figure 8 are the group data from the seven experimental sessions for all six cats, using the data for all interleaved and all synchronous IFMS exp periments. The median number of episodes unntil the two-degree criterion could no longer be acchieved by interleaved IFMS was 18.5, whereeas the median number of episodes for synchronous IFFMS was 9.5, a difference in rate of fatigue thhat was significant at the p < .05 level (Wilcoxon m matched-pairs signed-ranks test, T = 0). It is nooted that the recovery from fatiguing episodes was nnot complete even with the ½ hour recovery pperiod: the number of stance maneuvers that could be achieved in the second trial of synchronouss or interleaved stimulation was less than was achieved in the first trial.
Figure 8.
Muscle fatigue prod duced with interleaved and synchronous IFM S in all six cats. The ordinate plots the number of ffatiguing episodes until the animal could no loonger rotate the trough by greater than the two-degreee criterion. The order of the bars for each animal indicates the order of the fatiguing sequences, and the color of the bar indicates whether interlleaved (light grey) or synchronous IFMS (dark greyy) was used. Cats 1–4 were implanted acutelyy, and cats 5 and 6 were implanted chronically. Fatigue experiments were conducted on cat 6 on days 24 and 71 post implant. In all animals, interleaved IFMS produced a slower rate of fatigue than did synchronous IFMS. Note that in cat 6, day 71, the fatiguing sequences were stopped at stance 100, even though the animal still produced stance exceeding the 2-degree criterion, so this value underestimates the number of stances the animal could have achieved. Overall (labeled `Group'), the median number of fatiguing episodes until the two-degree criterion could not be met was significantly greater for interleaved IFMS than for synchronous IFFMS (p < .05, *).
3.5. Stance in the chronically implanted animals
The surgical implantation of three USEAs, and the characterization of the respponses evoked by up to 288 USEA electrodes was a time-consuming process. In the four acutely implanted cats, these procedures required 25.5 +/− 1 h. It was only after these procedures were completed that the fatiguing sequences were delivered to the animals. The fatiguing sequences took an additional 3–5 h to complete. In order to allow more clinically relevant test conditions, free of the immediate effects of long-term surgery and the prolonged anesthesia required for USEA implantation and electrode-response characterization, two animals were chronically implanted with two and three USEAs and were stimulated on post-implantation day 14 for animal 5 and on days 24 and 71 for animal 6. As we previously observed in other chronically implanted animals over these prolonged periods, the chronically implanted animal did not manifest any lasting behavioral deficits due to the implanted USEAs, the lead wires, or the percutaneous connectors. Further, stimulation remained viable, and we were able to obtain robust sit-to-stand fatiguing sequences (animals 5 and 6 in Figure 8). As with the acute preparations, interleaved IFMS produced more stance episodes than did synchronous stimulation. However, the chronic preparation also manifested a gradual degradation in muscle responses across repeated fatiguing sequences, indicating that this decline represented residual fatigue, rather than run-down in the quality of the preparation as a consequence of the prolonged surgical and electrode characterization procedures.
4. Discussion
The objectives of this study were met. 1) Activation of most of the extensors of the hip, knee, and the plantar flexors of the ankle joint was achieved with surgical implantations of two or three USEAs in three nerves: the muscular branch of the sciatic nerve, the femoral nerve, and the main branch of the sciatic nerve. 2) Semi-automated algorithms were developed and used to efficiently characterize forces evoked by the 96 to 288 electrodes of the two to three implanted USEAs, and for the selection of an appropriate subset of electrodes for functional experimentation. 3) The generation of muscle force was achieved via stimulation strategies that mimic those producing natural skeletal movements [21]. 4) Behaviorally relevant, three-joint torques were produced in both acutely and chronically implanted animals. 5) Interleaved IFMS produced sit-to-stand maneuvers with kinematics that were similar to those shown in Figure 3, but with less fatigue than was produced with whole-nerve stimulation techniques that would be achieved with more conventional electrode architectures. Taken together, these results illustrate the potential value of intrafascicular multielectrode stimulation as a promising approach that could be used to restore motor function in paralyzed individuals. However, a number of issues will need further work before this approach could be considered as a practicable means to restore stance in human subjects who have lost this capability due to spinal cord injury, stroke, or disease.
