Abstract
The migratory response of astrocytes is essential for restricting inflammation and preserving tissue function after spinal cord injury (SCI), but the mechanisms involved are poorly understood. Here, we observed stimulation of in vitro astrocyte migration by the new potent glycogen synthase kinase-3 (GSK-3) inhibitor Ro3303544 and investigated the effect of Ro3303544 administration for 5 days following SCI in mice. This treatment resulted in accelerated migration of reactive astrocytes to sequester inflammatory cells that spared myelinated fibres and significantly promoted functional recovery. Moreover, the decreased extent of chondroitin sulphate proteoglycans and collagen IV demonstrated that scarring was reduced in Ro3303544-treated mice. A variety of in vitro and in vivo experiments further suggested that GSK-3 inhibition stimulated astrocyte migration by decreasing adhesive activity via reduced surface expression of β1-integrin. Our results reveal a novel benefit of GSK-3 inhibition for SCI and suggest that the stimulation of astrocyte migration is a feasible therapeutic strategy for traumatic injury in the central nervous system.
Keywords: astrocyte, glial scar, GSK-3, migration, spinal cord injury
INTRODUCTION
Spinal cord injury (SCI) currently has no satisfying cure. While the therapeutic potential of stem/progenitor cells from various sources, including induced pluripotent stem (iPS) cells (Tsuji et al, 2010), in cell replacement strategies is uncontested (Okano, 2010), numerous obstacles including their potential tumourigenicity (Miura et al, 2009) must be overcome. Alternative, potentially complementary, therapeutic strategies are thus still required.
Recently, using several conditional knock-out mice targeting STAT3 signalling in reactive astrocytes, we and others have observed that the compaction and seclusion of infiltrating inflammatory cells in the lesion centre by migrating reactive astrocytes during the sub-acute phase of SCI is associated with improved locomotor recovery (Herrmann et al, 2008; Okada et al, 2006). These findings suggest that reactive astrocyte migration may constitute a new therapeutic target for the early phase of SCI (Renault-Mihara et al, 2008).
Glycogen synthase kinase-3 (GSK3)-α and β are serine/threonine kinases originally identified as regulators of glycogen synthase. Based on their involvement in several signalling pathways (Forde & Dale, 2007), they are considered potential therapeutic targets for several diseases (Chico et al, 2009). The fibroblast-specific genetic deletion of GSK3-β is associated with accelerated skin wound closure in mice (Kapoor et al, 2008). Furthermore, GSK-3 inhibition was reported to be beneficial for SCI, possibly by reducing apoptosis and promoting axonal growth (Cuzzocrea et al, 2006; Dill et al, 2008).
Taking advantage of a novel, potent specific inhibitor of GSK-3, Ro3303544, we investigated the effects of GSK-3 inhibition on astrocyte migration capability. The observation that sustained inhibition of GSK-3 stimulated astrocyte migration in vitro led us to administer Ro3303544 after contusive SCI in mice and examine the effects of this treatment in vivo.
RESULTS
Ro3303544 is more potent than a previously utilized GSK-3 inhibitor
Although the potency and specificity of Ro3303544 have been evaluated in kinase assays (Adachi et al, 2007), its potency was evaluated in more detail in primary cultures of hippocampal neurons, which are recognized as very sensitive to any toxicity. In the absence of Wnt-induced signalling, cytoplasmic β-catenin is constitutively phosphorylated by GSK-3 and degraded by the ubiquitin-proteasome system (Inestrosa & Arenas, 2010). Upon initiation of the Wnt signal and subsequent inhibition of GSK-3 activity, β-catenin accumulates in the cytoplasm, translocates into the nucleus and promotes the transactivation of various genes. Treatment of E17.5 hippocampal neurons with 1 µM Ro3303544 for 48 h resulted in a strong nuclear and peri-nuclear accumulation of β-catenin as expected (Fig 1A).
Figure 1. Ro3303544 is more potent than a previously utilized GSK-3 inhibitor.

- Treatment of E17 rat hippocampal neurons in vitro with 1 µM Ro3303544 for 48 h resulted in dramatic nuclear and perinuclear accumulation of β-catenin. Scale bars: 20 µm.
- Complete abrogation of Thr514-CRMP2 phosphorylation with Ro3303544 under the same conditions as in A confirmed the higher potency of Ro33034544 compared to SB415286. Data represent mean ± SEM of three independent experiments. ***p < 0.001; *p < 0.05.
- Treatment of E17 rat hippocampal neurons in vitro with Ro3303544 for 72 h significantly promoted neurite outgrowth. Green: βIII-tubulin, blue: Hoechst. Scale bar: 50 µm. Data represent mean ± SD of three independent experiments performed in triplicate. ***p < 0.001.
The potency of Ro3303544 was then compared to another GSK-3 inhibitor, SB415286 (Coghlan et al, 2000), previously used in vivo (Dill et al, 2008). To quantify the level of GSK-3 inhibition at various concentrations chosen according to their respective IC50s (0.6 and 78 nM for Ro3303544 and SB415286, respectively), the phosphorylation level of collapsin response mediator protein 2 (CRMP2) at Thr514, a specific site for phosphorylation by GSK-3 (Yoshimura et al, 2005), was examined in hippocampal neurons. Ro3303544 at 500 nM drastically reduced phosphorylation, in contrast to a partial effect of SB415286 at 10 µM (Fig 1B).
