Abstract
Background:
Cutaneous leishmaniasis (CL) is still a major health problem in many countries including Saudi Arabia. Patients with CL are seen, not only by dermatologists, but also by pediatricians and community physicians. Knowledge of available treatment options is essential.
Design:
A literature review utilizing PubMed and Cochrane evidence-based library was undertaken in the last five years.
Results:
Several medications and therapeutic modalities are currently in use, though the gold standard remains systemic antimonials. Drug resistance and serious side effects preclude the use of available medications. Newer therapies like liposomal amphotericin B, miltefosine and pentamidine are being used; while it is hoped that other drugs like imiquimod, tamoxifen, PDT and pentamidine structural analogs being tested would offer better efficacy, easier administration and lower toxicity.
Conclusion:
After decades of little advance in the treatment of leishmaniasis, there are now several options with newer compounds and combinations of these.
Keywords: Leishmaniasis, Leishmaniasis treatment
INTRODUCTION
Cutaneous leishmaniasis (CL) which is associated with considerable morbidity is still a major health problem in the world especially in developing countries. Over the years, many medications and procedures have been tried (Tables 1 and 2). Their number attests to the fact that none of these has been satisfactory. Evidence-based studies are few and it is difficult to determine what the best treatments are.1 For many decades, pentavalent antimonials have remained the gold standard of treatment. In a recent study, a meta-analysis of treatment of New World Cutaneous Leishmaniasis showed a 76.5% cure rate in 1150 patients treated with pentavalent antimonials and concluded that the pentavalent antimonials were as effective as pentamidine.2 In the most recent studies, cure rates have not gone beyond 80%.3 Several factors, most important of which are the strain of leishmania and host's immune system, determine the biologic behaviour of the disease. In assessing the efficacy of treatment, the natural course of the disease has to be taken into consideration as well as the fact that CL often heals spontaneously within one week to three years, usually within one year. To be considered also when the best mode of treatment is being determined are the cost, availability of drugs, adverse effects and the local experience. Over the last 50 years, though only a few drugs for cutaneous leishmaniasis have emerged, drug resistance has increased. In this review, the newly introduced medications and those being developed will be discussed.
Table 1.
Current treatment options of CL
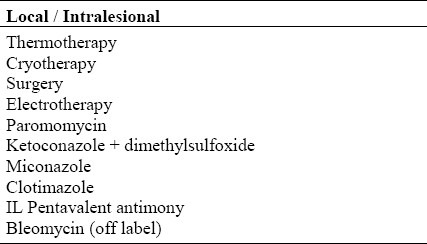
Table 2.
Current treatment options of CL
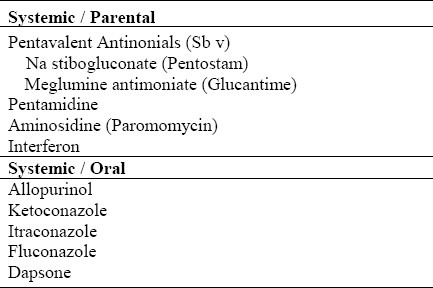
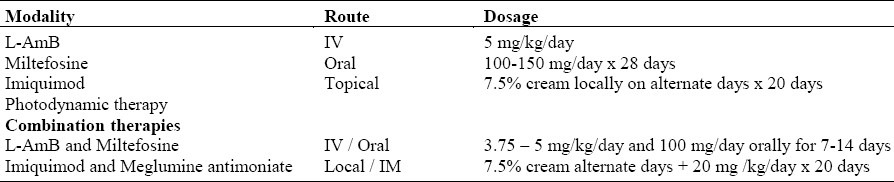
NEWLY INTRODUCED MEDICATIONS (Table 3)
Table 3.
Newly introduced medications/procedures for treatment of CL
Liposomal Amphotericin B ( L-AmB )
Amphotericin B is the standard drug for the treatment of systemic fungal infections. It has also been used as the second line of treatment for visceral leishmaniasis (VL) resistant to pentavalent antimonials. Amphotericin B, however, has a low therapeutic index, high acute toxicity and necessitates parenteral administration. To avoid these drawbacks, a new lipid-associated formulation of the original polyene amphotericin B, liposomal amphotericin B has been introduced. This formulation is better tolerated and has less infusion-related toxicity and nephrotoxicity than AmB, but has the same efficacy as the original formulation. It is administered intravenously in a dose 3 – 10 mg/kg/day for 5 – 7 days. The lower dosage and shorter duration are as effective as the higher dosages and longer duration of treatment. The recently introduced L- AmB formulation has shown proven efficacy in Old World and New World VL ( OWVL and NWVL ),4–5 However, its use in Old and New World CL has been limited. This is a truly promising new medication.