4.1. Surgical issues and long-term biocompatibility of USEAs
The implantations of the USEAs required only two surgical openings: one that exposed the bifurcation of the upper trunk of the sciatic nerve and the muscular branch of the sciatic nerve, and one that exposed the femoral nerve. The routing of the lead wires for the sciatic nerve implant was straightforward, but the routing required for the femoral nerve implantation was more complex. The length of the lead wires for the femoral nerve implant, and their proximity to the muscles of the hip and abdomen are likely to have reduced the stability of this implant site in the chronically implanted animal. Tethering forces on the USEA implanted in the femoral nerve associated with hip and abdominal motions could tend to dislodge the implanted array and any micromotion due to these tethering forces could produce a chronic inflammatory response at the implant site. The development of wireless intrafascicular electrode arrays would greatly mitigate this complication, and such device development is ongoing [39, 40].
Continuing problems with all implanted neural interfaces include the tissue response to the implant system, and the response of the implant system to the neural tissue and to the passing of stimulation currents via the microelectrodes [41]. Biphasic stimulation is usually desired to minimize electrode and tissue damage, but the stimuli used in this study were monophasic (with charge balance achieved with capacitor coupled outputs). Virtually every study that has investigated the long-term stability of neural implants has found a gradual decrease in the neural recording capability of the electrodes [31–33, 42], and a gradual increase in stimulation thresholds required to evoke behavioral responses. Further, histological studies on tissues implanted with penetrating microelectrode arrays also show both a proliferation of reactive astrocytes and macrophages that extends for tens to hundreds of microns around the electrode tips [43–45]. These immunological responses will impact the functional longevity of the neural implant system, but in studies performed in the central nervous system, neural implats have been reported to retain limited function over a number of years [46, 47]. Given that all materials that are implanted in the nervous system have been shown to evoke such immunological responses [48–50], it is unclear at this point in the development of chronically implantable neural interfaces how to mitigate this problem. This problem is further exacerbated by the dense vascularity of the nervous system where implantation of devices with complex 3-D architectures will certainly cause localized damage to the blood vessels and provoke a localized immune response [44, 45]. Although there is less vascularity in the peripheral nervous system than in the central nervous system, there are larger tissue displacements and motions in the peripheral nerves due to the proximity of the surrounding contracting musculature. It is expected that these problems will not be fully solved until new electrode array architectures and materials have been developed. However, the results of the experiments described herein are expected to motivate the development of `next-generation' electrode array architectures.
4.2. Monotonic degradation of the preparation
A major difficulty in the measurement of muscle fatigue with either interleaved or synchronous IFMS was the gradual degradation of forces that were evoked with application of multiple fatiguing sequences. The 30-min muscle restoration period was less than optimal in restoring the extensor muscles back to their pre-fatigued strength, but the use of longer restoration periods was confounded by the gradual degradation of the preparations. It is unclear what was the source of this degradation, but it is possible that it resulted from the 25 h of anesthetic delivery required for each of these prolonged experiments [51]. Another contribution to the degradation could be the prolonged immobilization of the hind limbs as they were suspended from the trough (periodic manual flexion of the hind limbs did not mitigate the degradation). It was anticipated that the shorter anesthetic delivery and experimental times made possible by the use of the chronically implanted animals would reduce the degradation of the preparation, but the 30-min muscle restoration period was still insufficient to restore the extensors back to their pre-fatigued status in these two animals.