The treatment of E17.5 rat hippocampal neurons with Ro3303544 for 72 h in vitro resulted in significantly increased neurite length (mean ± SD; 58.83 ± 12.24%; Fig 1C). Together, these experiments demonstrated the high potency of Ro3303544 and its lack of toxicity at the concentrations used.
Sustained inhibition of GSK-3 stimulates the migration of astrocytes in vitro
Since the compaction of inflammatory cells in the lesion epicentre by reactive astrocytes during the sub-acute phase of SCI is associated with enhanced locomotor recovery (Herrmann et al, 2008; Okada et al, 2006), the stimulation of astrocyte migration is an attractive approach for the treatment of SCI during the early phase (Renault-Mihara et al, 2008). Considering that activation of the Wnt/β-catenin pathway results in the increased migration of numerous cell types in a variety of pathophysiological contexts, inhibition of GSK-3 leading to activation of β-catenin was speculated to stimulate astrocyte migration. This hypothesis was first tested in vitro.
Inhibition of GSK-3 by Ro3303544 was found to compromise the recolonization of a wounded area (Supporting Information, Fig S1A and Supplemental Movie 1), in agreement with the role of GSK-3 in polarization (Etienne-Manneville & Hall, 2003). Sustained GSK-3 inhibition before the migration assay was reasoned to be necessary for activating β-catenin-responsive genes. Treatment for 24 h with Ro3303544 resulted in the nuclear accumulation of β-catenin in astrocytes (Fig 2A) in a dose-dependent manner (Fig S2A and S2B). In agreement with the mitogenic effect of activated β-catenin in various cell types, these doses of Ro3303544 were observed to promote bromodeoxyuridine (BrdU) incorporation in astrocytes in vitro (Fig S2C).
Figure 2. Sustained, but not acute, inhibition of GSK-3 by Ro3303544 stimulates the migration of astrocytes and reduces their spreading in vitro.

- Treatment for 24 h with 1 µM Ro3303544 resulted in the strong nuclear accumulation of β-catenin in astrocytes (white arrowheads). Scale bar: 20 µm.
- The sustained inhibition of GSK-3 by 48-h pretreatment with either Ro3303544 or SB415286 greatly increased astrocyte migration in a transwell assay. Wash-out of Ro3303544 normalized the migration index. Data represent mean ± SD of three independent experiments. ***p < 0.001.
- Pretreatment for 48 h with Ro3303544 before seeding reduced the spreading of astrocytes onto coverslips coated with 10 µg/ml laminin. Green: F-actin labelled with phalloidin; red: α-tubulin; blue: Hoechst nuclear staining. Scale bars: 50 µm.
Considering that additional treatment time would allow the completion of downstream events, we attempted to extend the Ro3303544 treatment time of astrocytes to 48 h before performing the wound scratch assay in the presence of aphidicolin, a potent antimitotic drug. However, Ro3303544 treatment sustained over 24 h was observed to disrupt the astrocytic monolayer without inducing toxicity (Fig S1B and S1C). Since intercellular contacts, mainly through adherens junctions (Dupin et al, 2009), are required for the effective recolonization of wounded astrocytic monolayers, this monolayer disruption prohibited the evaluation of ‘β-catenin-activated’ astrocyte migration in this assay. Therefore, a modified Boyden's chamber assay or ‘transwell’ assay was used, which quantifies the migration of dissociated cells through a porous membrane. Treatment of astrocytes for 48 h with 1 µM Ro3303544 before the transwell assay resulted in a 2.73 ± 0.33-fold increase in cell migration compared to control-treated astrocytes (Fig 2B and Fig S2D). A similar increase observed upon pre-treatment with 10 µM SB415286 (2.15 ± 0.30-fold increase) indicated that the stimulation of astrocyte migration was indeed due to the sustained inhibition of GSK-3, rather than to the effect of Ro3303544. The acute inhibition of GSK-3 by Ro3303544 from 30 min prior to the transwell assay until its end (15 h), a time window similar to that for the wound assay, had no significant effect on cell migration (Fig S2E) indicating that the effect of GSK-3 depended on the cell migration mode.
Dysregulated activation of the Wnt/β-catenin pathway is a common phenomenon in numerous tumours and is associated with metastatic potential (Nguyen et al, 2009). To investigate whether sustained GSK-3 inhibition promoted an irreversible, cancerous transformation of the astrocytes, Ro3303544 was removed after the initial 48 h treatment, and the astrocytes were maintained in control medium for an additional 2 days before testing their migration properties. This wash-out procedure completely normalized the migratory ability of the cells (Fig 2B), suggesting that the pro-migratory effect of sustained Ro3303544 treatment was not related to cancerous cell transformation.
