Abstract
Objective:
This study was designed to assess various dietary factors and the nutritional status of hospitalized patients with colorectal cancer.
Materials and Methods:
A case-controlled study of fifty newly-admitted patients at King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia diagnosed with colorectal cancer were interviewed to collect data on various dietary factors and their nutritional status. Their data were compared with a sex-matched control group aged fifty.
Results:
The consumption of meat high in fat, fried eggs and whole fat dairy products, and diet low in fibers 2-3 times or above per week increased the risk of colorectal cancer, while the consumption of whole wheat products, vegetables and fruits, and diet low in animal fats at the same rate per week may play a protective role against colorectal cancer in both men and women when compared to controls.
Conclusions:
The higher consumption of meat and fat from animal sources could increase the risk of colorectal cancer. The high consumption of whole wheat bread, fruits and vegetables with high fiber content could play a protective role against the risk of colorectal cancer in the Saudi society. Additional studies are needed in different regions of the Kingdom of Saudi Arabia to verify or refute these results.
Keywords: Colorectal cancer, life style, diet, nutrition, case-control study
INTRODUCTION
Colorectal cancer, a major health problem, is associated with significant morbidity and mortality in the western world.1,2 It is the fourth most common cancer and the second most common cause of cancer death in the USA. Age-standardized incidence rates vary from 23-35 per 100,000 population in North America to 5-15 per 100,000 in the developing countries of Africa and Asia. Rates in Hawaiian Japanese (37 per 100,000) are higher than in any other US population.3 Moreover, Japanese immigrants to the United States showed an increasing incidence of colorectal cancer both in succeeding generations and close relatives of patients with carcinoma of the colon.4 African Americans are reported to have higher incidence and higher mortality from colorectal cancers, which might be due to racial differences.5 However, the disease is uncommon in Africa, Asia and South America suggesting a possible link with a diet rich in animal fat in the developed nations.6 This epidemiological trend is now changing in Asian countries where the incidence of colorectal carcinomas is on the rise.7 The global geographic variations in the incidence of colorectal adenoma and cancer are thought to be due to multiple factors, particularly diet and genetics. Hereditary factors play a definite role, but gene-environment interactions are also more important in the pathogenesis of colorectal cancer.8,9 Epidemiologic investigations have consistently demonstrated a two-to-four-fold increased risk of colon cancer in persons who had a first-degree relative with this carcinoma, as compared to the general population. Overall, 3% to 5% of colon cancer occurs in genetically defined high-risk colon cancer family syndromes.10,11 On the other hand, approximately 70% of colorectal cancer could be explained by environmental factors, and their identification may prevent the development of colorectal cancer to a greater extent.12 Other risk factors for colorectal cancer include age over 50, hereditary poly- and non-polyposis colorectal family syndromes, ulcerative colitis or Crohn's disease, a diet low in fiber and high in fat and from animal sources, hypertriglyceridemia, physical inactivity, and obesity and high body mass index and body size, type II diabetes mellitus, alcohol, and smoking and others.13–19
Conversely, the protective factors for colorectal cancers include physical activity, regular exercise, younger age, higher education, hormone (estrogen) replacement therapy, calcium, vitamin D, folate, and some antioxidant vitamins and minerals such as gamma-tocopherol and selenium, nonsteroidal anti-inflammatory drugs, and the most diverse diet including the use of yogurt and resistant starches.20–22 Women whose diet were rich in vegetables had 20 percent lower risks for colon cancer than women who did not consume vegetables. Modifications in life style such as increasing physical activity, reducing obesity and diet rich in high fibers are ways of primary prevention of colorectal cancer.13,18,21
Literature on colorectal cancer in the Arabian Gulf countries as a whole is scanty. The Kingdom of Saudi Arabia (KSA) is a low risk country for colorectal cancer.23,24 Between June 1975 and December 1989, 622 patients were registered with malignant colorectal and anal tumors in King Faisal Specialist Hospital and Research Center, Riyadh. There were 383 males and 239 females and the average ages were 53.5 and 47.8 years, respectively. The majority of patients were Saudis. The single most common site for large bowel malignancy was the rectum. The greatest majority of patients came from either Makkah or Riyadh. Malignant tumors of the large bowel and anus were the 14th most common tumors (3.3%) registered in KSA.
