Abstract
The ethanolic extract of Acalypha indica was tested for its biopotency on membrane bound enzymes and marker enzymes in urolithiasis in male wistar albino rats. Calcium oxalate urolithiasis was induced by 0.75% ethylene glycol in drinking water for 30 days. There was a significant decrease in membrane bound enzymes such as Ca2+ ATPase, Mg2+ ATPase, Na+K+ ATPase and marker enzymes Aspartate Transaminase (AST), Alanine Transaminase (ALT), Acid phosphatase (ACP) and Alkaline Phosphatase (ALP) in liver and kidney. The AST, ALT, ACP and ALP were increased in serum and urine of rats. Therapeutic treatment with plant extract (200mg/kg b.wt.dose-1 day-1 oral-1) has significantly ameliorated to near normalcy in the curative group. These results of the present study concluded that A. indica can play an important role in the prevention of disorders associated with kidney stone formation.
Keywords: Marker enzymes, membrane bound enzymes, Urolithiasis, ethylene glycol, Acalypha indica
Introduction
Urinary stone is one of the oldest and common afflictions of humans. A large number of people are suffering from urinary stone problem all over the globe. Not only the humans but animals and birds also suffer from the urinary stone problem. The occurrence in some areas is so alarming that they are known as ‘Stone Belts (Menon et al., 1998). In USA urinary stones are responsible for about 1.3 million medical consults each year with treatment costing about $2 billion annually in medical expenditure. 6 lacs Americans are suffering from urinary stone problem every year. Whereas, in India 11% people are expected to be having urinary stone problem and out of them 50% may lead to loss of kidneys or renal damages. Also, nearly 15% population of the northern India is suffering from kidney stone problem (Bethesda, 2009).
Kidney stones modify the victim's behavior with great fear of intense pain and threaten with failure of the kidneys. Not all standard pharmaceutical drugs used to prevent urolithiasis are effective in all patients, and many have adverse effects that compromise their long-term use. On the other hand the traditional system of Indian medicine “Ayurveda” recommends several medicinal plants for the treatment of urolithiasis (Mohamed farook et al., 2009).
Ehylene glycol was reported to induce calcium oxalate type of urolithiasis by the formation of oxalate, because of the intermediates of glycolate and glyoxalate in the rat liver (Hala be et al., 2003). Oxalate causes peroxidation of membrane lipids and oxidation of proteins, initiates the loss of membrane integrity and activities of membrane bound enzymes (Selvam, 2002).Membrane ATPases may play an important role in ionic gradients between the intra cellular / extra cellular compartments of the cell. Membrane Na+K+ ATPase plays an important role in active transport of Na+ and K+ ions, across the pasma membrane similarly, Ca2+ ATPase is clearly linked with Ca2+ pump and transport of Ca2+ while Mg2+ activated ATPase is distributed in all renal cell compartments.
The Mg2 ions forms Mg2+ ATPase complex, which is the substrate for the enzyme. Mg2+ ATPase is to control the intra cellular Mg2+ concentration, changes which can modulate the activity of Mg2+ dependent enzymes and regulate rates of protein synthesis and cell growth (Stekhoren and Bonting, 1981).
Lipid proxidation leads to cell damage leading to the release of marker enzymes namely Aspartate Transaminase (AST), Alanine Transaminase (ALT), Alkaline Phosphatase (ALP), Acid phosphatase (ACP) into blood circulation and urine. most of the urinary enzymes originating in the kidneys are localized to specific regions and cellular components of the nrphron, thereby studies pertaining to these enzymes will show the pathological status of the kidney(Srinivasan et al.,2004). current -day medical management of urolithiasis mainly involves the surgical removal of stones by the techniques such as ESWL(Extracorporeal shock -wave lithotripsy), PCNL(Percutaneous nephrolithotomy), but they do not assure thr prevention of the stone. they cause side effects such as heamorrhage, hypertension, tubular necrosis and subsequent fibrosis of the kidney. Hence, the search of effective herbal drugs for the treatment of urolithiasis with no side effects is necessary.