Other formulations such as Amph B colloidal dispersion (ABCD) and Amph B lipid complex (ABLC) have been developed. These have been investigated primarily for the treatment of invasive fungal infections and are effective and less toxic than amph B. Investigations of the use of these formulations for the treatment of leishmaniasis are limited, so more trials are needed.
Miltefosine
Miltefosine is the first recognized oral treatment of leishmaniasis. It was registered in 2002 in India for the treatment of visceral leishmaniasis and currently in use in the full range of clinical leishmaniasis.7–11 A review of PubMed articles from 2005 – 2008 dealing with the use of miltefosine in leishmaniasis shows that it is an effective recommended treatment for Indian, Ethiopian VL, and Columbian and Bolivian CL. It is also the drug of choice for unusual forms of leishmaniasis that require long periods of treatment as in diffuse cutaneous leishmaniasis (DCL) and post-kala-azar dermal leishmaniasis (PKADL).7
Of special interest, is the study of the pharmacokinetics of miltefosine in the treatment of Old World CL (OWCL). In this recent study, miltefosine was given to 31 Dutch military personnel who contracted leishmaniasis while serving in Afghanistan, at a dose of 150 mg/day for 28 days with a maximum follow up duration of 202 days after initiation of treatment. The concentration of miltefosine was determined in the blood samples during the period of treatment and up to 5 months post treatment. Results showed the first elimination half-life to be 7.05 days and the terminal elimination half-life of 30 days with a mean concentration of 30,800ng/ml in the last week of treatment (22-28 days). All analyzed samples contained concentrates of miltefosine above the lower limit of qualification (LLOQ) of 4ng/ml. Miltefosine was detected in human plasma samples for up to 5-6 months after cessation of treatment. The implication of the detection of subtherapeutic concentrations of miltefosine in the blood beyond five months post treatment may indicate emerging resistant parasites as well as risks of teratogenicity.12
The efficacy and safety profile of miltefosine in the treatment of zoonotic cutaneous leishmaniasis (ZCL) caused by L. major in Iran has been studied and compared with meglumine antimoniate in a randomized open-label study. Complete clinical response was defined as 100% re-epithelialization of the lesion. Definitions of lesion cure and failure were based on both clinical and parasitological criteria two weeks after the end of treatment, and clinical recovery three months after the parasitological cure period. Miltefosine was given to 32 subjects with ZCL, while 31 received IM meglumine antimoniate in a dose of 20 mg sb (5) kg/daily for 14 days. Of the 28 subjects who completed treatment with miltefosine, 26 were cured at 3 months corresponding to a 92.9% cure rate. There was one treatment failure (3.1%), one relapse (3.1%) and four drop-outs as a result of lack of tolerance of the drug. In the antimonial group, 25 of the 31 subjects were cured (83.3%); five failed (16.1%) and one (3.2%) was lost at three months of follow up. No relapses were observed in the group who received the IM meglumine antimoniate at six months follow-up after the cessation of treatment. Both treatment protocols were tolerated but nausea (32.2%) and vomiting (21.5 %) were observed within the first two weeks of initiation of the oral miltefosine regimen. Other gastrointestinal and musculoskeletal and total adverse reactions were not significant in the two groups. No abnormalities were detected in the hematological, liver enzymes or creatinine levels. The study concluded that miltefosine was as effective as meglumine antimoniate in the treatment of ZCL.13
Imiquimod
This is a recently introduced topical immunomodulator initially approved for the treatment of genital warts. Subsequently, it has been used in a wide spectrum of diseases, both inflammatory and neoplastic.