To our knowledge, no study has been carried out in KSA to investigate the correlation between dietary habits and colorectal cancer. Therefore, this study was designed to investigate the association between the Saudi diet and colorectal cancer only.
MATERIAL AND METHODS
The sample of this case-control design study consisted of fifty Saudi patients newly diagnosed with colon cancer. They were consecutively selected from the inpatients in King Faisal Specialist Hospital and Research Center (KFSH & RC), a national tumor registry center in the Kingdom. Fifty matched controls were selected consecutively from the outpatients in KFSH & RC. All cases and controls were Saudis over 30 years of age, and represented both sexes. The selection of controls was matched to the cases in relation to both age and sex. A case was defined as a newly-diagnosed colon cancer patient who is free from other chronic diseases such as diabetes, hyperlipidemia, hypertension, cardiac, liver and renal diseases. Female cases were not pregnant or lactating. Controls were free from cancer or chronic diseases, but were patients who attended outpatient's clinics of KFSH & RC. The study was approved by the ethical committee of KFSH & RC, and all patients and controls gave informed, written consent to participate in the study.
DIETARY ASSESSMENT
The level of intake of energy, fat, and fibers in the diet was assessed for patients and controls using, 1) The 24-hour recall method. Collection of the cases of 24-hour food recall was of the day before they were diagnosed with colorectal cancer. The 24-hour food recall had a table for recording food items, portion size, and details of food and drinks in accordance to the timings, and 2) Food Frequency Questionnaire (FFQ). Both controls and patients were interviewed to assess the dietary habits with particular emphasis on the frequency of consumption of 98 food items per week over the past year.
The Food Frequency Questionnaire was not used to give quantitative estimates of the dietary intake, but a rough estimate of the qualitative aspects of the dietary intake. The 24-hour recall method, however, was used for the quantitative dietary estimate. Although the 24-hour food recall method does not reflect the usual dietary intake, but is a reliable estimation of the usual dietary intake when combined with the FFQ.25,26 This method is valid if biochemical investigations show that serum values reflect the diet intake during the past 24 hours.25,26
STATISTICAL ANALYSIS
Data analysis was done using SPSS 14.0 version. Frequency distribution and descriptive statistics for each variable were calculated. The odds ratio (OR) which is the odds of cases exposure divided by the odds of controls exposure and t-test were used to determine the significance of the differences between cases and controls. A p-value less than 0.05 was considered statistically significant.
RESULTS
Energy, Fat, Protein, Carbohydrates, and Fibers
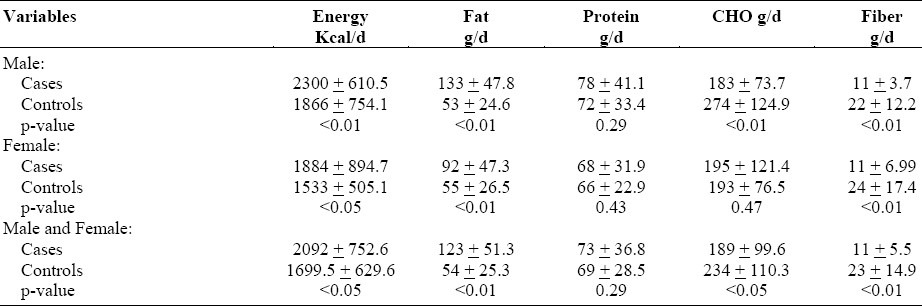
The estimated means of energy, fat, protein, carbohydrate and fiber intake from the 24-hour recall were calculated using food composition tables (Table 1). Significant differences were observed in males and females for both cases and controls for energy, fat and fiber (p<0.05). This significance was not seen in their protein intake. Male controls had significantly more carbohydrates than the male cases (p<0.01); however, there were no significant differences between female cases and controls in this regard (p>0.5). When the means of combined male and female cases were compared with combined male and female controls, there were significant differences with regard to energy, fat (p<0.01), carbohydrates (p<0.5) and fiber intake (p<0.01), but no such differences in relation to protein intake (p>0.05). Thus, cases consumed higher calories and fatty foods but their diet was lower in carbohydrates and fibers as compared to controls while there was no difference between the two groups in their intake of protein.
Table 1.
Mean energy, fat, protein, carbohydrate, and fiber intake for cases and controls (mean ± SD)
Frequency of consumption of food items on weekly basis
Table 2 shows the frequency of consumption of different food items among all cases and controls on a weekly basis. The foods were classified into two categories, 1) high fat and 2) high fiber foods. Odds ratio of the comparison of the consumption of each food item 2-3 times per week were calculated with never; it was also calculated with the consumption of the food item 4 times and more, compared to 2-3 times.