Acalypha indica is a small erect herb up to 60 cm tall or a little more, with a few ascending branches, these angled and pubescent; leaves broadly ovate, subdeltoid, rather coarsely toothed, on petioles as long as or longer than the 3-5 cm long blades; nerves 3-5 from base, thereafter pinnately arranged; stipules minute; flowers sessile on erect axillary spikes longer than the leaf; male flowers minute, crowded distally; stamens 8, female flowers scattered along the inflorescence axis, each subtended by a conspicuous semicupular foliaceous toothed green bract nearly mm long; capsule hispid, 1 mm broad, 3-locular (Stone and Benjamin,1970). The present study aims to give data highlighting the present trends in research of medicinal plants accredited with antiurolithiatic activity. This may help investigators to identify and develop appropriate lead compounds or plant products beneficial in the management of urolithiasis.
Materials and Methods
Collection of the Plant Material
Acalypha indica was collected from Maruthamalai hills, Coimbatore district, Tamil nadu, India during the month of March to May, 2009. The plant was identified and authenticated by Taxonomist Dr. K. Arumugasamy, Lecturer (SG), Department of Botany, Kongunadu Arts and Science College, Coimbatore, Tamilnadu, India.
Selection of Animals
For the purpose of urolithiatic studies, adult male wistar albino rats weighing about 150 to 200 g were collected from animal breeding centre, Kerala Agricultural University, Mannuthy, Thrissur, Kerala, India. The rats were kept in properly numbered large polypropylene cages with stainless steel top grill having facilities for pelleted food. The animals were maintained in 12 hours light and dark cycle at 28° C ± 2° C in a well ventilated animal house under natural conditions in large polypropylene cages and they were acclimatized to laboratory conditions for 10 days prior to the commencement of the experiment. The animals were fed with standard pelleted diet supplied by AVM foods, Coimbatore, Tamilnadu, India. All animal experiments were performed according to the ethical guidelines suggested by the institutional animal ethics committee (IAEC).
Stone Induction
Animals were fed with 0.75% (0.75ml of ethylene glycol in 100 ml of drinking water) of rats for a period of 30 days to induce urolithiasis. The urolithiatic rats were then used for the study.
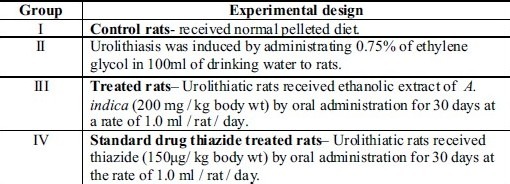
Experimental design of animals for antiurolithiatic activity
The rats were divided into four groups of six animals each.
Experimental Design
Collection of Urine Sample
Before the day of sacrifice the rats were placed in metabolic cages and urine was collected for 24 hours. Urine was freed from faecal contamination. Rats were provided with water but no feed. Urine collected in 50 ml beaker maintained at 0° C in an ice bath. The collected urine samples were centrifuged for 10 minutes and any sediment present was discarded. The urine was used for further analysis.
Collection of Serum Sample
After the experimental regimen the animals were sacrificed by cervical decapitation under light ether anesthesia. Blood was collected and centrifuged for 10 min. at 2500 rpm. The serum supernatant was collected and then diluted with water in the ratio of 1:10. Aliquots of the diluted serum were then used for the determination of serum constituents and serum enzymic activities.
Collection of Liver and Kidney Samples
The experimental animals were sacrificed, liver and kidney were removed immediately, washed with ice cold saline10% tissue homogenate was prepared by homogenizing 1.0g of chopped liver or kidney tissue in 10ml of 0.1M tris HCl homogenizing buffer at pH 7.5. The homogenate was used for assaying the enzyme activities.
Chemicals
All the chemicals used in the present study were of analytical reagent grade.
Biochemical Assays
The total homogenate was used for assaying membrane bound enzymes like Na+K+ ATPase (Bonting, 1970), Ca2+ ATPase (Hjertan and Pan, 1983), Mg2+ ATPase (Ohnishi et al. 1982) activities. The activies of AST (Reitman & Frankel (1957), ALT (Reitman & Frankel (1957), ACP (King (1965), ALP (King & Armstrong, 1934), in kidney, liver, serum and 24 urine were assayed.