Sustained inhibition of GSK-3 reduces astrocytic spreading in vitro
Since alterations in cell migratory properties are often associated with morphological changes, the effects of prolonged Ro3303544 treatment on astrocytic morphology were examined by staining for F-actin and α-tubulin. Ro3303544 or control solution was applied for 48 h, and then the cells were replated at a low density onto laminin-coated coverslips. After 15 h of culture, the astrocytes treated with Ro3303544 remained rounded with intense peripheral actin rings (Fig 2C), in contrast to their control counterparts, which adopted a typical astrocytic morphology. Quantitative morphometric analysis confirmed a significant reduction of the mean area per cell after treatment with Ro3303544 (4774 ± 3650 µm2 and 2866 ± 2064 µm2, n = 977 and 756 analysed cells in the control and Ro3303544 group, respectively), thus demonstrating that sustained inhibition of GSK-3 reduces the spreading of astrocytes.
Inhibition of GSK-3 by Ro3303544 promotes the compaction of infiltrated inflammatory cells after spinal cord injury
Next, the in vivo effects of Ro3303544 after SCI were examined. To focus on the compaction of inflammatory cells by reactive astrocytes, the protocol consisted of intraperitoneal administration of Ro3303544 for only the first 5 days after thoracic contusive SCI in mice (Fig 3A). This is in contrast to a previous report in which Dill et al (2008) administered SB415286 for 3–4 weeks after SCI and reported increased axonal growth and improved functional recovery. Axonal growth is a delayed event that commences after the inflammatory reaction has subsided, while the compaction of inflammatory cells by reactive astrocytes occurs during the sub-acute phase of SCI, namely the first 2 weeks after injury in mice (Okada et al, 2006).
Figure 3. In vivo administration of Ro3303544 for the first 5 days after SCI in mice is effective.
- Experimental design.
- Immunoblot of spinal cord lysates at 5 DPI revealed that intraperitoneal injections of Ro3303544 potently increased the levels of a phosphorylated active form of β-catenin in vivo. Histogram displays the mean ± SEM of one experiment (four mice per group) (*p < 0.05, Wilcoxon rank-sum test).
- At 4 DPI, confocal analysis showed that while active β-catenin is weakly expressed and localized exclusively to the cytoplasm of neurons in the spinal cords of control mice (arrows), injections of Ro3303544 resulted in drastic upregulation and nuclear accumulation of β-catenin in neurons (arrowheads). Note that the relative upregulation of active β-catenin is even more pronounced in reactive astrocytes (asterisks).
First, the efficiency of the protocol was evaluated. Analysis by confocal microscopy revealed that at 4 days post-injury (DPI), while phosphorylated active β-catenin (van Noort et al, 2002) was weakly expressed and localized exclusively to the cytoplasm of neurons in the spinal cords of control mice, administration of Ro3303544 resulted in β-catenin upregulation and nuclear accumulation in neurons and reactive astrocytes (Fig 3C). Immunoblotting of spinal cord lysates collected at 5 DPI quantitatively confirmed this β-catenin activation in vivo after administration of Ro3303544 (Fig 3B).
The effect of Ro3303544 on the compaction of inflammatory cells after SCI was then investigated. As previously observed (Okada et al, 2006), CD11b-positive inflammatory cells appeared as a diffuse infiltrate at the lesion centre of the injured spinal cord parenchyma at 7 DPI in both groups (Fig 4A) and were progressively compacted by the surrounding GFAP-positive reactive astrocytes at 14 and 42 DPI. Three-dimensional measurement of the lesion volume through the analysis of GFAP-negative areas in serial sagittal sections revealed that while the initial infiltration of inflammatory cells at 7 DPI was similar in both groups, the compaction of inflammatory cells at 14 DPI was significantly accelerated by Ro3303544 administration, consistent with the in vitro stimulation of astrocyte migration by Ro3303544 (Fig 4B). At 14 DPI, confocal microscopic examination of the boundary between reactive astrocytes and the lesion centre visualized through laminin confirmed the potent walling off of the lesion by reactive astrocytes in the Ro3303544-group (Fig 4C).
Figure 4. Administration of Ro3303544 after SCI accelerates the compaction of infiltrated inflammatory cells by stimulating reactive astrocyte migration.
- The compaction of CD11b-positive inflammatory cells surrounded by GFAP-positive reactive astrocytes was significantly accelerated upon Ro3303544 administration. Right panels display the separate immunostaining at 14 DPI. Red: GFAP; green: CD11b; blue: Hoechst nuclear staining. Scale bars: 500 µm.
- Lesion volume was significantly reduced at 14 DPI in the Ro3303544 group compared to control. Data represent mean ± SEM. *p = 0.011 (Mann–Whitney test, n = 5 mice per group at 7 DPI and 42 DPI, n = 12 and 10 mice at 14 DPI in the control and Ro3303544 groups, respectively.).
- At 14 DPI, confocal imaging of the boundary between reactive astrocytes and the lesion centre visualized through laminin revealed reactive astrocytes potently walling off the lesion in Ro3303544 group. Scale bar: 50 µm.
- Quantification of BrdU incorporation during the first 2 weeks after the lesion did not evidence any significant difference between groups, indicating that the increased compaction did not rely on increased astrocyte proliferation in vivo. Confocal imaging (middle panel) illustrates BrdU/GFAP positive cells at high magnification. Histogram data represent mean ± SEM. *p < 0.05 (Wilcoxon rank-sum test, n = 5 and 6 mice in the control and Ro3303544 groups, respectively). Red: GFAP; green: BrdU. Scale bars: 50 and 10 µm in the left and middle panels, respectively.