Table 2.
Frequency of consumption of different food items on a weekly basis for combined cases and controls
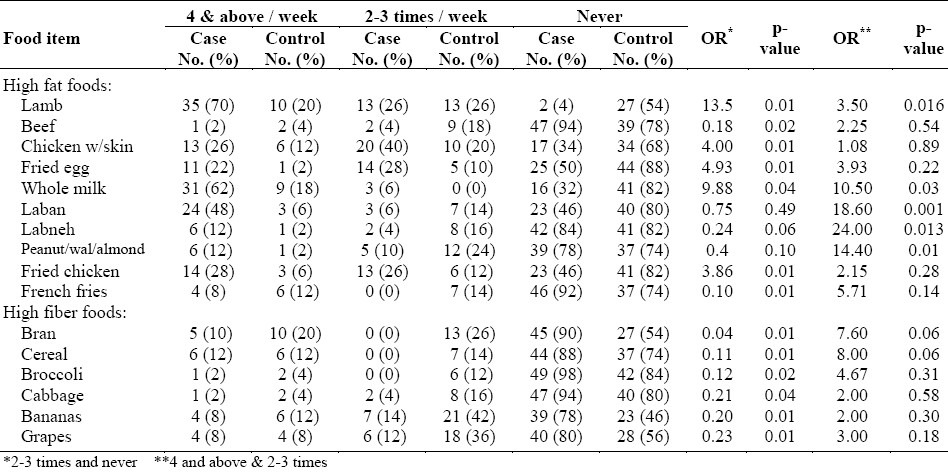
In the high fat food category, the following observations were made. Consumption of lamb increased the risk of having colorectal cancer (OR=13.5, p<0.01), an increase in the consumption resulted in a further increase in the risk (OR=3.5, p<0.02). The consumption of chicken with the skin and fried eggs increased the risk (OR=4, p<0.01) and (OR=4.93, p<0.01), respectively. However, the consumption of beef reduced the risk of developing colorectal cancer (OR=0.18, p<0.02).
Consumption of milk was associated with increased risk (OR=9.88, p< 0.04) and (OR=10.5, p<0.03). Although the consumption of laban 2-3 times a week did not show any significant risk of having colorectal cancer, its increased consumption to 4 times or above a week resulted in an increase in the risk of colorectal cancer to more than 18 times (OR=18.6, p<0.01) when compared to having it only 2-3 times a week. Labnah showed the same trend, but its increased consumption to more than 4 times a week resulted in increasing the risk of colorectal cancer to 24 folds compared to 2-3 times a week (OR=24, p<0.02).
Consuming peanuts, walnuts, and almonds 2-3 times a week did not show any significance.
Yet, their increased consumption to more than 4 times a week resulted in increasing the risk (OR=14.4, p<0.01). Fried chicken and French fries increased the risk (OR=3.86, p<0.01) and (OR=0.1, p<0.01), respectively.
In the high fiber category, the following findings were observed. Consumption of bran 2-3 times per week showed a protective effect against the risk of having colorectal cancer (OR=0.04, p<0.01). The same trend was seen with breakfast cereals (OR=0.11, p<0.01), broccoli (OR=0.12, p<0.02), cabbage (OR=0.21, p<0.04), banana (OR=0.2, p<0.01), and grapes (OR=0.23, p<0.01). However, their increased intake to more than 4 times a week gave no further protection.
Gender and diet effects on the risk of developing colorectal cancer are shown in Tables 3 & 4. Table 3 shows the frequency of consumption of different food items by male cases and controls on a weekly basis. In the high fat food category, we observed the following results. Increasing the consumption of lamb to more than 4 times a week as compared to only 2-3 times a week, increased the risk of colorectal cancer in men (OR=19.8, p<0.01). The consumption of chicken with the skin 2-3 times a week increased the risk in men (OR=4.28, p<0.04) and fried eggs showed the same trend (OR=7, p<0.01). Beef, however, reduced the risk when men consumed it 2-3 times a week (OR=0.13, p<0.04).
Table 3.