Statistical Analysis
The results of the biochemical estimations were reported as mean ± SD of six animals in each group. Total variations, present in a set of data were estimated by one way Analysis Of Variance (ANOVA) followed by the analysis of level of significance between different groups based on ANOVA using AGRES statistical package (Version 3.1). Difference among means were analysed by least significant difference (LSD) at 5% level (p<0.05).
Results
One of the major functions of kidney is the transport of sodium and other solute across the tubular epithelium. Generation of free radicals such as peroxyl, alkoxyl and aldehyde radicals can cause severe damage to the membrane bound enzymes such as Ca2+ ATPase, Mg2+ ATPase and Na+K+ ATPase, (Pragasam et al., 2005).
Table 1 and Figure 1 represent the activities of kidney and liver membrane bound enzymes such as Ca2+ ATPase, Mg2+ ATPase and Na+K+ ATPase of control and experimental rats.
Table 1.
Effect of A.indica extract on membrane bound enzymes in kidney of control and experimental rats
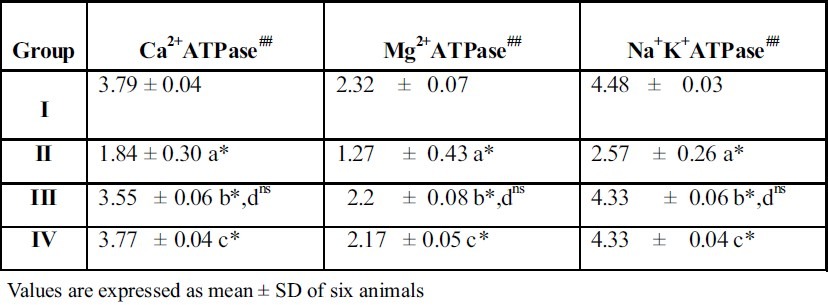
From the table 1 & 2 it is evident that the activity of Ca2+ ATPase, Mg2+ ATPase, Na+K+ ATPase and were significantly reduced (p < 0.05) in ethylene glycol induced urolithiic rats (Group II) when compared with control rats (Group I).
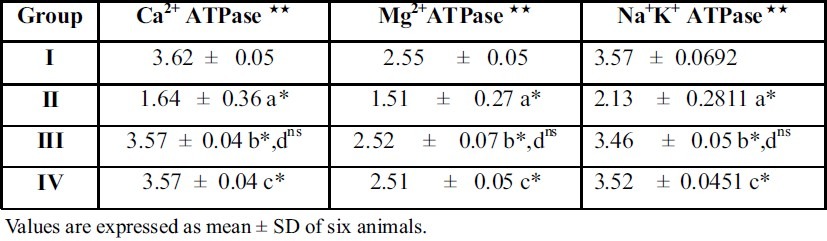
Table 2.
Effect of A. Indica on Na+K+ ATPase, Mg2+ ATPase and Ca2+ ATPase in liver of control and ethylene glycol urolithiatic rats
Experimental Design
Group I: Control rats received normal pelleted diet
Group II: Received 0.75% ethylene glycol in water for 30 days
Group III: Treated rats Urolithiasis induced rats received A.indica extract (200 mg / kg body weight) by oral administration for 30 days at a rate of 1.0 ml / rat / day
Group IV: Standard drug thiazide treated rats Urolithiasis induced rats received thiazide (150 μg / kg body weight) by oral administration for 30 days at a rate of 1.0 ml / rat / day.
Comparison between the groups
‘a’ represents compari-son between II and I
‘b’ represents comparison between III and II
‘c’ represents comparison between IV and II
‘d’ represents comparison between III and IV
The symbols represent statistical significance p* < 0.05, ns not significant
Units
##μmoles of phosphorus liberated / min / mg protein
Experimental design and comparison between the groups are as in table 1
The symbols represent statistical significance p* < 0.05, ns not significant
Units
##μmoles of phosphorus liberated / min / mg protein
Treatment with the plant extract restored the levels and it brought back the values to near normal range in group III rats. When A.Indica extract treated rats (Group III) were compared with thiazide treated rats (Group IV), there was no significant difference between these groups of rats. This result gives a supportive evidence for the antiurolithiatic activity of ethanolic extract similar to standard drug thiazide. The activities of the marker enzymes such as aspartate transaminase (AST) and alanine transaminase (ALT) alkaline phosphatase (ALP), acid phosphatase (ACP), in serum, urine, kidney and liver of control and experimental rats are represented in tables 3, 4, 5 and 6. Activities of the enzyme were significantly increased in serum and urine whereas they were decreased in kidney and liver of urolithiatic rats (group II). The activity of these enzymes were reverted to near normal in A.indica ethanolic extract administrated rats (group III) in serum, urine, kidney and liver.