To examine the possibility that the increased compaction of inflammatory cells upon Ro3303544 administration in vivo resulted from enhanced proliferation of reactive astrocytes, BrdU incorporation experiments were performed. Mice in the control and Ro3303544 groups received daily intraperitoneal injections of BrdU for 14 days after the injury. Quantitative analysis revealed no significant difference in the number of BrdU-labelled reactive astrocytes surrounding the lesion, implying that the increased compaction of infiltrated inflammatory cells by Ro3303544 results from the migration of reactive astrocytes rather than from astrocyte proliferation (Fig 4D).
Treatment with Ro3303544 reduces the size of the lesion scar and demyelination
The lesion scar in traumatic SCI consists of a fibrous scar at the lesion core surrounded by a glial scar. Among the numerous molecules that are upregulated in CNS lesions (Sofroniew, 2009), chondroitin sulphate proteoglycans (CSPG) were examined first. Seminal studies have shown that CSPG staining overlapped with areas of inflammatory cell infiltration (Fitch & Silver, 1997). Quantitative analysis indicated that the area of CSPG immunoreactivity was significantly reduced in the Ro3303544 group at 14 DPI (Fig 5A) but not at 42 DPI (not shown). Considering that mice harbouring a fibroblast-specific deletion of GSK-3β exhibit accelerated wound closure as well as excessive scarring characterized by elevated collagen production (Kapoor et al, 2008), immunostaining for collagen IV, a major component of the fibrous scar (Klapka & Muller, 2006), was performed. A significant reduction of collagen IV was observed in the Ro3303544 group at 14 DPI (Fig 5B), but again not at 42 DPI (not shown). The reduction of both collagen IV- and CSPG-immunoreactive areas confirms that the stimulation of astrocyte migration by Ro3303544 is associated with reduced scar formation.
Figure 5. Treatment with Ro3303544 reduces the size of the lesion scar at 14 DPI.

- The extent of CSPG was significantly reduced at 14 DPI in Ro3303544-treated mice.
- The fibrous scar, assessed by collagen IV, was also significantly reduced at 14 DPI in Ro3303544-treated mice. In A and B, data represent mean ± SEM. *p < 0.05 (unpaired t-test, n = 6 mice per group). Scale bars: 100 µm.
An association between compaction of inflammatory cells by migrating reactive astrocytes and a reduction in delayed neuronal damage, such as demyelination, has been previously reported (Herrmann et al, 2008; Okada et al, 2006). Accordingly, while eriochrome cyanine blue staining revealed severe demyelination, as expected, at the lesion level in the control group at 42 DPI, significantly reduced demyelination was observed in the mice treated with Ro3303544 (Fig 6A).
Figure 6. Administration of Ro3303544 reduces demyelination and promotes functional recovery after contusive SCI.

- Quantitative analysis of eriochrome cyanine-positive areas in the ventral white matter at 42 DPI revealed that treatment with Ro3303544 reduced injury-associated demyelination. Data represent mean ± SEM (**p < 0.01, *p < 0.05, n = 5 mice per group). Scale bars: 250 µm.
- Hindlimb movement evaluated using the Basso mouse scoring scale improved significantly in the Ro3303544 group compared to the control group from 21 DPI. Data represent mean ± SEM. (*p < 0.05, **p < 0.01, ***p < 0.001; 2-way repeated measures ANOVA followed by Bonferroni post hoc test, n = 12 and 13 mice in the control and Ro3303544 groups, respectively.)
Administration of Ro3303544 improves motor function recovery after spinal cord injury
The recovery of motor function was then monitored over 42 days using the Basso Mouse Scale open-field score (BMS) (Basso et al, 2006). The mice in the Ro3303544 group exhibited a tendency for greater motor function recovery compared to the control group as early as 7 DPI (1.08 ± 0.97 vs. 1.85 ± 1.12 in the control and Ro3303544 groups, respectively). Control mice, with a mean BMS score of 2.95 ± 1.21 at 42 DPI, could not support their weight on their hind limbs. By contrast, mice in the Ro3303544 group had a mean BMS score of 5.00 ± 2.05 at 42 DPI, and many mice in this group were able to walk with forelimb–hindlimb coordination. The BMS score of the Ro3303544 group was statistically better than that of the control group (two-way repeated measures ANOVA: p-value related to an effect of the treatment = 0.0151), and Bonferroni's multiple comparisons test at each time-point demonstrated statistical significance from 21 DPI until the end of the observation period, i.e. 42 DPI (Fig 6B).
Stimulation of astrocyte migration by Ro3303544 is mediated by unknown mechanisms
What is the mechanism by which sustained Ro3303544 stimulates the migration of astrocytes? The pro-migratory effect of GSK-3β depletion in fibroblasts relies on the enhanced expression of endothelin-1 (Kapoor et al, 2008). In the case of astrocytes, the concomitant blockade of both endothelin receptors A and B by BQ-123 and BQ-788 (each 1 µM) had no effect on the pro-migratory effect of Ro3303544 (Fig S3A), indicating that different molecular mechanisms mediate the pro-migratory effect of GSK-3 inactivation/inhibition in astrocytes.
