Abstract
We describe a procedure for controlled, periodic, reversible modulation of selected regions of the blood–brain barrier (BBB) or the inner-blood–retina barrier (iBRB) based on incorporation into an AAV-2/9 vector of a doxycycline-inducible gene encoding shRNA targeting claudin-5, one of 30 or so proteins constituting the BBB and iBRB. The vector may be introduced stereotaxically into pre-selected regions of the brain or into the retina, rendering these regions permeable to low-molecular weight compounds up to approximately 1 kDa for the period of time during which the inducing agent, doxycycline, is administered in drinking water, but excluding potentially toxic higher molecular weight materials. We report on the use of barrier modulation in tandem with systemic drug therapy to prevent retinal degeneration and to suppress laser-induced choroidal neovascularization (CNV), the latter being the hallmark pathology associated with the exudative, or wet, form of age-related macular degeneration (AMD). These observations constitute the basis of a minimally invasive systemic therapeutic modality for retinal diseases, including retinitis pigmentosa and AMD, where, in early stage disease, the iBRB is intact and impervious to systemically administered drugs.
Keywords: age-related macular degeneration, blood–brain barrier, blood–retina barrier, drug delivery, RNAi/claudin-5
→See accompanying Closeup by John J. Rossi DOI http://dx.doi.org/10.1002/emmm.201100132
INTRODUCTION
The tight junctions associated with the blood–brain and inner blood–retina barriers (BBB/iBRB) are comprised of a series of up to 30 proteins, interacting to provide a tight seal between adjacent endothelial cells lining the neuronal and inner retinal microvasculatures. They have evolved for the specific purpose of protecting neuronal tissues from potentially damaging blood-borne agents and, hence, breakage of such barriers, for the purposes of systemic drug delivery or for other purposes, would have serious adverse effects (Campbell et al, 2009). However, remodelling of the BBB/iBRB by selectively modulating levels of tight junction proteins, such as to render these barriers controllably and reversibly permeable to low-molecular weight compounds, could have substantial therapeutic potential given that the vast majority of low-molecular weight drugs (an estimated 98%) cannot gain access to neuronal tissues from the peripheral circulation. Moreover, those drugs that can gain access and are in clinical use (e.g. lipophilic antipsychotic drugs) may require dosages sufficiently high as to cause serious adverse clinical reactions—Clozapine, for example (MW 327 Da), can require daily dosages of up to 1 g (Kane et al, 1988). We have previously shown in mice that systemic administration (tail vein injection) of siRNA targeting transcripts derived from the claudin-5 gene, encoding one of the protein components of the tight junctions of the neuronal and retinal microvasculatures, renders the BBB and iBRB reversibly permeable to compounds up to approximately 1 kDa for periods of up to 36 h, starting 24 h post-siRNA delivery (Campbell et al, 2008, 2009). Importantly, however, molecules greater than approximately 1 kDa do not passively diffuse from the blood to the brain or retina when claudin-5 is suppressed, an observation made more significant by the fact that many low-molecular weight drugs lie within this size range, with larger substances, such as antibodies, blood-borne soluble enzymes, anaphylatoxins and pathogens being excluded.
Here, we describe an adeno-associated virus (AAV-2/9 serotype; Foust et al, 2009, 2010) expressing a doxycycline-inducible shRNA targeting transcripts encoding claudin-5. The process allows for localized and inducible modulation of the BBB or iBRB independently of each other and provides a means of delivery to pre-selected neuronal regions of low-molecular weight compounds up to approximately 1 kDa in molecular weight for the period of time during which the inducing agent is administered. The process has no negative impact on neuronal function, nor does it induce neuronal oedema. Using the retina as a model system for assessment of neuronal function, we have validated the barrier modulation approach to systemic drug delivery by demonstrating protection of photoreceptors against light-induced damage by treatment with the calpain inhibitor ALLM. We have also shown that laser-induced choroidal neovascularization (CNV), the latter being the hallmark of vision threatening disease pathology in age-related macular degeneration (AMD), can be suppressed by systemic drug therapy using the low-molecular weight compounds, Sunitinib malate (Sutent®, Pfizer) and 17-AAG (Bristol-Myers Squibb) following induced barrier modulation. These observations form the basis of a novel and minimally invasive means of drug delivery to neuronal tissues while also representing a valuable experimental tool for the evaluation and screening of low-molecular weight drug efficacy for neurodegenerative conditions. The translational potential of this technology is potentially large and could be applied to a range of neurodegenerative and neuromalignant conditions.
RESULTS
Assessment of the efficacy of CLDN5 AAV-2/9
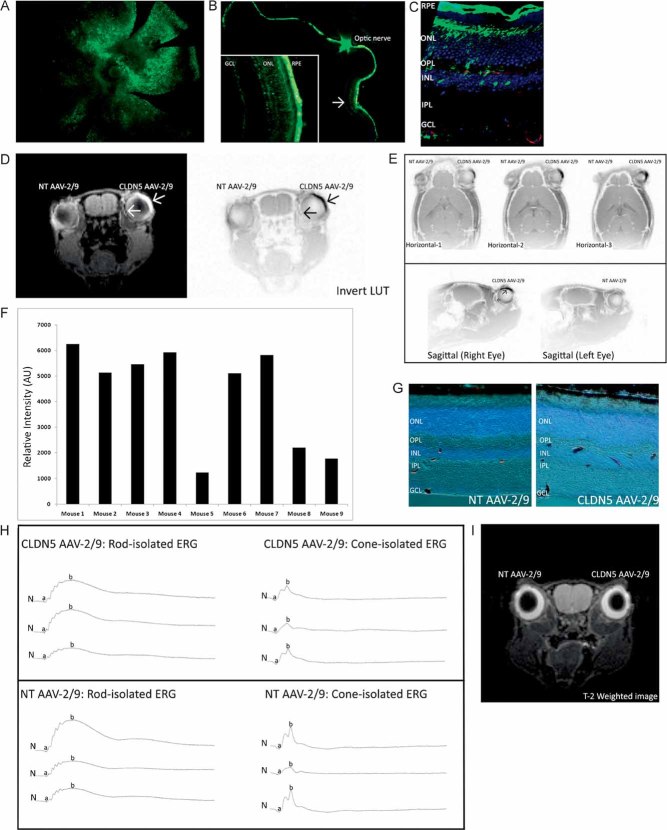
The system described here allows for reversible modulation of the iBRB with the exclusion of the BBB, and vice versa. To achieve this, we incorporated shRNA targeting claudin-5 into a doxycycline-inducible plasmid system. Subsequently, this was incorporated into the genome of an AAV-2/9 vector (these viruses have recently been shown to transduce endothelial cells of the neuronal microvasculature with high efficiency; Fig 1A and B; Foust et al, 2009, 2010). Viral purity was assessed using SDS–PAGE (Supplementary Fig 1).