Frequency of consumption of different food items on a weekly basis for male cases and controls
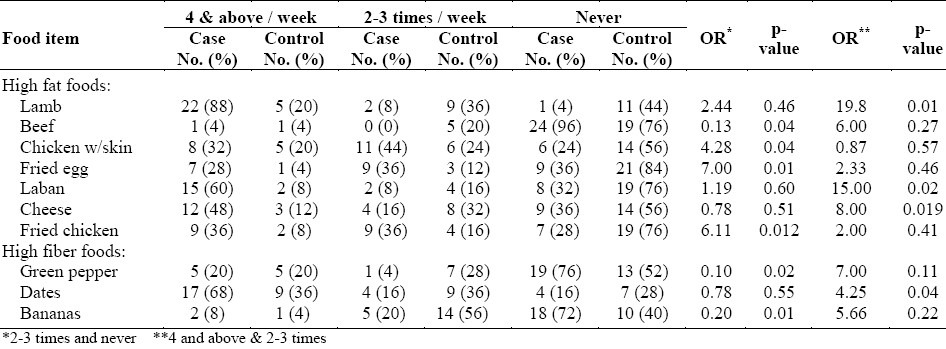
Table 4.
Frequency of consumption of different food items on a weekly basis for female cases and controls
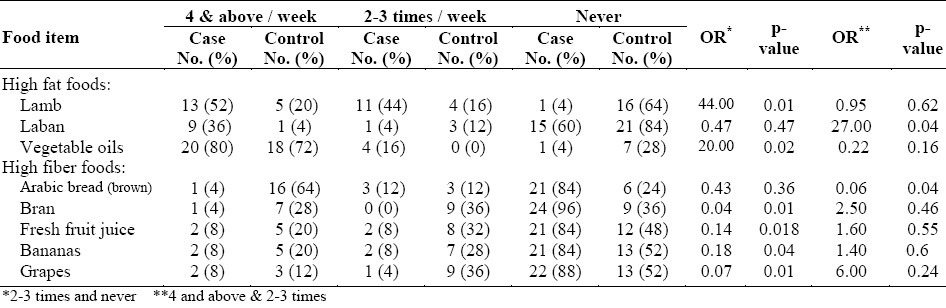
Consuming laban 4 times or above a week resulted in increasing the risk of colorectal cancer in men (OR=15, p <0.02) when compared to having it only 2-3 times a week. The same observations were made on cheese (OR=8, p<0.02). Fried chicken was also a risk factor (OR=6.11; P<0.02).
In the high fiber food category, the following findings were observed. Consumption of green pepper 2-3 times a week played a protective role against colorectal cancer in men (OR=0.1, p<0.02). Any increase in consumption failed to show any stronger protective effect. Bananas followed the same trend as a protective factor in men (OR=0.2, p<0.01). The consumption of dates was shown to be a risk factor only at 4 times or above per week in men (OR=4.25, p<0.04).
Table 4 shows the frequency of consumption of different food items by female cases and controls on a weekly basis. In the high fat food category, we observed that lamb was a strong risk factor in women (OR=44, p<0.01). Laban was found to be a high risk factor in women only when consumed 4 times or above per week (OR=27, p<0.04). The consumption of vegetable oil also showed significant statistical differences in female cases and controls as a risk factor at a consumption rate of 2-3 times a week (OR=20, p<0.02).
In the high fiber food category, the following results were observed. The consumption of Arabic Brown Bread 4 times or above a week gave protection against colorectal cancer in women (OR=0.06, p<0.04). The consumption of 2-3 times per week was also protective in women (OR=0.04, p<0.01). The same observations were made on fruit juice (OR=0.14, p<0.02), banana (OR=0.18, p<0.04), and grapes (OR=0.07, p<0.01). However, their increased intake to more than 4 times a week did not increase the protection in women. Overall, the consumption of high fat meat, fried eggs and whole fat dairy products 2-3 times or above per week could increase the risk of having colorectal cancer, while the consumption of whole wheat products, vegetables and fruits at the same rate could play a protective role in both men and women when compared to controls.