Next, considering that hypoxia-inducible factor 1-α (HIF-1α) promotes cell migration (Le et al, 2004) and that GSK-3 inhibition stabilizes HIF-1α (Flugel et al, 2007), Ro3303544 treatment was investigated to determine whether it could activate HIF signalling. Glioma cells were transfected with reporter constructs in which the expression of firefly luciferase was driven by hypoxia-responsive elements (HRE), and luciferase reporter assays were performed at various time-points during Ro3303544 treatment. While transfection with positive controls, i.e. two different gain-of-function HIF-1α mutants, enhanced HIF-dependent luminescence, treatment with Ro3303544 had no effect at any time-point (Fig S3B). This experiment thus ruled out the possible involvement of HIF signalling in the pro-migratory effect of Ro3303544. These results led to the conclusion that the stimulation of astrocyte migration by the sustained inhibition of GSK-3 could involve previously unreported mechanisms.
DNA microarray analysis suggests a global attenuation of integrin signalling in astrocytes by Ro3303544
To gain insight into the molecular mechanism underlying the pro-migratory effect of Ro3303544 treatment, DNA microarray analysis was performed using astrocytes in primary culture treated for 48 h with 1 µM Ro3303544 or control. Considering that wash-out of Ro3303544 normalized astrocyte migration (Fig 2B), this condition was also included as a control for genes that remained highly up- or downregulated after the wash-out period, which would therefore not be relevant to the Ro3303544-enhanced migration.
Figure 7A illustrates the changes in gene expression after treatment with Ro3303544 and demonstrates that the wash-out procedure partially reestablished the normal pattern. Among the 26,734 flags expressed in astrocytes, 1601 and 1638 genes were up- or downregulated more than twofold, respectively, in Ro3303544-treated astrocytes compared to control astrocytes (Fig 7B). In the wash-out versus vehicle groups, 1148 and 591 genes were up- or downregulated, respectively, more than twofold. Only 211 genes were upregulated and 573 genes downregulated in both the Ro3303544-treated and wash-out astrocytes compared to the control cells. Of these overlapping genes, all those for which the ratio of intensity (i.e. expression level) between the Ro3303544 and washout groups was below twofold were excluded.
Figure 7. Gene microarray analysis examining the effects of sustained GSK-3 inhibition by Ro3303544 on primary astrocyte cultures.
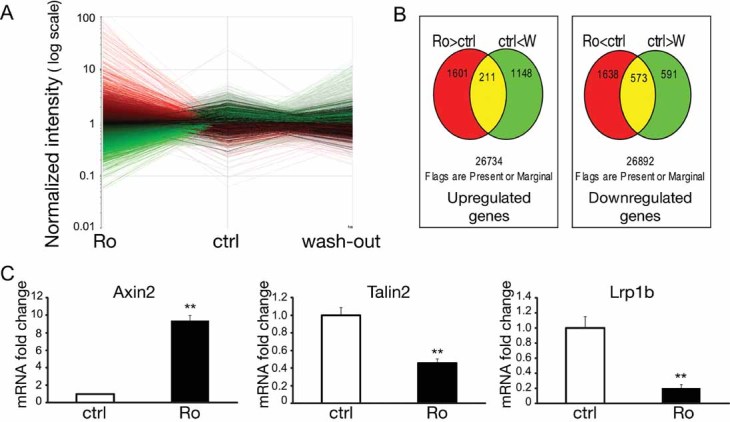
- Treatment for 48 h with 1 µM Ro3303544 resulted in a drastic change in the gene expression pattern that could be partially reversed by a 48-h wash-out, n = 2 per group. Red: upregulated genes; green: downregulated genes.
- Diagram illustrating the overlap in genes that were upregulated (left) or downregulated (right) more than twofold in the Ro3303544-treated (Ro: red) and -wash-out (W: green) groups compared to control astrocytes.
- Significant changes in Axin2, Tln2 and Lrp1b mRNA levels detected by qPCR confirmed activation of the Wnt/β-catenin pathway in astrocytes upon prolonged treatment with Ro3303544, as well as the variations in molecules known to modulate β1-integrin signalling. Data represent mean ± SEM. **p < 0.01, n = 5 (Mann–Whitney test).
To summarize these findings, strong variations were first observed in molecules belonging to the Wnt/β-catenin pathway (Table 1), consistent with the activation of this pathway by the sustained GSK-3 inhibition of Ro3303544. As an example, real-time polymerase chain reaction (qPCR) confirmed the upregulation of Axin2 (Fig 7C). Considering that the prolonged administration of Ro3303544 drastically affected cell spreading and morphology (Fig 2C), genes related to cell adhesion were then focused on. Integrins are prototypical adhesion receptors that link the extracellular matrix (ECM) to the intracellular actin cytoskeleton. They are heterodimers consisting of an α- and a β-subunit. Within the integrin family, Ro3303544 led to downregulation of the genes for α1, α3, α6, β5, and β1-like integrins, and no member of this family was upregulated (Table 1). Moreover, the expression levels of three genes previously reported to modulate β1-integrin maturation were significantly affected: N-acylsphingosine amidohydrolase 3-like (Asah3l, also named Acer2; 4.01-fold increase), talin2 (Tln2; 52% decrease) and low-density lipoprotein-related protein 1B (Lrp1b; 72% decrease).