Figure 1. Assessment of the efficacy of CLDN5 AAV-2/9.
- A,B. The plasmid incorporating the inducible system with claudin-5 shRNA (A) or a NT luciferase shRNA (B) was cloned into the plasmid pAAV-MCS, such as to incorporate L-ITR and R-ITR. Abbreviations: tTS, tetracycline-inducible transcriptional suppressor; β-globpA, beta-globin promoter; pTRE-U6, Tet-responsive U6 promoter; f1 ori, f1 origin of replication; Amp, ampicillin selection; pUC, pUC origin of replication. AAV-2/9 was then generated using a triple transfection system in a stably transfected HEK-293 cell line for the generation of high-titre viruses. tTS is a fusion of the Tet repressor and the Kid-1 KRAB-AB silencing domain. In the absence of doxycycline, tTS repressor binds to Tet operator (TetO) elements in a modified polIII promoter (pTRE-U6), inhibiting expression of claudin-5 (or NT luciferase) shRNAs. In the presence of doxycycline, tTS no longer binds to the promoter, allowing expression of shRNA.
- C. 3 weeks post-sub-retinal inoculation of 3 µl of 5 × 1011 viral particles/ml of the NT AAV-2/9 or CLDN5 AAV-2/9 and subsequent supplementation in the drinking water of mice of 2 mg/ml doxycycline with 5% sucrose.
- D,E. Strong and significant suppression of claudin-5 was observed at both the protein (***p = 0.0005) and transcript level (*p = 0.0218).
- F,G. Qualitative assessment of claudin-5 expression in retinal flatmounts showed a continuous and strong pattern of staining at the endothelial cell margins in the microvasculature of the retinas of mice injected with the NT AAV-2/9. However, following sub-retinal inoculation of the CLDN5 AAV-2/9, this pattern of staining, while not completely ablated, was discontinuous and fragmented, with large immunoreactive precipitates of claudin-5 manifesting in the microvasculature.
- H. Analysis of claudin-5 expression in retinal cryosections of mice injected with the NT AAV showed claudin-5 expression associated with the microvessels of the retina, permeating as far as the OPL (Arrows). Mice injected with the CLDN5 AAV-2/9 showed a significant decrease in expression and localization of claudin-5 in each layer permeated by microvessels. Outer nuclear layer (ONL), inner nuclear layer (INL), IPL and GCL.
An experimental group of C57/Bl6 mice received a sub-retinal inoculation of 3 µl of 5 × 1011 viral particles/ml of CLDN5 AAV-2/9 in the right eye. Mice received a non-targeting (NT) AAV-2/9 in their left eye. These animals were then supplemented in their drinking water with 2 mg/ml doxycycline and 5% sucrose for 3 weeks prior to analysis. Western blot analysis showed significant decreases (***p = 0.0005) in the levels of expression of claudin-5 in retinas of mice injected with the CLDN5 AAV-2/9 compared to the NT AAV-2/9 injected retinas (Fig 1C and D). This suppression was also manifested by significant decreases (*p = 0.0218) in claudin-5 transcript levels in retinal RNA samples (Fig 1E). Qualitative retinal flatmount analysis of claudin-5 expression showed a distinct and continuous pattern of expression in the NT AAV-2/9-injected retinas compared to the CLDN5 AAV-2/9-injected retinas (Fig 1F and G), and this was also observed in retinal cryosections, where decreased levels of expression and a discontinuous, fragmented pattern of staining was observed in the vascularized retinal layers as far as the outer plexiform layer (OPL; up to five mice analysed/group; Fig 1H).
Phenotype assessment of CLDN5 AAV-2/9
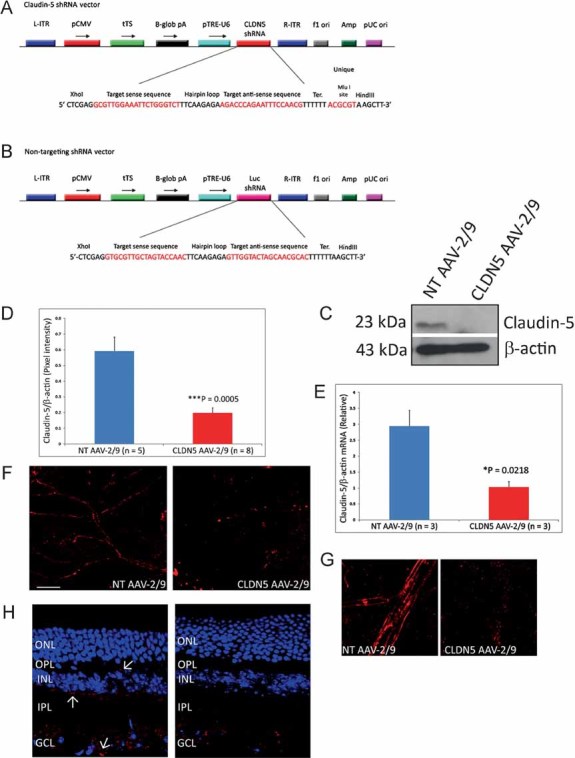
Although in use both experimentally and clinically, AAV serotypes will have differing transduction patterns when injected sub-retinally (Bainbridge et al, 2008; Hauswirth et al, 2008; Maguire et al, 2008). In this regard, we wished to assess the pattern of transduction of AAV-2/9 using an eGFP reporter gene. Mice sub-retinally injected with this AAV showed widespread transduction when a retinal whole-mount was prepared (Fig 2A). Indeed, transduction was widespread throughout the entire retinal pigment epithelium (RPE), which as a point of interest does not express claudin-5 (Rizzolo et al, 2007). Efficient transduction of the neural retina was, however, localized to the site of injection (arrow in Fig 2B and inset image) and following retinal cryosectioning and staining with an endothelial cell-specific isolectin (Griffonia-simplicifolia isolectin-Alexa 568), we observed co-labelling of eGFP and endothelial cells in the ganglion cell layer (GCL), inner plexiform layer (IPL), and OPL. While not all microvessels were transduced by AAV-2/9, the degree of transduction was sufficient enough to allow for significant modulation of claudin-5 expression (Fig 2B and C). Importantly, and unlike other AAV serotypes, for instance AAV-2/8 (Stieger et al, 2008), there was no transduction of AAV-2/9 along the optic nerve, which highlights an initial safety profile of this serotype. Transduction appeared to be retained solely to retinal tissue (Supplementary Fig 2).