DISCUSSION
The present case-control study assessed the nutritional status of colorectal cancer patients and investigated the retrospective dietary practices and food intake that might promote colorectal cancer. In an unpublished National Nutrition Survey that was conducted in 1994 among Saudi population, the daily per capita energy, fat, protein and carbohydrate intake was estimated to be 3082 kcal, 145 gm/head, 115 gm/head and 300 gm/head, respectively.27 These values were higher than what was found in this study among cases as well as controls. Furthermore, according to this study the controls were very close to the per capita intake of fiber for Saudis27 but the cases had only half of that. These findings could be explained by the timings of the two investigations (1994 versus 2003) and the campaign in KSA in the awareness of the effect of obesity, regular exercise and balanced diet. The projection is that if this trend continues, the incidence and prevalence rates of colorectal cancer might continue to decline in Saudi society.
This study found that increasing the consumption of lamb and red meat was associated with colorectal cancer, which supports the results of other researchers.28–30 Alternatively, decreasing the intake of red meat and processed meat will contribute to a reduction in the incidence of cancers of the digestive and respiratory tracts. However, in this study, the association with beef was not seen, probably because the consumption of beef is uncommon among Saudis. When eaten, it is the lean cuts of beef, which are associated with reduced risks of small colorectal adenomas.31 Some researchers have reported opposite views32 of the lack of association of the consumption of red meat with colorectal cancer.
According to this study, the consumption of chicken without skin had no effect on the risk of having colorectal cancer. However, the high consumption of red meat, chicken with skin and fried chicken showed some associations, which is in accord with other studies33,34 that reported a positive association between the risk of colorectal polyps and the consumption of meat and a negative association with fish or the consumption of poultry/fish. Furthermore, an increase in the ratio of the consumption of red meat to the consumption of fish/chicken was associated with an increased risk in the colorectal polyps. Frequent raw/cooked fish intake may decrease the risk of colorectal cancer.35 In this study, the consumption of whole milk by the combined cases (62%) was close to the national average of 60%.27 Interestingly, only 18% of the controls had whole milk; and, in this study the increased consumption of laban (cultured butter milk) resulted in the increased risk of having colorectal cancer. On the other hand, the increased consumption of dietary calcium is reported to inhibit the development of colonic tumors.36 Similarly, the fermented dairy products with their lactic acid bacteria are considered potential cancer preventing agents in the diet enhancing the cancer fighting ability of the microflora in the gut.20,37 Our findings might be due to the fact that these dairy products had high fat content. Low fat dairy products have been recently introduced into the Saudi market, especially the cultured butter milk which is known to be a dairy product low in fat.
Our study found that the consumption of almonds and other nuts reduced the risk of colon cancer risk as evidenced in a rat model.38 Butyrate, a short-chain fatty acid produced in the colon by microbial fermentation of fiber, inhibits the growth of colonic carcinoma cells while inducing differentiation. In the same vein, resveratrol, a plant polyphenol found in peanuts, has been shown to exert chemopreventive properties on colon cancer cells.39 At a cautious level, our findings also showed that when the intake of peanuts was increased to more than four times a week, there was increased risk of colorectal cancer. This stresses the importance of moderation which is one of the basic recommendations in the healthy diet.
In our study, the consumption of French fries had no effect on colorectal cancer. However, Fung et al40 found a significant positive association between the western dietary patterns characterized by the higher consumption of red and processed meats, sweets, desserts, French fries, and refined grains with colorectal cancer. On the other hand, Mucci et al41 found that moderate to high levels of acrylamide were unexpectedly detected in widely consumed food items notably French fries, potato crisps, and bread, which were not associated with large bowel cancer. In findings similar to those in this study, fats and oils were reported to have a link with colorectal cancer.42 Even vegetable oils, considered to be healthier than animal fats, led to increased risk of colorectal cancer.43 More importantly, when these vegetable oils are used for frying, they become more saturated and therefore aggravate the risk of having colorectal cancer.44
One of the findings of this study is the inverse relationship between the consumption of rich food sources of dietary fiber such as bran, Arabic brown bread, broccoli, bananas, green peppers and other fruits and vegetables versus colorectal cancer which supports the results of previous epidemiological studies.45–50 There are many possible mechanisms of the protection of dietary fiber against colorectal cancer. These include: 1) Shortening the transit time, 2) Formation of short chain fatty acids such as acetate, butyrate, and propionate in the colon upon the fermentation of dietary fiber, 3) Reduction of the ability of bile acids to act as carcinogens by the resistant starch and indigestible oligosaccharides, 4) Suppression of cancer cell proliferation by selenium from cereals which functions as a cofactor for glutathione peroxidase, an enzyme that protects against oxidative tissue damage, 5) The inverse association of folic acid from fruits with cancer of the colon and rectum, 6) Presence of anticarcinogenic components such as carotenoids, vitamin C, resveratrol, flavonoids, organosulfides, isothiocyanates, and protease inhibitors in fruits and vegetables. These mechanisms should be interpreted carefully because an intervention study of a diet rich in fruits, vegetables, and fibers failed to decrease the risk of new colorectal adenoma formation.51 Surprisingly, our study found that a high consumption of fiber containing dates may increase the risk of colorectal cancer. Since there have been no reports in support of this finding, more studies are required.