In a study in Peru, imiquimod cream 7.5% was used on alternate days for 20 days in a group with newly diagnosed CL. Even though, there was an initial response, there was a relapse on discontinuation of treatment.14
Photodynamic Therapy (PDT)
Photodynamic therapy is a recently introduced modality approved for the treatment of actinic keratoses. It involves the topical application of a porphyrin precursor, aminolevulinic acid (ALA) or methyl-aminolevulinate (MAL), followed by irradiation with laser or Intense Pulsed Light (IPL). Since its first introduction, PDT has been utilized in various cutaneous neoplasias. More recently, this therapeutic modality has been utilized in non-neoplastic conditions including cutaneous leishmaniasis.15
Few mechanistic studies have addressed the principles underlying the use of PDT for the treatment of CL.16–18
In vitro and mechanistic studies of ALA-PDT against CL did not demonstrate any parasiticidal effects on amastigotes and no differences in ALA-derived PpIX levels were detected between leishmania-infected and non-infected J774.2 cells.16
In contrast, in vivo topical ALA-PDT performed on a murine CL model resulted in extensive tissue destruction and significant reduction of parasite load. A dramatic decrease of macrophages and increased levels of interleukin six was observed in infected skin. These findings suggest that the parasiticidal effect of ALA-PDT for cutaneous leishmaniasis is indirect and mediated through the killing of host cells rather than the direct destruction of parasites.16,17
A review of six clinical studies investigating the use of PDT in 39 patients with a total of 77 lesions for the treatment of Old World CL showed that PDT with the porphyrin precursors is relatively effective. However, the data is still limited and PDT cannot be recommended in routine clinical practice at this point. Even though it is considered a promising therapeutic modality, additional controlled trials and further investigations are needed.15
COMBINATION THERAPIES
Liposomal Amphotericin B (L-AmB) and Oral Miltefosine
In an attempt to find an effective and alternative treatment regimen for the treatment of Indian VL, where drug resistance to pentavalent antimony is high, a new approach using the combination of a single infusion of L-AmB followed by a short course of oral miltefosine was tested in a randomized, non-comparative, group sequential, triangular design. A total of 181 patients participated in the study and were assigned to four treatment groups with 5mg/kg of L-AmB alone (group 1; 45 patients); 5mg/kg of L-AmB followed by miltefosine for 10 days (group 2; 46 patients); or 14 days (group 3; 45 patients); or 3.75 mg/kg of L-AmB followed by miltefosine for 14 days (group 4; 45 patients). All four treatment regimens proved to be effective. An additional 45 non-randomized patients were assigned to receive 5 mg/kg of L-AmB followed by miltefosine for 7 days (group 5).
All 226 subjects assigned to each of the five regimens showed an initial cure response, and a final cure rate nine months post treatment in the five groups ranged from 91-98%. The results of this study suggest that combination therapy with a single dose L-AmB followed by 7-14 days of miltefosine is effective in Indian Kala Azar.19
Imiquimod and Parenteral Meglumine Antimoniate
In a pilot study in Peru, the use of imiquimod alone and in combination with parenteral meglumine antimoniate in the initial treatment of CL was compared. Results of the therapy of seven patients with CL treated with topical imiquimod cream 7.5% combined with intravenous meglumine antimoniate at a dose of 20 mg/kg/day for 20 days showed a cure rate of 100% compared to 57% in the seven patients who received parenteral meglumine antimoniate alone.14 These results attest to the effectiveness of this combined therapy especially as initial treatment of cutaneous leishmaniasis. However, additional larger studies are necessary for this promising regimen.