Figure 2. Phenotype assessment of CLDN5 AAV-2/9.
- An AAV-2/9 with an eGFP reporter gene was injected sub-retinally in an adult C57/Bl6 mouse and 3 weeks post-injection, a retinal whole-mount showed the pattern of transduction to be widespread (green).
- Although transduction was observed throughout the retina, it was apparent that this transduction was heavily localized just to the neural retina (30–50% transduction) at the site of injection (arrow and high magnification inset), with the entire RPE being transduced. There was no transduction of AAV-2/9 observed along the optic nerve.
- High-magnification microscopy of retinal cryosections injected sub-retinally with the eGFP AAV-2/9 (green) in the region of neural retina transduction and subsequent staining with a griffonia-simplicifolia isolectin-Alexa-568 (endothelial cell staining—red), showed the transduction efficiency of the AAV-2/9 serotype for endothelial cells.
- Contrast-enhanced MRI showed extravasation of the MRI contrasting agent Gd-DTPA (MW 742 Da) in mice injected in the right eye with CLDN5 AAV-2/9, but not in the left eye, injected with NT AAV-2/9, when animals were supplemented with the inducing agent doxycycline (2 mg/ml with 5% sucrose in their drinking water) for 3 weeks post-injection. The inverted (LUT) image highlights the localized extravasation of Gd-DTPA in the right eye compared to the contra-lateral control (arrows).
- Both horizontal and sagittal MRI show the pattern of extravasation of Gd-DTPA localized to the site of sub-retinal inoculation but that Gd-DTPA permeates the entire retina of the CLDN5 AAV-2/9-injected eye.
- This extravasation was a consistent observation (***p = 0.0001) in all mice analysed (n = 9), with the background pixel intensity from the NT AAV-2/9-injected eye being subtracted from the CLDN5 AAV-2/9-injected eye.
- Retinal cryosections of CLDN5 AAV-2/9-injected mice perfused with microperoxidase (1881 Da) showed this tracer molecule to be confined within the retinal microvasculature, without signs of extravasation (n = 4).
- Rod and cone isolated ERGs showed typical ERG tracings expected 3 weeks post-sub-retinal injection and no differences between the NT AAV-2/9-injected eyes and the CLDN5 AAV-2/9-injected eyes.
- T-2-weighted coronal MRI analysis revealed no signs of retinal oedema in either NT AAV-2/9 or CLDN5 AAV-2/9 injected eyes (n = 9).
Following confirmation of the efficiency of suppression of the inducible CLDN5 AAV-2/9, we undertook a series of magnetic resonance imaging (MRI) experiments to allow a phenotypic assessment of the system. In mice receiving CLDN5 AAV-2/9 sub-retinally in the right eye and a NT AAV-2/9 in the left eye, contrast-enhanced MRI showed significant quantities of Gd-DTPA (742 Da) extravasation specifically in the CLDN5 AAV-2/9-injected eye (Fig 2D). The pattern of extravasation appeared greatest in the region of sub-retinal injection, and this pattern was similar in horizontal and sagittal orientations (***p = 0.0001; Fig 2E and F; Supplementary Fig 3). Each quantitative bar represents the enhanced contrasting observed in the CLDN5 AAV-2/9-injected eye when the background from the contra-lateral NT-AAV-2/9-injected eye was subtracted. As Gd-DTPA has a molecular weight of 742 Da, we wished to determine the size-selectivity of iBRB modulation, so we perfused mice with a solution of microperoxidase (1881 Da). Following retinal cryosectioning, microperoxidase was observed to be confined in the retinal microvasculature with suppression of claudin-5 (Fig 2G). Therefore, while the iBRB was modulated to a molecule of 742 Da, the iBRB remains impermeable to a molecule of 1881 Da. This phenotype produced no adverse effects on the rod or cone isolated electroretinogram (ERG) readouts of mice and importantly, (Fig 2H) barrier modulation using CLDN5 AAV-2/9 produced no signs of retinal oedema as assessed by high-resolution T-2-weighted MRI analyses (Fig 2I).
Systemic and stereotaxic injection of CLDN5 AAV-2/9
Systemic administration of CLDN5 AAV caused a phenotype similar to that observed at the iBRB, and as expected also manifested in increased permeability of Gd-DTPA across the BBB correlated with suppression of brain capillary claudin-5 (Supplementary Fig 4). Moreover, a stereotaxic inoculation of 2 µl of the CLDN5 AAV-2/9 (5 × 1011 vp/ml) in the region of the right hippocampus showed a localized and inducible BBB modulation site-specifically when mice were supplemented with doxycycline (2 mg/ml) in their drinking water. This localized modulation of the BBB caused enhanced passive diffusion of Gd-DTPA from the blood to the brain while causing no signs of oedema formation. Specifically, dark contrasting observed in the right hippocampus of Fig 3E was manifested as intensely high-contrast blue in the pseudo-coloured image of Fig 3F. Each of nine individual mice injected had significantly higher Gd-DTPA contrasting in their right hippocampus compared to the left (Supplementary Fig 5). Importantly, the inducibility of the barrier modulating system was highlighted when doxycycline was removed from the drinking water and no barrier permeability was observed (Supplementary Fig 6).
Figure 3. Systemic and stereotaxic injection of CLDN5 AAV-2/9.
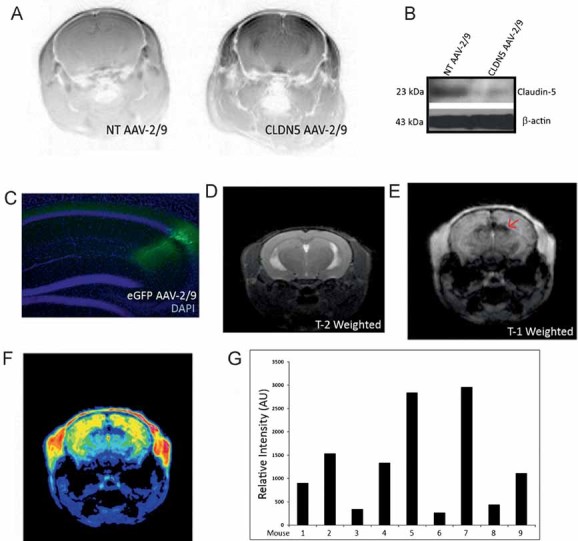
- BBB modulation to Gd-DTPA was observed throughout the brain and in the region of the hippocampus, extravasation of Gd-DTPA was manifested by dark contrasting in inverted LUT MR images following systemic injection of AAV.