This study suggests that the intake of high fiber diets such as whole wheat products, fruits and vegetables might reduce the risk of colorectal cancer while red and processed meat and high fat consumption might increase the risk of colorectal cancer among Saudis. The consumption of chicken without skin, fish and low fat milk might not be a colorectal cancer risk. In view of this study and reviewed literature, we recommend the followings: an education program on nutrition to increase public awareness of the protective role of high fiber diets and limited fat and red meat intakes; the inclusion of education programs on nutrition health promotion activities at primary health care centers; screening of patients more than 50 years of age with average risk factors for the disease. In addition, more studies with larger samples from different regions of Saudi Arabia are needed to determine the effect of dietary factors and colorectal cancer risk.
REFERENCES
- 1.Dove-Edwin I, Thomas HJ. The Prevention of colorectal cancer: Review article. Aliment Pharmacol Ther. 2001;15:323–36. doi: 10.1046/j.1365-2036.2001.00934.x. [DOI] [PubMed] [Google Scholar]
- 2.Wallace HM, Caslake R. Polyamines and colon cancer. Eur J Gastroenterol Hepatol. 2001;13:1033–9. doi: 10.1097/00042737-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg ER, Baron JA, Tosteson TD, Freeman DH, Jr, Beck GJ, et al. A clinical trial of antioxidant vitamins to prevent colorectal adenoma: Polyp Prevention Study Group. N Engl J Med. 1994;21(331):141–7. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- 4.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer: A population-based study. N Engl J Med. 1990;1(323):1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 5.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer and race: understanding the differences in outcomes between African Americans and whites. Med Clinics North Am. 2005;89:771–93. doi: 10.1016/j.mcna.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Willet W, Stampfer MJ, Colditz CA, Rosner BA, Speizer FE. Relation of meat, fat and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990;13(323):1664–72. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- 7.Sung JJ, Lau JY, Goh KL, Leung WK. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871–6. doi: 10.1016/S1470-2045(05)70422-8. [DOI] [PubMed] [Google Scholar]
- 8.Heavy PM, McKenna D, Rowland IR. Colorectal cancer and the relationship between genes and the environment. Nutr Cancer. 2004;48:124–41. doi: 10.1207/s15327914nc4802_2. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson BM, Finan PJ, Gascocyne J, Garbett F, Murday VA, Bishop DT. Frequency of familial colorectal cancer. Br J Surg. 1991;78:1162–66. doi: 10.1002/bjs.1800781005. [DOI] [PubMed] [Google Scholar]
- 10.Burt RW, Neklason DW. Genetic testing for inherited colon cancer. Gastroenterol. 2005;128:696–1716. doi: 10.1053/j.gastro.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 11.Grandy WM. Genetic testing for high-risk colon cancer patients. Gastroenterol. 2003;124:1574–94. doi: 10.1016/s0016-5085(03)00376-7. [DOI] [PubMed] [Google Scholar]
- 12.Saikali J, Picard C, Freitas M, Holt P. Fermented milks, probiotic cultures and colon cancer. Nutr Cancer. 2004;49:14–24. doi: 10.1207/s15327914nc4901_3. [DOI] [PubMed] [Google Scholar]
- 13.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55:285–91. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jass JR. What is new in hereditary colorectal cancer? Arch Pathol Lab Med. 2005;129:1380–4. doi: 10.5858/2005-129-1380-WNIHCC. [DOI] [PubMed] [Google Scholar]