TREATMENT OF IMMUNOCOMPROM-ISED PATIENTS
Organ Transplant
Leishmaniasis is occasionally reported amongst transplant recipients and most cases are observed in the Mediterranean area. Although, this is most commonly associated with kidney transplantation (77%), it also occurs amongst patients who have undergone liver, heart, lung, pancreas and bone marrow transplantation. The most frequently observed clinical presentation in such patients is VL followed by mucosal leishmaniasis and very rarely CL. In such patients, the first line of treatment is L-AmB.20–22
HIV and AIDS
Patients with HIV/AIDS infection are prone to opportunistic infections such as leishmaniasis which occurs mainly in the Mediterranean region, Southern Europe and Brazil.8,23–26 The clinical manifestations of leishmaniasis in patients with HIV are varied and include disseminated, visceral, diffuse cutaneous and post kala azar dermal leishmaniasis (PKDL). Furthermore, these infections are usually resistant to treatment with pentavalent antimonials, resulting in high rate of failure and relapse. The drugs that are commonly used for HIV patients with leishmaniasis are the pentavalent antimonial compounds, AmB, L-AmB and miltefosine.8,10,23–25,27 The efficacy of the pentavalent antimonial compounds was compared with that of AmB in HIV patients with leishmaniasis and showed similar initial cure rates of 66% vs. 62% respectively. However, relapse is the norm.23
Apart from being an opportunistic infection in patients with AIDS, leishmaniasis seems to be an emerging complication in immune reconstitution inflammatory syndrome (IRIS) following HAART therapy.28
DRUGS ON THE HORIZON
Tamoxifen
This is an anti-estrogen that has been used in the treatment and prevention of breast cancer for several years. In a recent study in which BALB/c mice infected with L. amazonensis, tamoxifen was administered at a dose of 20 mg/kg intraperitoneally daily for 15 days. This resulted in a decrease in lesion size and ulcer formation, a sustained reduction in the number of parasites and extreme susceptibility. This is a promising drug that needs to be further tested.29 Similar results were achieved testing the efficacy of tamoxifen in L. braziliensis-infected BALB/c mice and in L. chagasi-infected hamsters.30
Pentamidine Structural Analogs
The pentamidine structural analogs are alkylphosphocholines used as anticancer treatment. They have recently been found to be very effective as oral treatment for leishmaniasis. These compounds act as membrane synthetic ether-lipid analogs and consist of alkyl chains in the lipid portions. The most promising of these are miltefosine (hexadecyl phosphocholine), eldofisine [ET-18-OCH (3)], ilmofosoine (BM41.440) and perofisine.31
Based on the proven efficacy of pentamidine and related dications against protozoal infections, 18 structural analogs of pentamidine were evaluated for in vitro anti-leishmanial activity of L. major and L. tropica. Pentamidine was the standard reference drug used for comparison. Results showed that the reversed amidine compounds were more active than the furan analogs against both leishmania species. DB 745 and DB 746 demonstrated the highest activity against L major, while DB 745 was more effective against L. tropica. Both compounds, however, exhibited 50% inhibitory concentrations (IC 50) below 1 nM for L. major. Amidine analogs were also tested. Of the 10 reversed amidine compounds, nine showed inhibitory growth effect on amastigote axenic model at a concentration below 1 nM. These studies show that dicationic compounds are potential new agents with less toxicity than the parent drug for the treatment of CL, but further studies are needed.32 Other pentamidine analogs tested for activity against leishmania organisms are parfuramidine, eflornithine, perifosine, edelfosine, ilmosfosine and sitamaquine.31,33–36
Parfuramidine is a novel diamidine already in Phase III clinical trial for early stage disease of human African trypanosomiasis (HAT).33
Perifosine, another novel alkylphospholipid, which recently entered Phase II clinical trials, demonstrated high in vitro activity against all tested strains of leishamania including L. amazonensis.34
Both edelfsoine and perifosine demonstrated in vivo activity against L. amazonensis as shown in a study in which edelfosine and perifosine were given to BALB/c mice in oral doses of 1 and 2.5 mg/kg/day during 28 days and 5 mg/kg/ day during 14 days, starting at two weeks after the first treatment scheme.34
An assay comparing miltefosine at the standard dose of 20 mg/kg/day during 28 days to in vivo treatment with perifosine at a dose of 5 mg/kg/day for 14 days demonstrated higher in vivo activity of perifosine than miltefosine against L. amazonensis. These findings show promise for a new treatment group in cutaneous leishmaniasis caused by L. amazonensis.34
Another drug, sitamaquine (WR 6026), an 8- aminoquinoline, currently being tested and in Phase II clinical trials has also shown promise as an effective oral agent in a dose of 1 mg/kg/day for 2 weeks for visceral leishmaniasis. Further studies are needed.36
The present focus is on identifying newer therapeutic targets in the parasite such as DNA topoismerases.31
Undoubtedly, in the coming years, a group of new medications safe and effective for the treatment of leishmaniasis will be found.