- Western blot analysis in a capillary isolated fraction of brain tissue showed suppression of claudin-5 in CLDN5 AAV-2/9-injected mice compared to NT AAV-2/9-injected mice.
- An eGFP expressing AAV-2/9 was also injected to identify the transduced region and showed efficient transduction of cells within the hippocampus (green), with retrograde transport observed in neuronal cells.
- High-resolution T-2-weighted MRI showed no signs of neuronal oedema at the site of injection.
- Post-Gd-DTPA injection, enhanced contrasting was observed within the hippocampus specifically at the site of injection.
- Extravasation of Gd-DTPA at the side of injection was highlighted in the pseudo-coloured image (arrows).
- Quantitative analysis of regions of interest in the right hippocampus of individual mice were assessed and compared to the contra-lateral hippocampus. In each mouse analysed, there was a significant enhancement of Gd-DTPA in the region of the hippocampus injected with CLDN5 AAV-2/9.
Prevention of retinal degeneration using barrier modulation
We chose initially to use a mouse model of retinal degeneration in order to assess the efficacy of neuronal barrier modulation using CLDN5 AAV-2/9 to enhance delivery of an anti-apoptotic agent to the retina. Light-induced retinal degeneration is a commonly used model to induce rapid photoreceptor cell death and we have previously shown that siRNA-mediated suppression of claudin-5 allows for enhanced delivery of the potent calpain inhibitor ALLM to the neural retina (Campbell et al, 2009). Calpain activity has been described as being central to light-induced photoreceptor cell degeneration and to this end, we injected albino BalB/c mice in their right eye with CLDN5 AAV-2/9 or in their left eye with a NT AAV-2/9. Again, mice were supplemented with the inducing agent doxycycline (2 mg/ml) in their drinking water and 24 h prior to light ablation all mice received an intraperitoneal (i.p.) injection of 20 mg/kg ALLM prior to dark adaptation. ALLM has a molecular weight of 401 Da and is a potent inhibitor of calpain I (Ki = 120 nM), calpain II (Ki = 230 nM), cathepsin-L (Ki = 0.6 nM), cathepsin-B (Ki = 100 nM), and the proteasome. Following dilation of their pupils, mice were then exposed to 7900 lux light for 2 h and 24 h later, eyes were harvested and stained for DNA fragmentation (TUNEL staining) for the identification of end stage dying photoreceptor cells and in effect the assessment of retinal degeneration. We observed up to 70% protection in the retinas of mice injected with CLDN5 AAV-2/9 compared to the NT AAV-2/9-injected retinas with i.p. injection of ALLM (Fig 4A and B). This degree of retinal protection has been shown previously, however, it was with intra-ocular injection of ALLM, in contrast to the systemic mode of delivery used here (Sanges et al, 2006). Systemic administration of anti-apoptotic agents in conjunction with CLDN5 AAV-2/9 appears to be highly efficient in preventing retinal degeneration. Moreover, decreases in cell death was concomitant with a distinct decrease in the amount of α-fodrin cleavage, a key substrate of calpain activity (Fig 4C).
Figure 4. Systemic drug delivery to the retina and inhibition of retinal degeneration.
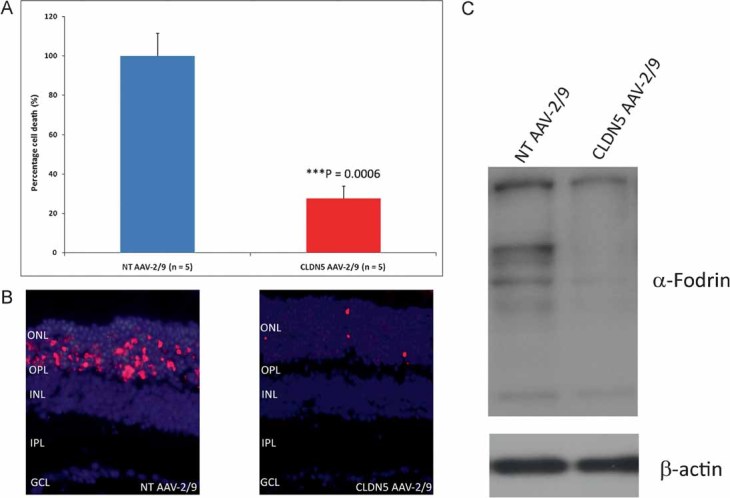
- Albino BalB/c mice were inoculated sub-retinally with either the NT AAV-2/9 in their left eye or the CLDN5 AAV-2/9 in their right eye. Mice were administered 2 mg/ml doxycycline in their drinking water for 3 weeks and 24 h prior to light ablation, they received an i.p. injection of 20 mg/kg ALLM. Mice were subsequently exposed to 7900 lux light for 2 h and 24 h post-light exposure, retinal cryosections were analysed for end stage apoptotic/necrotic photoreceptors by counting of TUNEL positive cells. Cell counts from the CLDN5 AAV-2/9-injected eyes and the corresponding NT AAV-2/9-injected eye were expressed as a percentage of each other as each individual animal received identical light ablation in each eye. Significant protection of photoreceptor cells was observed in the right eyes (CLDN5 AAV-2/9) of mice compared to the left eyes (NT AAV-2/9; ***p = 0.0006).
- TUNEL positive cells were shown to be consistently localized in large numbers to the ONL of NT AAV-2/9-injected retinas compared to CLDN5 AAV-2/9-injected retinas.
- Extensive cleavage of the calpain substrate α-fodrin was observed in mice receiving the NT AAV compared to mice receiving the CLDN5 AAV with ALLM prior to light ablation.