- 15.Jarvinen HJ. Genetic testing for polyposis and ethical aspects. Gut. 2003;52(Suppl 2):ii19–22. doi: 10.1136/gut.52.suppl_2.ii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo HH, Loeb LA. Tumbling down a different pathway to genetic instability. J Clin Investig. 2003;112:1793–5. doi: 10.1172/JCI20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulrich CM, Bigler J, whinon JA, Bostick R, Fosdick L, Potter JD. Epoxide hydrolase Try1l3His Polymorphism is associated with elevated risk of colorectal polyps in the presence of smoking and high meat intake. Cancer Epidemiol Biomarkers Prev. 2001;10:875–82. [PubMed] [Google Scholar]
- 18.Wu AH, Shibata D, Yu MC, Lai MY, Ross RK. Alcohol, physical activity and other risk factors for colorectal cancer: a prospective study. Br J Cancer. 2001;55:687–94. doi: 10.1038/bjc.1987.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sial SH, Catalano MF. Gastrointestinal tract cancer in the elderly. Gastroenterol Clinics North Am. 2001;30:565–90. doi: 10.1016/s0889-8553(05)70196-5. [DOI] [PubMed] [Google Scholar]
- 20.Adolfsson O, Meydani SN, Russell RM. Yogurt and gut function. Am J Clin Nutr. 2004;80:245–56. doi: 10.1093/ajcn/80.2.245. [DOI] [PubMed] [Google Scholar]
- 21.Belza B, Warms C. Physical activity and exercise in women's health. Nurs Clinics North Am. 2004;39:181–93. doi: 10.1016/j.cnur.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Humphries KH, Gill S. Risks and benefits of hormone replacement therapy: the evidence speaks. CMAJ. 2003;168:1001–10. [PMC free article] [PubMed] [Google Scholar]
- 23.Isbister WH. Malignant neoplasia of the colon, rectum and anus at the King Faisal Specialist Hospital and Research Center. Ann Saudi Med. 1992;12:429–33. doi: 10.5144/0256-4947.1992.429. [DOI] [PubMed] [Google Scholar]
- 24.El-Sheikh MAR, Al-Karawi MA, Koreich OM. Incidence of colorectal cancer and colonic polyps in Saudi patients. Ann Saudi Med. 1990;10:19–21. [Google Scholar]
- 25.Burke BA, Staurt HC. A method of diet analysis. J. Pediatr. 1938;12:493–503. [Google Scholar]
- 26.Block GA. A review of validations of dietary assessment methods. Am J Epidemiol. 1982;115:492–505. doi: 10.1093/oxfordjournals.aje.a113331. [DOI] [PubMed] [Google Scholar]
- 27.Al Kanhal M, Osman AK. WHO Symposium on “The epidemiologic transition/transaction and health in developing countries.”. College of Medicine, K.S.U., Riyadh: 1994. Nov 27-29, Changing food consumption pattern and health in Saudi Arabia. [Google Scholar]
- 28.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2003;31:925–43. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 29.Seow A, Quah SR, Nyam D, Straughan PT, Chua T, Aw TC. Food groups and the risk of colorectal cancer in an Asian population. Cancer. 2002;1(95):2390–6. doi: 10.1002/cncr.10971. [DOI] [PubMed] [Google Scholar]
- 30.Norat T, Riboli E. Dairy products and colorectal cancer: A review of possible mechanisms and epidemiological evidence. Eur J Clin Nutr. 2003;57:1–17. doi: 10.1038/sj.ejcn.1601522. [DOI] [PubMed] [Google Scholar]
- 31.Seneese P, Boutron MC, Faivre J, Chatelain N, Belghiti C, Meance S. Foods as risk factors for colorectal adenomas: a case-control study in Burgundy (France) Nutr Cancer. 2002;44:7–15. doi: 10.1207/S15327914NC441_2. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed FE. Effect of diet, life style, and other environmental chemopreventive factors on colorectal cancer development, and assessment of the risks. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2004;22:91–147. doi: 10.1081/LESC-200038263. [DOI] [PubMed] [Google Scholar]
- 33.Butler LM, Sinha R, Millikan RC, Martin CF, Newman B, et al. Heterocyclic amines, meat intake, and association with colon cancer in a population-based study. Am J Epidemiol. 2003;157:434–45. doi: 10.1093/aje/kwf221. [DOI] [PubMed] [Google Scholar]