Recent work on sand-fly saliva has identified maxadilan, a vasoactive peptide that acts on the PAC-1 receptor.37 This results in vasodilation and consequently enhances infectivity. Possibilities of identifying medications that affect the PAC-1 receptor can help in the treatment of leishmaniasis, as well as in the production of a vaccine that will protect against this infection.
Undoubtedly, there are other aspects of the management of leishmaniasis in endemic areas. Once the infection is present, vaccines and vector control are paramount in addition to therapeutic measures, but these are beyond the scope of this review.
CONCLUSION
At present, the pentavalent antimonial compounds remain the mainstay of the treatment for cutaneous leishmaniasis. However, because of the high toxicity associated with this group and the recent emergence of drug resistant strains, alternative therapeutic options to be considered are the pentamidines and liposomal Amphotericin B. The most effective alternative to antimonials are combination therapies using L-AmB and miltefosine. Photodynamic therapy, imiquimod, and tamoxifen show promise for the near future. In immunocompromised individuals, miltefosine alone or in combination with the antimonials and L-AmB have proved to be effective.
Amphotericin B and the pentavalent antimonials are relatively cheaper but the efficacy and toxicity of these outweigh the costs of the newer agents.
The choice of anti-leishmanial therapy should be based on the geographic location, availability of the drug, host immune status and expertise of the treating physician.
These new treatments are not expected to be available at the PHC but rather in specialized centers/hospitals.
REFERENCES
- 1.Tuon FF, Amato VS, Graf ME, Siqueira AM, Nicodemo AC, Amato Neto V. Treatment of New World cutaneous leishmaniasis- a systematic review with a meta-analysis. Int J Dermatol. 2008;47(2):109–24. doi: 10.1111/j.1365-4632.2008.03417.x. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez U, Pinart M, Reveiz L, Alvar J. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev. 2008;(4):CD005067. doi: 10.1002/14651858.CD005067.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Van der Meide WF, Sabajo Lo, Jensema AJ, Peekel I, Faber WR, Schallig HD, et al. Evaluation of treatment with pentamidine for cutaneous leishmaniasis in Suriname. Int J Dermatol. 2009;48(1):52–8. doi: 10.1111/j.1365-4632.2009.03883.x. [DOI] [PubMed] [Google Scholar]
- 4.Seaman J, Boer C, Wilkinson R, de Tong J, de Wild E, Sondorp E, et al. Liposomal amphotericin B (AmBisome) in the treatment of complicated kala-azar under field conditions. Clin Infect Dis. 1995;21(1):188–93. doi: 10.1093/clinids/21.1.188. [DOI] [PubMed] [Google Scholar]
- 5.Davidson R, diMartino L, Gradoni L, Giacchino R, Gaeta GB, Pempinello R, et al. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome) Clin Infect Dis. 1996;22(6):938–43. doi: 10.1093/clinids/22.6.938. [DOI] [PubMed] [Google Scholar]
- 6.Sundar S, Murray H. Cure of antimony-unresponsive Indian visceral leishmaniasis with amphotericin B lipid complex. J Infect Dis. 1996;173(3):762–5. doi: 10.1093/infdis/173.3.762. [DOI] [PubMed] [Google Scholar]
- 7.Bermann JJ. Treatment of leishmaniasis with miltefosine: 2008 status. Expert Opin Drug Metab Toxicol. 2008;4(9):1209–16. doi: 10.1517/17425255.4.9.1209. [DOI] [PubMed] [Google Scholar]
- 8.Perez C, Solias Y, Rodriguez G. Diffuse cutaneous leishmaniasis in a patient with AIDS. Biomedica. 2006;26(4):485–97. [PubMed] [Google Scholar]