Prevention of choroidal neovascularization using neuronal barrier modulation
In order to assess the effects of enhanced delivery of therapeutics for the treatment of CNV using CLDN5 AAV-2/9, we chose to use a laser-induced model of CNV. This model involves the use of a targeted and intense laser burn to the back of the eye to cause a localized disruption of the RPE and Bruch's membrane. Localized increases in VEGF at the site of the laser burn have previously been reported to cause a CNV-like lesion and represent the best available animal model of the central pathology associated with wet AMD (Sakurai et al, 2003). Moreover, these lesions are extremely poorly perfused as outlined in Fig 5A, with green fluroescence representing perfused fluorescein isothiocyanate (FITC)-conjugated dextran (FITC-dextran), while red staining represents the actual endothelial cells of the CNV. To this end, we wished to determine the effects of barrier modulation in tandem with systemic treatment of two well characterized and clinically relevant VEGF inhibitors, 17-AAG (MW: 585 Da; Sausville et al, 2003) and Sunitinib malate (Sutent®) (MW: 532 Da; Motzer et al, 2007). Mice were administered a sub-retinal injection of either CLDN5 AAV-2/9 in the right eye and a NT AAV-2/9 in the left eye. Mice were subsequently treated with 2 mg/ml doxycycline in their drinking water for 3 weeks prior to the administration of targeted laser burns (three/eye) in each eye. All animals were assessed for CNV formation 14 days post-burn and in the intervening period, animals received two i.p. injections of either 17-AAG (31.25 mg/kg) or Sunitinib malate (20 mg/kg). CNV volumes were assessed following removal of the neural retina and staining of eyecups with a Griffonia-simplicifolia isolectin-Alexa-568 molecule in order to stain endothelial cells. Confocal scanning laser microscopy (CSLM) was used to create Z-stacks of each CNV by assessing the fluorescence in each layer of the CNV. These CNV's were then compiled into a 3D image and subsequently assessed using dedicated Imaris® software which will allow for the determination of both CNV area and, importantly, depth. We observed significant decreases in CNV volumes in CLDN5 AAV-2/9-injected eyes with 17-AAG (Fig 5A and B) or Sunitinib malate (Sutent®; Fig 5D and E) when these drugs were administered systemically. Animals injected with 17-AAG also showed significant decrease in expression of VEGFR-2 (Fig 5C). Importantly, each CLDN5 AAV-2/9-injected eye was compared to a contra-lateral NT AAV-2/9-injected eye so each eye was exposed to identical quantities of active compound (n = 5–7 mice/group).
Figure 5. Inhibition of CNV using barrier modulation.
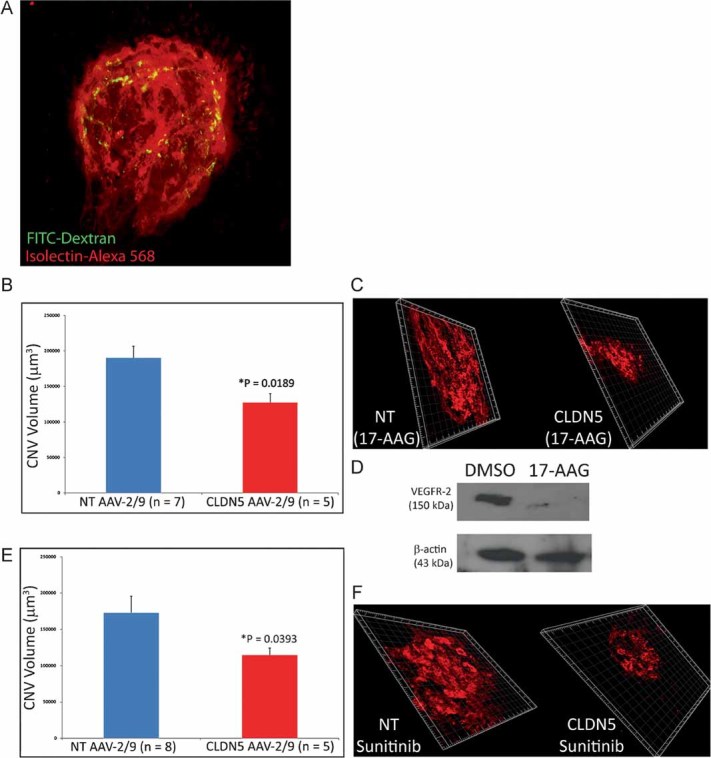
- Mice perfused with FITC-dextran-200 (FD-200) prior to preparation of choroidal flatmounts, displayed poorly perfused lesions (green) at the site of the laser burn when compared to the density of endothelial cells in the region stained with isolectin-Alex 568 (red).
- C57/Bl6 mice were administered NT AAV-2/9 in their left eye and CLDN5 AAV-2/9 in their right eye with doxycycline supplementation (2 mg/ml) in their drinking water for 3 weeks. CNV was induced with a targeted laser burn (140 mW, 100 mS, 50 µm spot size) in either the NT AAV-2/9 or CLDN5 AAV-2/9 injected eye and 4 days post-laser burn, mice were injected with 31.25 mg/kg 17-AAG i.p. Mice were injected again 4 days later. CNV volumes in the eyes of mice receiving the CLND5 AAV-2/9 were significantly reduced when compared to the NT AAV-2/9 injected eyes (*p = 0.0189).
- CNV formation in mice inoculated sub-retinally with CLDN5 AAV-2/9 were shown to be consistently reduced when compared to the NT AAV-injected eyes (n = 5–7 mice per experimental group).
- 17-AAG is a potent inhibitor of VEGFR-2 expression via the inhibition of hsp-90.
- In the same manner as described above, mice were injected with 20 mg/kg Sunitinib malate (Sutent®) i.p. on days 4 and 8 post-laser burn and CNV volumes were shown to be significantly reduced in CLDN5 AAV-2/9-injected mice compared to NT AAV-2/9 controls (*p = 0.0393).
- Again, confocal Z-stack images showed consistently decreased CNV volumes in the CLDN5 AAV-2/9-injected eyes compared to the NT AAV-2/9-injected eyes.
DISCUSSION
In terms of the safety profile of using barrier-modulating technology, we have seen very few differentially regulated genes in neuronal tissues when claudin-5 is suppressed (Campbell et al, 2009). It must also be considered that a relatively small number of drugs can cause ocular complications when administered systemically and, therefore, modulation of the barriers could exacerbate such complications (Abdollahi et al, 2004). However, it must be stressed that these agents are relatively few in number, are extremely well documented and could easily be avoided by individuals receiving a sub-retinal or stereotaxic inoculation of CLDN5 AAV-2/9.
CNV's are very badly perfused and similar to the blood–tumour barrier (Giannini et al, 2005), systemic drug treatment is limited as active compound needs to be exposed to a large area of tissue to be efficacious. Tet elements have also been used previously in a long-term safety study in the retinas of macaques and have shown to be an efficient and safe means to regulate gene expression. Repeated induction of gene expression was also observed for 2.5 years after initial injection (Stieger et al, 2006). Therapeutically, claudin-5 shRNA would be expressed for very short periods of time and given that the half-life of claudin-5 is approximated to be 30 h, the barrier would reform once shRNA expression is prevented upon removal of doxycycline. Given the lack of impact on retinal electrophysiology and retinal histology, it is highly unlikely that shRNA is expressed in sufficient quantities to interfere with the endogenous miRNA pathways. Indeed, miRNA are involved in the control of expression of up to 30% of genes and from a previous microarray study (Campbell et al, 2009), we have observed very few differentially regulated genes when claudin-5 is suppressed using siRNA. Moreover, we have observed no up-regulation of IL12 or RANTES expression in the neural retinas of mice administered NT AAV-2/9 or CLDN5 AAV-2/9, demonstrating that innate immune system activation is not an issue with this method of delivery (Supplementary Fig 6).