Table 3.
Effect of A.indica extract on marker enzymes in serum of control and experimental rats
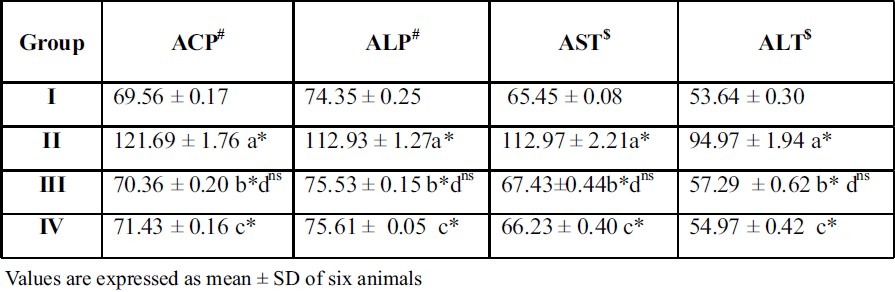
Table 4.
Effect of A.indica extract on marker enzymes in urine of control and experimental rats
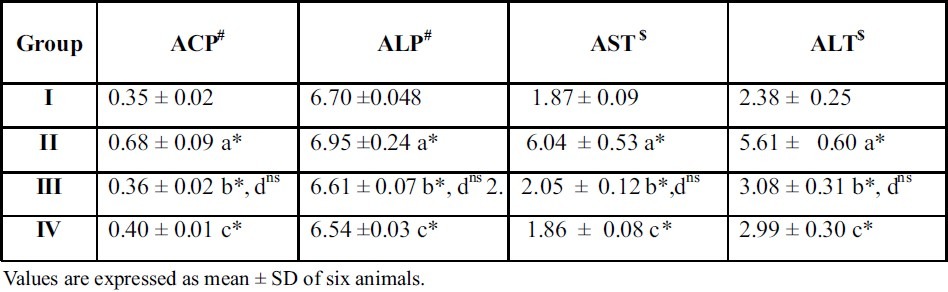
Table 5.
Effect of A.Indica extract on marker enzymes in kidney of control and ethylene glycol induced urolithiatic rats
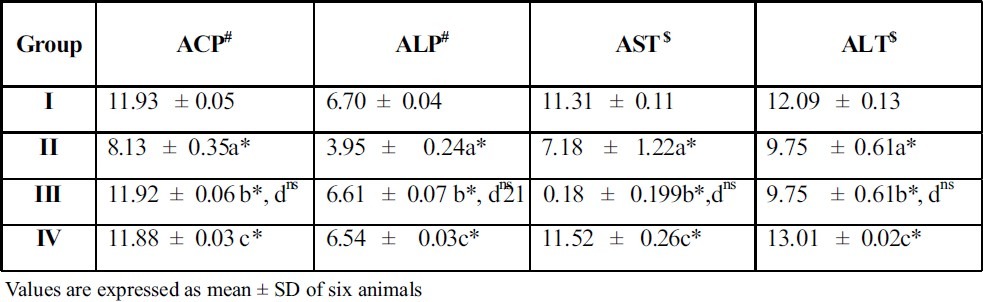
Table 6.