- 9.Berman J, Bryceson AD, Croft S, Engel J, Gutteridge W, Karbwang J, et al. Miltefosine: issues to be addressed in the future. Trans R Soc Trop Med Hyg. 2006;100(Suppl 1):S41–4. doi: 10.1016/j.trstmh.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Marques N, Sa R, Coelho F, Oliveira J, Da Cunha J Saraiva, Melico-Silvestre A. Miltefosine for visceral leishmaniasis relapse treatment and secondary prophylaxis in HIV-infected patients. Scand J Infect Dis. 2008;40(6-7):523–6. doi: 10.1080/00365540701787800. [DOI] [PubMed] [Google Scholar]
- 11.Ameen M. Cutaneous leishmaniasis: therapeutic strategies and future directions. Expert Opin Pharmacother. 2007;8(16):2689–99. doi: 10.1517/14656566.8.16.2689. [DOI] [PubMed] [Google Scholar]
- 12.Dorlo TP, van Thiel PP, Huirtema AD, Keizer RJ, de Vries HJ, Beijnen JH, et al. Pharmacokinetics of Miltefosine in Old World cutaneous leishmaniasis patients. Antimicrob Agents Chemother. 2008;52(8):2855–60. doi: 10.1128/AAC.00014-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohebali M, Fotouhi A, Hooshmand B, Zarei Z, Akhnoundi B, Rahnema A, et al. Comparison of Miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. 2007;103(1):33–40. doi: 10.1016/j.actatropica.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Arevalo I, Tulliano G, Quispe A, Spaeth G, Matlashewski G, Lianos-Cuentas A, et al. Role of imiquimod and parenteral meglumine antimoniate in the initial treatment of cutaneous leishmaniasis. Clin Infect Dis. 2007;44(12):1549–54. doi: 10.1086/518172. [DOI] [PubMed] [Google Scholar]
- 15.van der Snoek E, Robinson D, van Hellemond J, Neumann H. A review of photodynamic therapy in cutaneous leishmaniasis. 2008 July 3. J Eur Acad Dermatol Venereol. 2008 Jul 3; doi: 10.1111/j.1468-3083.2008.02805.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Akilov OE, Kosaka S, O’Riordan K, Hasan T. Parasiticidal effect of delta-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis is indirect and mediated through the killing of the host cells. Exp Dermatol. 2007;16(8):651–60. doi: 10.1111/j.1600-0625.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 17.Kosaka S, Akilov OE, O’Riordan K, Hasan T. A mechanistic study of delta-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis. J Invest Dermatol. 2007;127(6):1546–9. doi: 10.1038/sj.jid.5700719. [DOI] [PubMed] [Google Scholar]
- 18.Tsai JC, Chen IH, Wong TW, Lo YL. In vitro/in vivo correlations between transdermal delivery of 5-aminolaevulinic acid and cutaneous protoporphyrin IX accumulation and effect of formulation. Br J Dermatol. 2002;146(5):853–62. doi: 10.1046/j.1365-2133.2002.04715.x. [DOI] [PubMed] [Google Scholar]
- 19.Sundar S, Rai M, Chakravarty J, Agarwal D, Agarwal D, Vaillant M, et al. New Treatment Approach in Indian Visceral Leishmaniasis: Single-Dose Liposomal Amphotericin B Followed by Short-Course Oral Miltefosine. Clin Infect Dis. 2008 Sep 9; doi: 10.1086/591972. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Antinori S, Cascio A, Parravicini C, Bianchi R, Corbellino M. Leishmaniasis among organ transplant receipients. Lancet Infect Dis. 2008;8(3):191–9. doi: 10.1016/S1473-3099(08)70043-4. [DOI] [PubMed] [Google Scholar]
- 21.Berenguer J, Gomez-Campdera F, Padilla B, Rodriguez-Ferrero M, Anaya F, Moreno S, et al. Review visceral leishmaniasis (Kala-Azar) im transplant recipients: case report and review. Transplantation. 1998;65(10):140–4. doi: 10.1097/00007890-199805270-00022. [DOI] [PubMed] [Google Scholar]
- 22.Basset d, Faraut F, Marty P, Dereure J, Rosenthal E, Mary C, et al. Review Visceral leishmaniasis in organ transplant recipients: 11 new cases and a review of the literature. Microbes Infect. 2005;7(13):1370–5. doi: 10.1016/j.micinf.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Laguna F. Treatment of leishmaniasis in HIV-positive patients. Ann Trop Med Parasitol. 2003;97(Suppl 1):135–42. doi: 10.1179/000349803225002606. [DOI] [PubMed] [Google Scholar]