Although doxycycline has previously been reported to cause up-regulation in systemic PEDF levels and subsequent decreases in CNV volumes (Samtani et al, 2009), we have controlled for this using the NT AAV-2/9-injected contra-lateral eye. Moreover, the levels of doxycycline used in this study (2 mg/ml with 5% sucrose in drinking water) to induce claudin-5 shRNA expression are well below that used to prevent CNV formation alone. All evidence to date indicates that AAV infection of neuronal tissues is long lasting or may even be permanent and, hence, repeated injections of this inducible barrier-modulating agent should not be required (Bainbridge et al, 2008; Hauswirth et al, 2008; Maguire et al, 2008). Indeed, endothelial cells of the iBRB and BBB, while possessing high-turnover rates and replication potential do not in fact undergo nuclear division, and AAV transduction should persist long-term following one injection. In this regard, CLDN5 AAV-2/9 used in conjunction with known anti-neovascular agents such as 17-AAG or Sunitinib malate could form the basis of a novel and pre-emptive therapeutic strategy for wet AMD. Indeed, the levels of active compound that would be needed would be far less than that currently used for these drugs and this would apply to other molecules with proven efficacies in treating molecular pathologies associated with other neurodegenerative conditions. The method described here has distinct advantages over BBB disruption using ultrasound or mannitol in that it is size-selective to low-molecular weight molecules (Muldoon et al, 1995; Yang et al, 2010). The two methods outlined above are not size-selective and will allow for extravasation of plasma constituents such as albumin or IgG. Moreover, the use of intra-carotid administered mannitol, which is used clinically to break down the BBB for drug delivery in Glioblastoma multiforme (GBM) treatment, can induce severe seizures in patients and is neither size-selective nor short-term, with effects being manifested for days after the infusion (Neuwelt et al, 1986). A direct comparison of mannitol infusion and AAV-mediated barrier modulation was not performed during the course of this study given the disparity between the two approaches. Mannitol infusion causes global BBB disruption, for short periods of time, and this is neither size-selective, nor is it localized to distinct neuronal regions. Our AAV approach allows for periodic and localized barrier modulation specifically at the site of injection of the virus and has been designed in such a way that barriers only become reversibly permeable to low-molecular weight material. While the use of mannitol has been described in relation to attempts to improve access of cytotoxic drugs to the brain for treatment of GBM, its administration must be performed under highly specialized surgical conditions and it cannot be used for chronic conditions such as those described here in view of the fact that repeated administration may produce abnormal neurological and renal side-effects (Helmy et al, 2007).
While further GLP/GMP grade safety/toxicology studies mandated for experimental systems such as that described here are currently on-going, to date, the AAV-2/9 barrier modulating system for systemic drug delivery of therapeutics appears safe, tolerable and represents a novel form of chronic preventative therapy. Indeed, this system may now have a very broad range of applications for other neurodegenerative conditions that could be amenable to small molecule therapeutics. We have established that enhanced delivery of anti-neovascular and anti-apoptotic compounds following reversible modulation of the iBRB provides a direct proof of principle of an AAV-mediated system for inducible neuronal barrier modulation in human subjects. The short periods of time during which shRNAs are sequentially expressed will reduce any negative effects that could be induced by their permanent expression within the retina. We have also demonstrated that the system can be readily adapted to the brain microvasculature, allowing in principle, localized and inducible modulation of any distinct region of the brain inoculated with the virus. We anticipate that inducible suppression of claudin-5 in humans will represent an attractive avenue for controlled delivery of low-molecular weight therapeutics currently deemed unusable as they do not cross the neuronal or retinal blood barriers. In the case of AMD, the initial target here (AMD is the most prevalent cause of blindness in the developed world) (deJong, 2006; Seddon et al, 2006; Tan et al, 2008; The Eye Disease Prevalence Group, 2004), it is envisioned that a human therapeutic regime would involve delivery to the endothelial cells of the inner retina of an AAV vector expressing an inducible claudin-5 transcript inhibitory system. More generally, this technology represents a fundamental platform for drug delivery protocols in a wide range of neurodegenerative conditions where the BBB can be similarly modulated.
MATERIALS AND METHODS
Animal and experimental groups
All studies carried out in the Ocular Genetics Unit in TCD adhere to the ARVO statement for the use of Animals in Ophthalmic and Vision Research. Mice were originally sourced from Jackson Laboratories and bred on-site at the Ocular Genetics Unit in TCD.
AAV production
shRNAs designed to target transcripts derived from mouse claudin-5 were incorporated into AAV-2/9 vectors. shRNA was cloned into the pSingle-tTS-shRNA (Clontech) vector. The plasmid incorporating the inducible system with claudin-5 shRNA was digested with BsrBI and BsrGI and ligated into the Not1 site of the plasmid pAAV-MCS, such as to incorporate left and right AAV inverted terminal repeats. (L-ITR and R-ITR). AAV-2/9 was then generated using a triple transfection system in a stably transfected HEK-293 cell line for the generation of high-titre viruses (Vector BioLabs; Tam et al, 2008 and 2010; Chadderton et al, 2009; O'Reilly et al, 2007).
Sub-retinal AAV injection
Sub-retinal injections were carried out in compliance with the ARVO statement for the use of animals in ophthalmic and vision research.
Stereotaxic injection of AAV in mice
Using stereotaxic co-ordinates of the mouse brain, high-titre inducible AAV particles containing claudin-5 shRNA were injected into the hippocampus of the mouse brain (2.5 mm posterior and 1.0 mm lateral to bregma and 2.2 mm depth) using a hamilton syringe at a rate of 0.1 µl/min over a 10 min period.
Murine models of choroidal neovascularization
CNV, in which the vascular bed proliferates into the retina, mimicking neovascular AMD, was induced in mice using a green 532 nm Iridex Iris laser (532 nm, 140 mW, 100 mS, 50 µm spot size, three spots/eye) incorporating a microscopic delivery system as described previously. Following administration of laser burns, mice were returned to their cages and each injected i.p. on days 4 and 8 post-laser burn with either 20 mg/kg Sunitinib malate (Sutent®) or 31.25 mg/kg 17-AAG. Mice were sacrificed 14 days post-experiment and the neural retina was removed. Eye-cups were cut radially and fixed for 2 h at room temperature with 4% PFA (pH 7.4). Eye-cups were then incubated with a Griffonia-simplicifolia-isolectin-Aleax-568 molecule (1:300) overnight at 4°C. Eye-cups were washed three times with PBS and flatmounted on a glass slide using aqua-polymount. CNV's were assessed by confocal microscopy and Imaris® 7.0 (Bitplane Scientific Software).