Effect of A.Indica extract on marker enzymes in liver of control and ethylene glycol induced urolithiatic rats
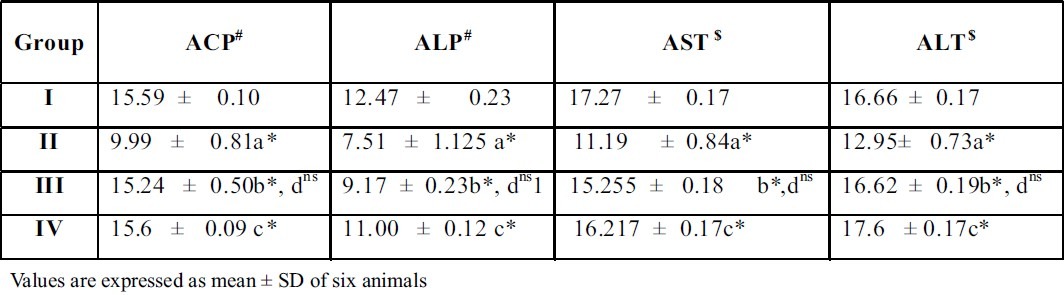
Units
#μ moles of phenol liberated / L
$μ moles of pyruvate liberated / L
Experimental design and comparison between the groups are as in table 1
The symbols represent statistical significance p* < 0.05, ns not significant
Units
#μ moles of phenol liberated / 24 hr urine
$μ moles of pyruvate liberated / 24 hr urine
Units
$μ moles of phenol liberated / min/ mg protein
#μ moles phenol liberated / min/ mg protein
Experimental design and comparison between the groups are as in table 1
The symbols represent statistical significance p* < 0.05, ns not significant
Units
$μ moles of phenol liberated / min/ mg protein
#μ moles phenol liberated / min/ mg protein
However, when the ethanolic extract of Acalypha indica (200 mg / kg body weight) administered group (group III) compared with standard drug, thiazide treated rats (group IV), no significant difference (p<0.05) was observed between these two groups of animals. This indicates that the antiurolithiatic activity of ethanolic extract of A.indica was comparable with the standardized drug thiazide, a commercially available antiurolithiative agent.
DISCUSSION
One of the major functions of the kidney is the transport of sodium and other solutes across the tubular epithelium. Generation of free radicals such as peroxyl, alkoxyl and aldehyde radicals can cause severe damage to the membrane bound enzymes such as Ca2+ ATPase, Mg2+ ATPase and Na+K+ ATPase (Pragasam et al., 2005).
Membrane Na+K+ ATPase play an important role in active transport of Na+ and K+ ions across the plasma membrane (Vani and Reddy, 2000). The enzyme Na+K+ ATPase utilizes the energy derived from ATP hydrolysis to pump out Na+ from inside the cell to transfer K+ from outside to cytosol. Its activity has been frequently used as a marker for plasma membranes and has been followed as a probe for monitering membrane integrity alteration in the physical state of various biological membranes (Stepherd, 1994).
Na+K+ ATPase activity markedly reduced in kidney and liver of ethylene glycol induced urolithic rats. This may be due to increased oxalate formation. Oxalate is known to interfere with a broad spectrum of solute transport processes in the renal tubule and inhibition of Na+K+ ATPase (Tulenko et al.,1988).
Ca2+ ATPase, the enzyme responsible for active calcium transport, is extremely sensitive to hydroperoxides and this may lead to its inhibition. Ca2+ ATPase activity was significantly lowered in the liver and kidney of ethylene glycol induced urolithic rats. The decreased activity of this enzyme may be due to the peroxide stress, which may act on the sulphhydryl groups present in the active sites of the Ca2+ ATPase (Srinivasan et al., 2004).
Mg2+ ATPase is to control the intracellular Mg2+ concentration which can modulate the activity of Mg2+ dependent enzymes and regulate rates of protein synthesis and cell growth (Sanu and Rubin, 1982). Ca2+ ATPase activity was significantly lowered in the kidney and liver of ethylene glycol induced urolithic rats. This may be due to free radical induced cell damage by oxalate and their severe cytotoxic effects, such as lipid peroxidation and protein oxidation in cell membrane followed by the alteration of the membrane fluidity, enzyme properties and ion transport.
Na+K+ ATPase, Mg2+ ATPase and Ca2+ ATPase in the plasma membrane keep the intracellular sodium low but intracellular magnesium and potassium high when compared with the levels in extracellular fluids (Pragasam and Kalaiselvi, 2005).