- 34.Yoon H, Benamouzig R, Little J, Francois M, Tome D. Systematic review of epidemiological studies on meat, dairy products and egg consumption and risk of colorectal adenomas. Eur J Cancer Prev. 2000;9:151–64. [PubMed] [Google Scholar]
- 35.Yang CX, Takezaki T, Hirose K, Inoue M, Huang XE, Tajima K. Fish consumption and colorectal cancer: a case-reference study in Japan. Eur J Cancer Prev. 2003;12:109–15. doi: 10.1097/00008469-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Lipkin M. Early development of cancer chemoprevention clinical trials: studies of dietary calcium as a chemopreventive agent for human subjects. Eur J Cancer Prev. 2002;11(2):S65–S70. [PubMed] [Google Scholar]
- 37.Burns AJ, Rowland IR. Anti-carcinogenicity of probiotics and prebiotics. Curr Issues Intest Microbiol. 2002;1:13–24. [PubMed] [Google Scholar]
- 38.Davis PA, Iwahashi CK. Whole almonds and almond fractions reduce aberrant crypt foci in a rat model of colon carcinogenesis. Cancer Lett. 2001;165:27–33. doi: 10.1016/s0304-3835(01)00425-6. [DOI] [PubMed] [Google Scholar]
- 39.Wolter F, Stein J. Resveratrol enhances the differentiation induced by butyrate in caco-2 colon cancer cells. J Nutr. 2002;132:2082–6. doi: 10.1093/jn/132.7.2082. [DOI] [PubMed] [Google Scholar]
- 40.Fung T, Hu FB, Fuchs C, Giovannucci E, Hunter DJ, Stampfer MJ, et al. Major dietary patterns and the risk of colorectal cancer in women. Arch Inter Med. 2003;163:309–14. doi: 10.1001/archinte.163.3.309. [DOI] [PubMed] [Google Scholar]
- 41.Mucci LA, Dickman PW, Steineck G, Adami HO, Augustsson K. Dietary acrylamide and cancer of the large bowel, kidney, and bladder; absence of an association in a population – based study in Sweden. Br J Cancer. 2003;13(88):84–9. doi: 10.1038/sj.bjc.6600726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B, Li X, Nakama H, Zhang X, Wei N, Zhang X, et al. A case-control study on risk of changing food consumption for colorectal cancer. Cancer Invest. 2002;20:458–63. doi: 10.1081/cnv-120002145. [DOI] [PubMed] [Google Scholar]
- 43.You WC, Jin F, Devesa S, Gridley G, Schatzkin A, Yang G, et al. Rapid increase in colorectal cancer rates in urban Shangahi in relation to dietary changes. J Cancer Epidemiol Prev. 2002;7:143–6. [PubMed] [Google Scholar]
- 44.Kim YI. Vegetables, fruits, and colorectal cancer risk: What should we believe? Nutr Rev. 2001;59:394–8. doi: 10.1111/j.1753-4887.2001.tb06969.x. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez CA. The European prospective investigation into cancer and nutrition. Public Health J. 2006;9:124–6. doi: 10.1079/phn2005934. [DOI] [PubMed] [Google Scholar]
- 46.Peters U, Sinha R, Chatterjee N, Subar AF, Ziegler RG, et al. Dietary fiber and colorectal adenoma in a colorectal cancer early detection program. Lancet. 2003;361:1491–5. doi: 10.1016/S0140-6736(03)13173-X. [DOI] [PubMed] [Google Scholar]
- 47.Scheppach W, Luelirs H, Menzel T. Beneficial health effects of long-digestible carbohydrate consumption. Br J Nutr. 2001;85:S23–S30. doi: 10.1079/bjn2000259. [DOI] [PubMed] [Google Scholar]
- 48.Lynn A, Collins A, Fuller Z, Hillman K, Ratcliffe B. Cruciferous vegetables and colorectal cancer. Porc Nutr Soc. 2006;65:135–44. doi: 10.1079/pns2005486. [DOI] [PubMed] [Google Scholar]
- 49.Bingham S. the fibre-folate debate in colorectal cancer. Porc Nutr Soc. 2006;65:19–23. doi: 10.1079/pns2005472. [DOI] [PubMed] [Google Scholar]
- 50.McGarr SE, Ridlon JM, Hylemon PB. Diet, anaerobic bacterial metabolism, and colon cancer: a review of the literature. J Clin Gastroenterol. 2005;39:98–109. [PubMed] [Google Scholar]
- 51.Giacosa A, Hill MJ, Davies GJ. Fibers and colorectal cancer: should we change our dietary advice on prevention? Dig Liver Dis. 2002;34:S121–S3. doi: 10.1016/s1590-8658(02)80178-5. [DOI] [PubMed] [Google Scholar]