Western blot analyses
Protein was isolated from total retinal tissue by homogenizing in lysis buffer containing 62.5 mM Tris, 2% SDS, 10 mM dithiothreitol, 10 µl protease inhibitor cocktail/100 ml (Sigma–Aldrich, Ireland). The homogenate was centrifuged at 10,000 × g for 20 min at 4°C, and the supernatant was removed for claudin-5 analysis (Campbell et al, 2009). Antibodies for Western blots were as follows: polyclonal rabbit anti-claudin-5 (Zymed Laboratories, San Francisco, CA) (1:500) and polyclonal rabbit anti-β-actin (Abcam, Cambridge, UK) (1:1000), mouse monoclonal anti-α-fodrin (Enzo Life Sciences) and VEGFR-2 (Abcam). Membranes were washed with TBS and incubated with a secondary anti-rabbit (IgG) antibody with horseradish peroxidase (HRP) conjugates (1:2500; Sigma–Aldrich), or anti-mouse (IgG) (1:1000; Sigma–Aldrich), for 3 h at room temperature. Immune complexes were detected using enhanced chemiluminescence (ECL). All Western blots were repeated a minimum of three times.
Real-time RT-PCR analysis
RNA was analysed by real-time RT-PCR using a Quantitect Sybr Green Kit as outlined by the manufacturer (Qiagen–Xeragon) under the following conditions: 50°C for 20 min; 95°C for 15 min; 38 cycles of 94°C for 15 s, 57°C for 20 s, 72°C for 10 s. Primers (Sigma–Genosys, Cambridge, UK) for the sequences amplified were as follows CLDN5 (Forward 5′-TTTCTTCTATGCGCAGTTGG-3′, Reverse 5′-GCAGTTTGGTGCCTACTTCA-3′), β-actin (Forward 5′-TCACCCACACTGTGCCCATCTA-3′, Reverse 5′ CAGCGGAACCGCTCATTGCCA-3′). IL12p40 (Forward 5′-CCACTTGGCCTTATGCTGTT-3′, Reverse 5′-TTGCATAATAGGGCCTGGTC-3′).
MRI analyses
Following AAV-mediated inducible RNAi of claudin-5, mice were analysed using a dedicated small rodent 7 T MRI system located at the Trinity College Institute of Neuroscience (TCIN), Dublin, Ireland (http://www.neuroscience.tcd.ie/technologies/mri.php). Mice were anesthetized with 3% isofluorane and maintained under sedation using 1%. For full MRI method, see SI text.
Indirect immunostaining of retinal flatmounts and retinal cryosections
Following fixation of eyes with 4% Paraformaldehyde (pH 7.4) for 4 h at room temperature and three subsequent washes with PBS, retinal cryosections were blocked with 5% normal goat serum (NGS) in PBS for 20 min at room temperature. Rabbit anti-Claudin-5 (Zymed), was incubated on sections overnight at 4°C. Secondary rabbit IgG-Cy3, (Jackson-Immuno-research, Europe) antibodies were incubated with the sections at 37°C for 2 h followed by three washes with PBS. All sections were counterstained with DAPI for 30 s at a dilution of 1:5000 of a stock 1 mg/ml solution. Analysis of stained sections was performed at room temperature with an epifluorescence microscope (Zeiss Axioplan 2, Oberkochen, Germany) or confocal laser scanning microscopy (Olympus FluoView TM FV1000).
The paper explained
PROBLEM:
Up to 98% of systemically deliverable low-molecular weight drugs, many of which could be used in treatment and prevention of the world's most common neurodegenerative and vision-threatening disorders, are not in current clinical use because they cannot cross the so-called BBB and iBRB.
RESULTS:
We have developed an AAV-2/9 vector, expressing an inducible shRNA designed to downregulate transcripts encoding claudin-5, a tight junction protein expressed in the brain and retinal microvasculatures. The AAV-2/9 vector was sub-retinally inoculated into the retinas of mice or stereotaxically injected into distinct regions of the brain. Subsequent treatment of inoculated mice with the inducing agent doxycycline turns on the genetic machinery that down-regulates claudin-5. Stopping doxycycline treatment shuts off the system. In this way, the iBRB and the BBB can be exclusively modulated—temporarily and reversibly—to allow passive diffusion of small molecules from the blood into the neural retina or brain.
IMPACT:
This form of therapy, using the inducible AAV-2/9 system, has the potential to be developed for use in humans, using one or more of a wide range of EMEA/FDA-approved anti-neovascular drugs in the case of wet AMD and a range of neuroprotective and anti-apoptotic agents for numerous other conditions with little or no current forms of therapy.
ERG analysis of mice
C57/Bl6 mice injected with wither a NT AAV-2/9 or a CLDN5 AAV-2/9 were dark-adapted overnight and prepared for electroretinography under dim red light. Pupillary dilation was carried out by instillation of 1% cyclopentalate and 2.5% phenylephrine. Animals were anesthetized by i.p. injection of ketamine (2.08 mg/15 g body weight) and xylazine (0.21 mg/15 g body weight). Standardized flashes of light were presented to the mouse in a Ganzfeld bowl to ensure uniform retinal illumination. The ERG responses were recorded simultaneously from both eyes by means of gold wire electrodes (Roland Consulting Gmbh) using Vidisic (Dr Mann Pharma, Germany) as a conducting agent and to maintain corneal hydration.
Light ablation of albino BalB/c mice
Mice were injected in their left eye with a NT AAV-2/9 or in their right eye with a CLDN5 AAV-2/9 virus. During the 3 weeks period when mice were supplemented in their drinking water with 2 mg/ml doxycycline/5% sucrose, animals were kept in cyclic light (12 h light and 12 h dark; 60 lux) prior to all experimentation. 24 h prior to light ablation, mice received ALLM i.p. (20 mg/kg) (Calbiochem, Germany) and were subsequently dark adapted for 24 h before being exposed to constant light.
TUNEL analysis
Eyes from mice injected with either the NT AAV-2/9 or the CLDN5 AAV-2/9 were fixed in 3.5% formaldehyde for 4 h followed by three washes in PBS. Eyes were cryoprotected using a sucrose gradient (10–30% sucrose in PBS), and subsequently 12 µm sections were cut using a cryostat. To detect cell death, sections were then incubated with staining mix (in situ cell death detection kit, TMR red, Roche) according to manufacturer's instructions.