Our findings are in accordance with the studies of Prins et al. (2002) who showed that supplementation of L-arginine highly controlled the depletion of ATPase enzyme levels in tissues and restored the values to near normal levels in ethylene glycol treated rats.
Increased activities of serum and urine AST and ALT levels in ethylene glycol induced urolithic rats were observed. This can be attributed to the damaged structural integrity of the renal and hepatic cells causing the enzymes which are located in the cytoplasm to be released into the circulation (Senthilkumar et al.,2003).If membrane of other organelles such as mitochondria is damaged, soluble enzymes such as compartmentalized AST will also released. The release of these enzymes into the circulation will indicate both plasma and organelle membrane damage.
Reduced AST, ALT, ACP and ALP activities in renal and hepatic tissues of ethylene glycol induced urolithic rats was observed. This might be due to leakage of the enzyme into the general circulation from the collatered circulation. The stone formation may acclude the ureter, leading to an increase in back pressure in the renal pelvis and because of ischeamia, may ultimately damage the tubular cells (Thind and Nath 1978).
The above results are in agreement with the findings of Farooq et al. (2004), who reported that serum ACP and ALP levels were increased due to administration of oxalate and their levels were maintained near normal in phycocyanin supplementation.
Poonkuzhali et al. (1994) showed a decrease of kidney and liver marker enzymes and their restoration to near normal levels by uric acid administration to sodium glycolate fed urolithiatic rats. Similar results were also observed in our studies.
CONCLUSION
In conclusion, the result of our study clearly revealed that the ethanolic extract of Acalypha indica have potent antiurolithiatic activities in ethylene glycol induced male wistar albino rats. Our results show that the anti urolithiatic effect of the plant may be due to its antioxidant, free radical scavenging properties of the secondary metabolites present in the plant.
ACKNOWLEDGEMENT
The authors are thankful to the college management for their guidance, valuable suggestion, encouragement and constant supporting during this investigation.[26]
REFERENCES
- 1.Menon M., Parulkar B.G., Drach G.W. Urinary lithiasis, etiology, diagnosis, and medical management. In: Campbell's Urology. 7th ed. Vol. 3. Philadelphia: WB Saunders publishers; 1998. pp. 2661–2733. [Google Scholar]
- 2.Bethesda M.D. Kidney and urologic diseases statistics for the United States. National Institute of Diabetes and Kidney Diseases. American Journal of Infectious Diseases. 2009;5(3):177–186. [Google Scholar]
- 3.Mohamed farook N.A, Rajesh S, Jamuna M. Inhibition of mineralization of urinary stone forming minerals by medicinal plants. E-Journal of Chem. 2009;6(3):938–942. [Google Scholar]
- 4.Halabe A, SHOR R, Wong N.L.M, Sutton R.A.L. Effect of vitamin D3 on the conversion of ethylene glycol to glycolate and oxalate in ethylene glycol- fed rats. Clin.Chim.Acta. 2003;330:135–139. doi: 10.1016/s0009-8981(02)00415-1. [DOI] [PubMed] [Google Scholar]
- 5.Selvam R, Kalaiselvi P., Govindaraj A., Balamurugan V., Satish A.S., Kumar Effect of Aerva lanata leaf extract and vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol.Res. 2002;43:89–93. doi: 10.1006/phrs.2000.0745. [DOI] [PubMed] [Google Scholar]
- 6.Stekhoren M.A., Bonting S.L. Transport ATPases: Properties and functions. Physiol. Rev. 1981;61:1–76. doi: 10.1152/physrev.1981.61.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan S., Pragasam V., Jenitha X., Kalaiselvi P., Muthu V., Varalakshmi P. Oxidative stress in urogenital tuberculosis patients: A predisposing factor for renal stone formation- amelioration by vitamin E supplementation. Clin.Chem.Acta. 2004;350(1-2):57–63. doi: 10.1016/j.cccn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Stone, Benjamin C. The flora of Guam. Micronesica. 1970;24(1-4):1–659. [Google Scholar]