4.3. The need for automated protocols for characterizing electrode properties
Even though only three (or in one acute and one chronically implanted animal, two) USEAs were implanted in the major hind limb nerves, this still presents the experimenter with the challenge of characterizing a large number (as many as 288) electrodes in each leg. We have developed a number of custom algorithms to automate this characterization problem, but these routines still required typically from three to four hours to complete. The use of the load cell attached to the animal's foot and the measurement of evoked 3-D force vectors allowed for the semi-automation of this process, and this technique has the advantage that it was non-invasive [18]. However, these algorithms need to be refined to reduce the time required for electrode characterization to something under one hour. A similar approach could be employed with human patients where only a minor degree of external restraint at the knee would be required.
4.4. The need for closed-loop feedback control of electrode stimulation
The strength of stimulation delivered via each of the selected electrodes activating a target muscle was adjusted on an ad hoc basis in the experiments described herein, an approach that was iterative and time consuming. A clinical neuroprosthesis system that would be used to produce stance in human subjects and that was based on interleaved IFMS strategies would need feedback information about the amount of force that was being generated by the stimulated leg extensor muscles. This produces additional challenges of how to acquire this information, and how to use it to control the amount of interleaved IFMS delivered via the intrafascicularly implanted electrodes. Ideally, one would want to monitor tendon forces in the major extensor muscles, but this would entail use of implantable sensors [52]. An alternative approach would be to sense joint angles which could be achieved with devices mounted externally to the leg [53]. Once one can either monitor muscle forces (or the consequences of muscle forces on joint angles), one must design strategies that use this data to control the stimulation delivered via multiple implanted electrodes. We have begun to investigate control strategies that could be used to control isometric forces in the gastrocnemius muscle, and this work has been described in a recent paper [54]. The problem of controlling the three hind limb joints to produce stance, or gait, is a more complex control problem that becomes even more challenging due to the biarticular nature of many hind limb muscles where activation of these muscles produces flexion around one joint, but extension around other joints.
5. Conclusions
The close apposition of USEA electrode tips to the motoneurons of the peripheral nerves, together with the large number of electrodes on each implanted USEA, make intrafascicular stimulation with this device a viable approach to recruiting muscle force based on physiological mechanisms [21]. A corollary benefit to intrafascicular stimulation with multielectrode arrays is the ability to interleave stimulation to independent motor units, which confers the ability to produce fatigue-resistant muscle forces, an important feature in a neuroprosthesis that will use IFMS to produce prolonged stance. Finally, the large number of electrodes in a USEA enables the development of new stimulation protocols that can evoke smooth yet powerful muscle forces. These features make the use of intrafascicularly implanted microelectrode arrays an attractive new approach to the field of motor neuroprostheses development.
Acknowledgements
We greatefully acknowledge the efforts of Drs. D.G. Everaert, L.N. MacFadden, and R.B. Stein in their measurements of the kinematics of back-stroke-evoked stance (Figure 3). This work was funded by support from: NIH R01-NS039677, NIH R01-NS064318-01A1, and DARPA N66001-06-C-8005.