Table 1.
Gene expression differences of selected gene clusters
 |
P, present; A, absent; (n = 2 per group)
The β1-integrin subunit is synthesized as an 87-kDa polypeptide that undergoes glycosylations in the endoplasmic reticulum and Golgi apparatus (Akiyama & Yamada, 1987). Only the most glycosylated form of β1-integrin, with a mass of ∼130 kDa, is found at the cell surface and functions in cell adhesion or cell signalling, justifying its denomination as the mature form. Importantly, the changes observed in Asah3l, Tln2 and Lrp1b suggested that Ro3303544 could cause the reduced maturation of β1-integrin. Asah3l overexpression is indeed known to inhibit β1-integrin maturation and thereby reduce cell adhesion to fibronectin or collagen (Sun et al, 2009). Furthermore, downregulation of Tln2 and Lrp1b is known to reduce β1-integrin maturation and cell adhesion (Albiges-Rizo et al, 1995; Salicioni et al, 2004). qPCR confirmed the downregulation of both Tln2 and Lrp1b after Ro3303544 administration (Fig 7C). Since the β1-integrin subunit is a component of most integrin receptors expressed by astrocytes (Takada et al, 2007), the role of this specific subunit was focused on next.
In vitro pro-migratory effect of Ro3303544 relies on decreased cell adhesion strength through a reduced surface expression of β1-integrin
Ro3303544 induced similar pro-migratory effects in astrocytes seeded on transwell membranes coated with laminin or fibronectin (Fig 8A), which are both ligands for β1-integrin-containing heterodimers. By contrast, sustained treatment with Ro3303544 did not stimulate astrocyte migration in the absence of any coating, indicating that the pro-migratory effect of Ro3303544 did not result from a switch to an adhesion-independent migration mode.
Figure 8. Increased migration of astrocytes in vitro upon prolonged Ro3303544 treatment relies on reduced cell adhesion with the ECM due to decreased surface expression of β1-integrin.

- The pro-migratory effect of prolonged Ro3303544 treatment was similar on fibronectin and laminin. By contrast, migration of astrocytes in the absence of ECM coating was limited regardless of Ro3303544 treatment.
- Treatment for 48 h with Ro3303544 dose-dependently decreased the expression level of mature β1-integrin in astrocytes. Histogram data represent mean ± SEM of a representative experiment performed in triplicate. ***p < 0.001.
- Flow cytometric analysis confirmed that 48-h treatment with Ro3303544 decreased the cell-surface expression of β1-integrin on astrocytes. The specificity of the immunostaining is shown at left. CD29 = β1-integrin. A representative experiment is shown. ***p < 0.001, n = 10 000 cells per sample (unpaired Student's t-test).
- Migration assays using a function-blocking antibody against β1-integrin demonstrated that functional β1-integrin was required for the migration of the astrocytes.
- The effect of Ro3303544 on in vitro astrocytes migration depended on the ECM concentration. While 48-h treatment with Ro3303544 resulted in decreased migration compared to control cells at low laminin concentrations, a pro-migratory effect was observed at higher concentrations. Data represent mean ± SD of three independent experiments. ***p < 0.001, n = 9.
Immunoblot analysis of total protein lysates prepared from astrocytes treated for 48 h with various concentrations of Ro3303544 revealed a dose-dependent decrease in the expression level of the ∼130-kDa mature β1-integrin (Fig 8B). The intensity of the ∼110-kDa band, corresponding to the precursor form of β1-integrin, was inversely correlated with that of the mature form, suggesting that the process of maturation through glycosylation was indeed inhibited by Ro3303544. Flow cytometry confirmed that the reduction in the ∼130-kDa mature form of β1-integrin seen with immunoblotting corresponded to reduced cell-surface expression (Fig 8C). Furthermore, the wash-out procedure partially reversed this reduction of cell-surface β1-integrin, consistent with its involvement in the effect of Ro3303544. Complete abrogation of astrocyte migration by a function-blocking monoclonal antibody against β1-integrin demonstrated that this subunit is necessary for the migration of astrocytes under the conditions in which Ro3303544 exerts its pro-migratory effect (Fig 8D).
In integrin-dependent two-dimensional migration models, the highest migration speeds result from an intermediate level of cell–substratum adhesion strength (net adhesion), which allows both rapid focal contact formation and the generation of traction forces (DiMilla et al, 1991). At low net adhesion, migration rates are indeed impaired because the reduced binding strength lowers the force generated at the leading edge (Gaudet et al, 2003; Palecek et al, 1997). Conversely, high net adhesion slows cells down and favours cell immobilization through delayed rear-process retraction. Three factors define net adhesion: substrate ligand level, integrin expression level, and the integrin–ligand binding affinity.
Reduced expression levels of integrins provide a pro-migratory advantage at high ECM protein concentrations (Palecek et al, 1997). Therefore, the effect of Ro3303544 on migration was examined with increasing concentrations of laminin (Fig 8E). At 2 µg/ml laminin, significantly more control than Ro3303544-treated astrocytes migrated. Although this observation may seem to contradict our initial observation of a Ro3303544 pro-migratory effect at 10 µg/ml ECM coating, it is in good agreement with the model: at above 5 µg/ml laminin, treatment with Ro3303544 provided a significant migratory advantage, consistent with the reduced cell-surface expression of β1-integrin in Ro3303544-treated cells. Together, these results suggest that the in vitro pro-migratory effect of Ro3303544 relies on decreased adhesion strength, especially through the reduced surface expression of β1-integrin.