- 9.Bonting S.L. Sodium potassium activated adenosine triphosphate cation transport in membrane and ion transport. Vol. 1. London: Wiley interscience; 1970. pp. 257–363. [Google Scholar]
- 10.Hjertan S, Pan H. Purification and characterization of two forms of a low affinity calcium ion-ATPase from erythrocyte membrane. Biochemistr Biophysics Acta. 1983;755:457–466. doi: 10.1016/0005-2736(83)90480-7. [DOI] [PubMed] [Google Scholar]
- 11.Ohinishi T, Suzuki T, Ozawa K. A comparative study of plasma membrane magnesium ion ATPase activities in normal, regenerating and malignant cells. Biochemistry Biophysics Acta. 1982;684:67–74. doi: 10.1016/0005-2736(82)90050-5. [DOI] [PubMed] [Google Scholar]
- 12.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetate, glutamic pyruvic transaminase. American Journal of Clinical Pathology. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 13.King J. The dehydrogenase or oxidoreductase-N lactate dehydrogenase. Practical clinical enzymology. 1965a, b:83–93. [Google Scholar]
- 14.King J, Armstrong The hydrolases, acid and alkaline phosphatase. Practical clinical enzymology. 1934:83–93. [Google Scholar]
- 15.Pragasam V., Kalaiselvi P., Sumitra K., Srinivasan S., Varalakshmi P. Counterction of oxalate induced nitrosative stress by supplementation of L- arginine, a potent antilithic agent. Clin. Chim.Acta. 2005;354:159–166. doi: 10.1016/j.cccn.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Vani M.L, Reddy K.P. Effects of fluoride accumulation on some enzymes of brain and gastrochemius muscle of mice. Fluoride. 2000;33:17–26. [Google Scholar]
- 17.Stephred G.M. Neurobiology. 3rd edn. New York: Oxford University Press; 1994. [Google Scholar]
- 18.Tulenko T.N., Robinwitz J.L., Cox R.H., Santamore W.P. Na+ K+ ATPase, cell Na+ and lipid profiles in canine arterial wall with chronic cigarette smoking. Inter.J.Biochem. 1988;20:285–289. doi: 10.1016/0020-711x(88)90352-7. [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan S., Pragasam V, Jenitha X., Kalaiselvi P., Muthu V., Varalakshmi P. Oxidative stress in urogenital tuberculosis patients: A predisposing factor for renal stone formation- amelioration by vitamin E supplementation. Clin.Chem.Acta. 2004;350(1-2):57–63. doi: 10.1016/j.cccn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Sanu H, Rubin H. Ions, cell proliferation and cancer. New York: Academic press; 1982. The role of magenisum in cell proliferation and transformation; pp. 517–537. [Google Scholar]
- 21.Pragasam V, Kalaiselvi P. Counteraction of oxalate induced nitrosative stress by supplementation of L-arginine, a potent antilithic agent. Clin. Chem. Acta. 2005;354(1-2):159–166. doi: 10.1016/j.cccn.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Prins H.A., Nijveldt R.J., Gasselt D.V., Kemenade V.F., Teerlink T., Vambalgen V.A.A. The flux of arginine ischemia reperfusion in the rat kidney. Kidney Inter. 2002;62(1):86–93. doi: 10.1046/j.1523-1755.2002.00409.x. [DOI] [PubMed] [Google Scholar]
- 23.Senthil kumar R, Ponmozhi M., Vishvanathan P., Nalini N. Activity of Cassia auriculata. 2003 [Google Scholar]
- 24.Leaf extract in rats with alcoholic liver injury. J.Nutr. Biochem. 14(8):452–458. doi: 10.1016/s0955-2863(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 25.Thind S.K, Nath R. Experimental urolithiasis. Part III- Comparative kinetic study of glyoxalase I, glycolate oxidase, alkaline phosphatas and LDH in the normal rat kidney and bladder and its alterations in urolithiasis. Indian J. Exp. Biol. 1978;16:66–70. [PubMed] [Google Scholar]
- 26.Poonkuzhali P, Saraswathi C.P., Rajalakshmi K. Effect of uric acid on sodium oxalate induced urolithiasis in rats - biochemical and histology evidences. Indian J Exp. Biol. 1994;32 [PubMed] [Google Scholar]