Footnotes
Present Addresses: Andrew Wilder and Scott Hiatt Ripple, LLC 2015 South 1100 East, Salt Lake City
PACS Electrode stimulation in neural prosthetics, 87.85.eg
Motor systems in neuroscience, 87.19.lu
References
- 1.Kralj A, Bajd T, Turk R. Electrical stimulation providing functional use of paraplegic patient muscles. Med Prog Technol. 1980;7(1):3–9. [PubMed] [Google Scholar]
- 2.Graupe D. An overview of the state of the art of noninvasive FES for independent ambulation by thoracic level paraplegics. Neurol Res. 2002;24(5):431–42. doi: 10.1179/016164102101200302. [DOI] [PubMed] [Google Scholar]
- 3.Akers JM, et al. Tissue response to chronically stimulated implanted epimysial and intramuscular electrodes. IEEE Trans Rehabil Eng. 1997;5(2):207–20. doi: 10.1109/86.593301. [DOI] [PubMed] [Google Scholar]
- 4.Nicolelis MA, et al. Reconstructing the engram: simultaneous, multisite, many single neuron recordings. Neuron. 1997;18:529–37. doi: 10.1016/s0896-6273(00)80295-0. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz AB, et al. Brain-controlled interfaces: movement restoration with neural prosthetics. Neuron. 2006;52(1):205–20. doi: 10.1016/j.neuron.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Hoffer JA, Kallesoe K. Nerve cuffs for nerve repair and regeneration. Progress in Brain Research. 2000;128:121–34. doi: 10.1016/S0079-6123(00)28012-6. [DOI] [PubMed] [Google Scholar]
- 7.Polasek KH, et al. Intraoperative testing of selectivity of spiral nerve cuff electrodes. Conf Proc IEEE Eng Med Biol Soc. 2004;6:4137–40. doi: 10.1109/IEMBS.2004.1404154. [DOI] [PubMed] [Google Scholar]
- 8.Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng. 2002;10(4):294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- 9.Boretius T, et al. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens Bioelectron. 2010;26(1):62–9. doi: 10.1016/j.bios.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Branner A, Normann RA. A multielectrode array for intrafascicular recording and stimulation in sciatic nerve of cats. Brain Res Bull. 2000;51(4):293–306. doi: 10.1016/s0361-9230(99)00231-2. [DOI] [PubMed] [Google Scholar]
- 11.Rutten WL, van Wier HJ, Put JH. Sensitivity and selectivity of intraneural stimulation using a silicon electrode array. IEEE Trans Biomed Eng. 1991;38(2):192–8. doi: 10.1109/10.76386. [DOI] [PubMed] [Google Scholar]
- 12.Stieglitz T, et al. A biohybrid system to interface peripheral nerves after traumatic lesions: design of a high channel sieve electrode. Biosens Bioelectron. 2002;17(8):685–96. doi: 10.1016/s0956-5663(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida K, Horch K. Selective stimulation of peripheral nerve fibers using dual intrafascicular electrodes. IEEE Trans Biomed Eng. 1993;40(5):492–4. doi: 10.1109/10.243412. [DOI] [PubMed] [Google Scholar]
- 14.Davis JA, Jr., et al. Preliminary performance of a surgically implanted neuroprosthesis for standing and transfers--where do we stand? J Rehabil Res Dev. 2001;38(6):609–17. [PubMed] [Google Scholar]
- 15.Mushahwar VK, et al. New functional electrical stimulation approaches to standing and walking. J Neural Eng. 2007;4(3):S181–97. doi: 10.1088/1741-2560/4/3/S05. [DOI] [PubMed] [Google Scholar]
- 16.Dowden BR, et al. Non-invasive method for selection of electrodes and stimulus parameters for FES applications with intrafascicular arrays. J Neural Eng. 2012;9(1):016006. doi: 10.1088/1741-2560/9/1/016006. [DOI] [PubMed] [Google Scholar]
- 17.Dowden B, Wilder A. Selective and Graded Recruitment of Cat Hamstring Muscles With Intrafascicular Stimulation. IEEE Trans Neural Syst Rehabil Eng. 2009 doi: 10.1109/TNSRE.2008.2011988. [DOI] [PubMed] [Google Scholar]