- 24.Lachaud L, Bourgeors N, Piourde M, Leprohon P, Bastien P, Ouellette M. Parasite susceptibility to Amphotericin B in failures of treatment for visceral leishmaniasis in patients coinfected with HIV type 1 and Leishmania infantum. Clin Infect Dis. 2009;48(2):e 16–22. doi: 10.1086/595710. [DOI] [PubMed] [Google Scholar]
- 25.Schonian G, Mauricio I, Gramiceia M, Canavate C, Boelaert M, Dujardin JC. Leishmaniasis in the Mediterranean in the era of molecular epidemiology. Trends Parasitol. 2008;24(3):135–42. doi: 10.1016/j.pt.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Lindoso JA, Barbosa RN, Posada-Vergara MP, Duarte MI, Oyafuso LK, Amato VS. Unusual manifestations of tegumentary leishmaniasis in AIDS patients from the New World. Br J Dermatol. 2009;160(2):311–8. doi: 10.1111/j.1365-2133.2008.08908.x. [DOI] [PubMed] [Google Scholar]
- 27.Ritmeijer K, Dejenie A, Assefa Y, Hundie TB, Mesure J, Boots G, et al. A comparison of miltefosine and sodium stibogluconate for treatment of visceral leishmaniasis in an Ethiopian population with high prevalence of HIV infection. Clin Infect Dis. 2006;43(3):357–64. doi: 10.1086/505217. [DOI] [PubMed] [Google Scholar]
- 28.Sinha S, Fernandez G, Kapila R, Lambert WC, Schwartz RA. Diffuse cutaneous leishmaniasis associated with the immune reconstitution inflammatory syndrome. Int J Dermatol. 2008;47(12):1263–70. doi: 10.1111/j.1365-4632.2008.03804.x. [DOI] [PubMed] [Google Scholar]
- 29.Miguel DC, Yokoyama-Yasunaka JK, Uliana SR. Tamoxifen is Effective in the Treatment of Leishmania amazonensis Infections in Mice. PLoS Negl Trop Dis. 2008;2(6):e249. doi: 10.1371/journal.pntd.0000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miguel DC, Zauli-Nascimento RC, Yokovama-Yasunaka JK, Katz S, Barbieri CL, Uliana SR. Tamoxifen as a potential antileishmanial agent: efficacy in the treatment of Leishmania braziliensis and Leishmania chagasi infections. J Antimicrob Chemother. 2009;63(2):365–8. doi: 10.1093/jac/dkn509. [DOI] [PubMed] [Google Scholar]
- 31.Mishra J, Saxena A, Singh S. Chemotherapy of leishmaniasis: past, present and future. Curr Med Chem. 2007;14(10):1153–69. doi: 10.2174/092986707780362862. [DOI] [PubMed] [Google Scholar]
- 32.Rosypal AC, Werbovetz KA, Salem M, Stephens CE, Kumar A, Boykin DW, et al. Inhibition by Dications of in vitro growth of Leishmania major and Leishmania tropica: causative agents of old world cutaneous leishmaniasis. J Parasitol. 2008;94(3):743–9. doi: 10.1645/GE-1387.1. [DOI] [PubMed] [Google Scholar]
- 33.Croft SL. Kinetoplastida: new therapeutic strategies. Parasite. 2008;15(3):522–7. doi: 10.1051/parasite/2008153522. [DOI] [PubMed] [Google Scholar]
- 34.Cabrera-Serra MG, Lorenzo-Morales J, Romero M, Valladares B, Pinero JE. In vitro activity of perifosine: a novel alkylphospholipid against the promastigote stage of Leishmania species. Parasitol Res. 2007;11(5):1155–7. doi: 10.1007/s00436-006-0408-4. [DOI] [PubMed] [Google Scholar]
- 35.Cabrera-Serra MF, Valladares B, Pinero JE. In vivo activity of perifosine against Leishmania amazonensis. Acta Trop. 2008;108(1):20–5. doi: 10.1016/j.actatropica.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Sangraula H, Sharma KK, Rijal S, Dwivedi S, Koirala S. Orally effective drugs for Kala-azar (visceral leishmaniasis): focus on miltefosine and sitamaquine. J Assoc Physcians India. 2003;51:686–90. [PubMed] [Google Scholar]
- 37.Reddy VB, Li Y, Lerner EA. Maxadilan, the PAC1 receptor, and leishmaniasis. J. Mol Neurosci. 2008;36(1-3):241–4. doi: 10.1007/s12031-008-9079-1. [DOI] [PubMed] [Google Scholar]