Acknowledgments
The Ocular Genetics Unit at TCD is supported by Science Foundation Ireland (SFI), The Wellcome Trust, Irish Research Council for Science Engineering and Technology (IRCSET) and Fighting Blindness Ireland (FB-Ireland). Health Research Board of Ireland (HRB), Enterprise Ireland (EI) and the US Department of Defense-Telemedicine and Advanced Technology Research Center (TATRC). We would like to thank Caroline Woods, Charles Murray, David Flynn and Rebecca Robertson for animal breeding and husbandry. The authors would also like to thank Dr Christian Kerskens for his advice with MRI experiments and the Research Foundation at the Royal Victoria Eye and Ear Hospital for assistance in the acquisition of the Iridex laser system.
Supporting information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author Contributions
MC designed and undertook experiments; MH generated AAV constructs; ATHN performed TUNEL assays and light ablation; OG performed MRI analysis; LCST performed experimental design and cloning; MS performed RT-PCR analyses; FH and EO analysed cell culture and RT-PCR; GJF and ASK performed experimental design cell culture and RT-PCR; PFK performed sub-retinal injections and ERG analyses. PH performed experimental design and data analysis.
For more information
Smurfit Institute of Genetics
Fighting Blindness
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Abdollahi M, Shafiee A, Bathaiee FS, Sharifzadeh M, Nikfar S. Drug-induced toxic reactions in the eye: an overview. J Infus Nurs. 2004;27:386–398. doi: 10.1097/00129804-200411000-00004. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Campbell M, Kiang AS, Kenna PF, Kerskens C, Blau C, O'Dwyer L, Tivnan A, Kelly JA, Brankin B, Farrar GJ, et al. RNAi-mediated reversible opening of the blood–brain barrier. J Gene Med. 2008;10:930–947. doi: 10.1002/jgm.1211. [DOI] [PubMed] [Google Scholar]
- Campbell M, Nguyen AT, Kiang AS, Tam LC, Gobbo OL, Kerskens C, Ni Dhubhghaill S, Humphries MM, Farrar GJ, Kenna PF, et al. An experimental platform for systemic drug delivery to the retina. Proc Natl Acad Sci USA. 2009;106:17817–17822. doi: 10.1073/pnas.0908561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton N, Millington-Ward S, Palfi A, O'Reilly M, Tuohy G, Humphries MM, Li T, Humphries P, Kenna PF, Farrar GJ. Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy. Mol Ther. 2009;17:593–599. doi: 10.1038/mt.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deJong PTVM. Age-related macular degeneration. N Engl J Med. 2006;355:1474–1485. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes AH. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL, Schroeder MA, James CD. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: intensive care management. Br J Anaesth. 2007;99:32–42. doi: 10.1093/bja/aem139. [DOI] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- Muldoon LL, Nilaver G, Kroll RA, Pagel MA, Breakefield XO, Chiocca EA, Davidson BL, Weissleder R, Neuwelt EA. Comparison of intracerebral inoculation and osmotic blood–brain barrier disruption for delivery of adenovirus, herpesvirus, and iron oxide particles to normal rat brain. Am J Pathol. 1995;147:1840–1851. [PMC free article] [PubMed] [Google Scholar]
- Neuwelt EA, Howieson J, Frenkel EP, Specht HD, Weigel R, Buchan CG, Hill SA. Therapeutic efficacy of multiagent chemotherapy with drug delivery enhancement by blood–brain barrier modification in glioblastoma. Neurosurgery. 1986;19:573–582. doi: 10.1227/00006123-198610000-00011. [DOI] [PubMed] [Google Scholar]
- O'Reilly M, Palfi A, Chadderton N, Millington-Ward S, Ader M, Cronin T, Tuohy T, Auricchio A, Hildinger M, Tivnan A, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81:127–135. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen CG, Fletcher AE, Donoghue M, Rudnicka AR. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom. Br J Ophthalmol. 2003;87:312–317. doi: 10.1136/bjo.87.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolo LJ, Chen X, Weitzman M, Sun R, Zhang H. Analysis of the RPE transcriptome reveals dynamic changes during the development of the outer blood–retinal barrier. Mol Vis. 2007;23:1259–1273. [PubMed] [Google Scholar]
- Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- Samtani S, Amaral J, Campos MM, Fariss RN, Becerra SP. Doxycycline-mediated inhibition of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50:5098–5106. doi: 10.1167/iovs.08-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanges D, Comitato A, Tammaro R, Marigo V. Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc Natl Acad Sci USA. 2006;103:17366–17371. doi: 10.1073/pnas.0606276103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausville EA, Tomaszewski JE, Ivy P. Clinical development of 17-allylamino, 17-demethoxygeldanamycin. Curr Cancer Drug Targets. 2003;3:377–383. doi: 10.2174/1568009033481831. [DOI] [PubMed] [Google Scholar]
- Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of age-related macular degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- Stieger K, Le Meur G, Lasne F, Weber M, Deschamps JY, Nivard D, Mendes-Madeira A, Provost N, Martin L, Moullier P, et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol Ther. 2006;13:967–975. doi: 10.1016/j.ymthe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Stieger K, Colle MA, Dubreil L, Mendes-Madeira A, Weber M, Le Meur G, Deschamps JY, Provost N, Nivard D, Cherel Y, et al. Subretinal delivery of recombinant AAV serotype 8 vector in dogs results in gene transfer to neurons in the brain. Mol Ther. 2008;16:916–923. doi: 10.1038/mt.2008.41. [DOI] [PubMed] [Google Scholar]
- Tam LC, Kiang AS, Kennan A, Kenna PF, Chadderton N, Ader M, Palfi A, Aherne A, Ayuso C, Campbell M, et al. Therapeutic benefit derived from RNAi-mediated ablation of IMPDH1 transcripts in a murine model of autosomal dominant retinitis pigmentosa (RP10) Hum Mol Genet. 2008;17:2084–2100. doi: 10.1093/hmg/ddn107. [DOI] [PubMed] [Google Scholar]
- Tam LC, Kiang AS, Campbell M, Keaney J, Farrar GJ, Humphries MM, Kenna PF, Humphries P. Prevention of autosomal dominant retinitis pigmentosa by systemic drug therapy targeting heat shock protein 90 (Hsp90) Hum Mol Genet. 2010;19:4421–4436. doi: 10.1093/hmg/ddq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115:334–341. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- The Eye Disease Prevalence Group. Prevalence of age-related macular degeneration in United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Yang FY, Lin YS, Kang KH, Chao TK. Reversible blood–brain barrier disruption by repeated transcranial focused ultrasound allows enhanced extravasation. J Control Release. 2010 doi: 10.1016/j.jconrel.2010.10.038. [Epub a head of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.