- 18.Dowden BR, et al. Non-invasive method for selection of electrodes and stimulus parameters for FES applications with intrafascicular arrays. J Neural Eng. 2012;9(1):016006. doi: 10.1088/1741-2560/9/1/016006. [DOI] [PubMed] [Google Scholar]
- 19.McDonnall D, Clark GA, Normann RA. Interleaved, multi-site electrical stimulation of cat sciatic nerve produces fatigue-resistant, ripple-free motor responses. IEEE Trans Biomed Eng. 2004;12(2):208–15. doi: 10.1109/TNSRE.2004.828425. [DOI] [PubMed] [Google Scholar]
- 20.McDonnall D, Clark GM, Normann RA. Selective motor unit recruitment via intrafascicular multielectrode stimulation. Canadian Journal of Physiology and Pharmacology. 2004;82:1599–1609. doi: 10.1139/y04-047. [DOI] [PubMed] [Google Scholar]
- 21.Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol. 1977;40(6):1432–43. doi: 10.1152/jn.1977.40.6.1432. [DOI] [PubMed] [Google Scholar]
- 22.Wise AK, et al. Fatigue in mammalian skeletal muscle stimulated under computer control. J Appl Physiol. 2001;90(1):189–97. doi: 10.1152/jappl.2001.90.1.189. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida K, Horch K. Reduced fatigue in electrically stimulated muscle using dual channel intrafascicular electrodes with interleaved stimulation. Ann Biomed Eng. 1993;21(6):709–14. doi: 10.1007/BF02368649. [DOI] [PubMed] [Google Scholar]
- 24.Rack PM, Westbury DR. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J. Physiology. 1969;204:443–60. doi: 10.1113/jphysiol.1969.sp008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh K, Richmond FJ, Loeb GE. Recruitment properties of intramuscular and nerve-trunk stimulating electrodes. EEE Trans Rehabil Eng. 2000;8(3):276–85. [PubMed] [Google Scholar]
- 26.Salmons S. Adaptive change in electrically stimulated muscle: a framework for the design of clinical protocols. Muscle Nerve. 2009;40(6):918–35. doi: 10.1002/mus.21497. [DOI] [PubMed] [Google Scholar]
- 27.Goslow GE, Jr., Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973;141(1):1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- 28.Smith JL, Carlson-Kuhta P, Trank TV. Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J Neurophysiol. 1998;79(4):1702–16. doi: 10.1152/jn.1998.79.4.1702. [DOI] [PubMed] [Google Scholar]
- 29.Trank TV, Chen C, Smith JL. Forms of forward quadrupedal locomotion. I. A comparison of posture, hindlimb kinematics, and motor patterns for normal and crouched walking. J Neurophysiol. 1996;76(4):2316–26. doi: 10.1152/jn.1996.76.4.2316. [DOI] [PubMed] [Google Scholar]
- 30.MacFadden LN, Everaert DG, Brown NAT. Musculoskeletal simulations of sit-to-stand transitions in the feline hindlimb for targeted functional electrical stimulation. in submission. [Google Scholar]
- 31.Ludwig KA, et al. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J Neural Eng. 2006;3(1):59–70. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- 32.Maynard EM, Fernandez E, Normann RA. A technique to prevent dural adhesions to chronically implanted microelectrode arrays. J Neurosci Methods. 2000;97(2):93–101. doi: 10.1016/s0165-0270(00)00159-x. [DOI] [PubMed] [Google Scholar]
- 33.Otto KJ, Johnson MD, Kipke DR. Voltage pulses change neural interface properties and improve unit recordings with chronically implanted microelectrodes. IEEE Trans Biomed Eng. 2006;53(2):333–40. doi: 10.1109/TBME.2005.862530. [DOI] [PubMed] [Google Scholar]
- 34.Parker RA, et al. The functional consequences of chronic, physiologically effective intracortical microstimulation. Prog Brain Res. 2011;194:145–65. doi: 10.1016/B978-0-444-53815-4.00010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rousche PJ, Normann RA. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann. Biomed. Eng. 1992;20:413–22. doi: 10.1007/BF02368133. [DOI] [PubMed] [Google Scholar]