Decreased expression of β1-integrin upon Ro3303544 treatment enhances the migration of reactive astrocytes in vivo
Since our proposed mechanism for the enhanced migration of astrocytes upon Ro3303544 administration implies the presence of high ECM concentrations, the changes in laminin expression, as an example ECM protein, were examined following SCI. In control animals, while the expression of laminin was initially restricted to blood vessels at 4 and 7 DPI, its expression at the lesion epicentre was drastically upregulated from 10 DPI and maintained at 14 DPI (Fig 9A). The time course of laminin upregulation following injury was not affected by Ro3303544 treatment (not shown). Then, the effect of Ro3303544 administration on β1-integrin expression levels was evaluated in vivo. Although the pattern of the different forms of β1-integrin in spinal cord lysates was much more complex than in astrocytes in vitro, a significant decrease in the expression level of the higher molecular-weight β1-integrin band in the Ro3303544-treated mice was observed at 5 DPI (Fig 9B). Considering that laminin was upregulated only from 10 DPI, the expression level of β1-integrin was next examined at this time-point. The results showed β1-integrin remain significantly reduced in Ro3303544-treated mice at 10 DPI (Fig 9B).
Figure 9. The Ro3303544-mediated reduction of β1-integrin expression in the spinal cord is concomitant with the spontaneous upregulation of laminin at 10 DPI.
- Laminin was upregulated at 10 DPI in the lesion centre of the injured spinal cord. Red: laminin; blue: Hoechst nuclear staining. Scale bars, 500 µm.
- At both 5 and 10 DPI, the higher-molecular-weight form of β1-integrin (arrowhead) was significantly decreased in the spinal cord of Ro3303544-treated mice compared to control mice. Data represent mean ± SEM. (**p < 0.01, *p < 0.05, unpaired t test, n = 4 mice per group and time-point.)
Together, the observed Ro3303544-mediated reduction in β1-integrin expression in the spinal cord and the concomitant spontaneous upregulation of laminin at 10 DPI suggests that the mechanistic model proposed on the basis of the in vitro data is relevant in vivo.
DISCUSSION
While the deleterious effects of reactive astrocytes and their associated glial scar after CNS injury are well established (Sofroniew, 2009), their beneficial roles have only been evidenced relatively recently (White & Jakeman, 2008). Using several conditional knock-out mice targeting STAT3 signalling in reactive astrocytes, we and others have previously observed that the compaction of inflammatory cells by migrating reactive astrocytes is associated with enhanced locomotor recovery after SCI (Herrmann et al, 2008; Okada et al, 2006). Here, we report that the pharmacological inhibition of GSK-3 using the novel, highly potent agent Ro3303544 successfully stimulated the migration of astrocytes both in vitro and in vivo and promoted functional recovery after SCI.
GSK-3 and cell migration
Current data about the role of GSK-3 in cell migration are contradictory (Etienne-Manneville & Hall, 2003; Kapoor et al, 2008). Our observations demonstrate first that the effect of GSK-3 depends on the migration mode involved (interested readers are invited to read the extended discussion in Supporting Information). Although the in vivo pattern of endogenous astrocyte migration following CNS injury is unknown, glial cells migrate as single cells during development (Klambt, 2009). Moreover, the observed increased compaction of inflammatory cells in vivo after Ro3303544 administration (Fig 4) suggests that the migration of astrocytes observed in the single-cell transwell assay more closely resembles the situation of in vivo reactive astrocytes and reveals that the in vitro wound scratch assay is not always relevant to in vivo CNS injury.
Mechanism of increased migration by the sustained inhibition of GSK-3
The historical model for describing the mesenchymal migration mode (DiMilla et al, 1991; Lauffenburger & Horwitz, 1996; Palecek et al, 1997) implies that cell migration speed depends on the strength of cell adhesion to the substratum. In agreement with the reduced surface expression of β1-integrin, the effect of Ro3303544 on migration was observed to depend on the concentration of the laminin coating. The apparent discrepancy between the administration period of Ro3303544 in vivo (the first 5 days after injury) and the observation that the compaction of inflammatory cells increased compared to control at 14, but not 7 DPI suggests that the pro-migratory effect of Ro3303544 in vivo indeed depends on the spontaneous upregulation of ECM proteins following SCI, which we have demonstrated occurs at 10 DPI. Given the complexity of the lesion environment, as well as the number of molecules that potentially modulate the migration of reactive astrocytes, it seems plausible that the actual mechanism for the in vivo enhancement of migration by Ro3303544 is more complex than our proposed model.