- 36.Hiatt S, et al. 1100-Channel neural stimulator for functional electrical stimulation using high-electrode-count neural interfaces. IFESS Abstract. 2010 Jul;26:1–3. [Google Scholar]
- 37.Wilder AM, et al. Automated stimulus-response mapping of high-electrode-count neural implants. IEEE Trans Neural Syst Rehabil Eng. 2009;17(5):504–11. doi: 10.1109/TNSRE.2009.2029494. [DOI] [PubMed] [Google Scholar]
- 38.Branner A, Stein RB, Normann RA. Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. J Neurophysiol. 2001;85(4):1585–94. doi: 10.1152/jn.2001.85.4.1585. [DOI] [PubMed] [Google Scholar]
- 39.Thurgood BK, et al. A wrieless integrated circuit for 100-channel charge-balanced neural stimulation. IEEE Trans Biomed Circuits and Systems. 2009;3(6):405–14. doi: 10.1109/TBCAS.2009.2032268. [DOI] [PubMed] [Google Scholar]
- 40.Musallam S, et al. A floating metal microelectrode array for chronic implantation. J Neurosci Methods. 2007;160(1):122–7. doi: 10.1016/j.jneumeth.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Grill WM, Norman SE, Bellamkonda RV. Implanted neural interfaces: biochallenges and engineered solutions. Annu Rev Biomed Eng. 2009;11:1–24. doi: 10.1146/annurev-bioeng-061008-124927. [DOI] [PubMed] [Google Scholar]
- 42.Branner A, et al. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans Biomed Eng. 2004;51(1):146–57. doi: 10.1109/TBME.2003.820321. [DOI] [PubMed] [Google Scholar]
- 43.Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195(1):115–26. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Biran R, Martin DC, Tresco PA. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J Biomed Mater Res A. 2007;82(1):169–78. doi: 10.1002/jbm.a.31138. [DOI] [PubMed] [Google Scholar]
- 45.Szarowski DH, et al. Brain responses to micro-machined silicon devices. Brain Res. 2003;983(1–2):23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- 46.Donoghue JP. Connecting cortex to machines: recent advances in brain interfaces. Nat Neurosci. 2002;5(Suppl):1085–8. doi: 10.1038/nn947. [DOI] [PubMed] [Google Scholar]
- 47.Hochberg LR, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442(7099):164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 48.Stensaas SS, Stensaas LJ. Histopathological evaluation of materials implanted in the cerebral cortex. Acta Neuropathol. 1978;41(2):145–55. doi: 10.1007/BF00689766. [DOI] [PubMed] [Google Scholar]
- 49.Yuen TG, Agnew WF, Bullara LA. Tissue response to potential neuroprosthetic materials implanted subdurally. Biomaterials. 1987;8(2):138–41. doi: 10.1016/0142-9612(87)90103-7. [DOI] [PubMed] [Google Scholar]
- 50.Mofid MM, et al. Biocompatibility of fixation materials in the brain. Plast Reconstr Surg. 1997;100(1):14–20. doi: 10.1097/00006534-199707000-00003. discussion 21–2. [DOI] [PubMed] [Google Scholar]
- 51.Richey MT, et al. Equine post-anesthetic lameness. A retrospective study. Vet Surg. 1990;19(5):392–7. doi: 10.1111/j.1532-950x.1990.tb01216.x. [DOI] [PubMed] [Google Scholar]
- 52.Ravary B, et al. Strain and force transducers used in human and veterinary tendon and ligament biomechanical studies. Clin Biomech (Bristol, Avon) 2004;19(5):433–47. doi: 10.1016/j.clinbiomech.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Zheng H, Black ND, Harris ND. Position-sensing technologies for movement analysis in stroke rehabilitation. Med Biol Eng Comput. 2005;43(4):413–20. doi: 10.1007/BF02344720. [DOI] [PubMed] [Google Scholar]
- 54.Frankel MA, et al. Multiple-input single-output closed-loop isometric force control using asynchronous intrafascicular multi-electrode stimulation. IEEE Trans Neural Syst Rehabil Eng. 2011;19(3):325–32. doi: 10.1109/TNSRE.2011.2123920. [DOI] [PubMed] [Google Scholar]