Mechanism of improved functional outcome by GSK-3 inhibition
The beneficial effect of GSK-3 inhibition in SCI using less potent and specific reagents has been previously reported (Cuzzocrea et al, 2006; Dill et al, 2008). This effect may involve reduced apoptosis and the direct promotion of axon outgrowth. While the direct stimulation of axon growth upon GSK-3 inhibition is still a matter of controversy in the literature (Alabed et al, 2010), we observed that Ro3303544 promoted the neurite outgrowth of embryonic hippocampal neurons in vitro, thereby demonstrating its lack of toxicity. However, to selectively evaluate the effects of enhanced astrocyte migration in vivo, Ro3303544 administration was restricted to the first 5 days after injury. This protocol allowed us to distinguish the observed results from possible direct axon growth-promoting effects of the drug, because axonal growth is a delayed event.
The observed decrease of astrocyte-devoid spaces filled with CD11b-positive inflammatory cells and the consistent reduction in CSPG- (Fitch & Silver, 1997) and collagen IV-positive areas demonstrated that GSK-3 inhibition at the acute phase of SCI accelerated the compaction of the lesion. We propose that this progressive seclusion of inflammatory cells by reactive astrocytes significantly contributes to the beneficial effect of GSK-3 inhibition after SCI. Although the local inflammatory reaction triggered by SCI is known to be capable of enhancing repair, the involvement of inflammatory cells in secondary neuronal damage such as demyelination is uncontested (Alexander & Popovich, 2009). The beneficial effect of walling off inflammatory cells by scar-forming reactive astrocytes is well established in innate (Bush et al, 1999; Faulkner et al, 2004; Herrmann et al, 2008; Myer et al, 2006; Okada et al, 2006) and adaptive inflammation (Voskuhl et al, 2009).
The major defects observed after ganciclovir-targeted death revealed the contribution of the dividing reactive astrocyte pool to the process of walling off leukocytes after brain and spinal cord injuries (Bush et al, 1999; Faulkner et al, 2004; Myer et al, 2006; Voskuhl et al, 2009). The mitogenic effect of Ro3303544 observed in vivo in brain progenitors (Adachi et al, 2007) and in vitro in astrocytes led us to investigate whether proliferation was involved in its in vivo effect. In vivo BrdU incorporation experiments suggest that the contribution of proliferation to the effect of Ro3303544 in vivo is not significant.
What is the mechanism whereby the accelerated compaction of inflammatory cells improves functional recovery? Although lesions ultimately demonstrated similar compaction levels at the last time-point examined (42 DPI), locomotor function was permanently superior in the Ro3303544 group compared to control. This observation highlights the critical role of this sub-acute period after the lesion in the recovery process, as previously suggested (Okada et al, 2006). The finding also strongly suggests that white matter sparing is crucial to recovery, as we and others have previously observed when reactive astrocytes wall off inflammatory cells (Faulkner et al, 2004; Herrmann et al, 2008; Okada et al, 2006). Whether remyelination also contributes to the increased myelin staining is difficult to analyse experimentally, as is the conflicting literature concerning the role of β-catenin signalling in remyelination (Azim & Butt, 2011; Fancy et al, 2009).
Given the wide actions of GSK-3 and Wnt/β-catenin, and the role of GSK-3 in inflammation (Jope et al, 2007), additional direct effects of Ro3303544 on inflammatory or immune cells are likely. Nevertheless, the normal infiltration of CD11b cells observed at 7 DPI, the peak of their invasion (Beck et al, 2010), suggests that Ro3303544 does not affect the recruitment of inflammatory cells.
A major question arising from this study is whether our current observation is relevant to other animal models or human SCI. In rat and human, cystic cavity formation is a common complication of brain and spinal cord damage. Unfortunately, these cystic cavities are not observed in most mouse strains, including the C57 BL6/J mice used for this study. In vitro studies have suggested that the development of these cavities is closely related to the relationship between inflammatory cells and reactive astrocytes (Fitch et al, 1999). While it is speculated that the physical contraction of the fibrous scar may also contribute to cavity formation in some previous reports (Klapka & Muller, 2006), two recent studies using different experimental approaches have observed that reduction of the scarring was associated with reduced cystic cavities in rat (Iannotti et al, 2006; Xia et al, 2008). However, further studies in relevant models are needed to examine whether the stimulation of reactive astrocyte migration through GSK-3 inhibition does indeed limit the development of these cystic cavities.
In conclusion, our findings reveal a novel beneficial effect of GSK-3 inhibition for SCI and suggest that the pharmacological stimulation of reactive astrocyte migration holds promise as a new therapeutic strategy for the treatment of SCI.
MATERIALS AND METHODS
Antibodies and reagents
The antibodies used are listed in Supporting Information. Ro3303544 was developed by Roche and kindly provided by Dr Gary Pelz (Department of Genetics and Genomics, Roche, Palo Alto, California, USA). The initial characterization of Ro3303544 has been reported elsewhere (Adachi et al, 2007). All the experiments reported in this study compared the effect of Ro3303544 dissolved in DMSO to a control solution that included an equivalent concentration of DMSO (0.1%). BQ-123, BQ-788 and SB415286 were from Sigma.
The paper explained
PROBLEM
Scarring is a general tissue response after injury to promote wound healing and to separate the injured tissue from the external environment. During the sub-acute phase of contusive SCI, reactive astrocytes migrate to the lesion epicentre and seclude infiltrating inflammatory cells there. This process has been shown to promote functional recovery using several strains of genetically modified mice and models of both innate and adaptive immunity. It is not yet known whether this property of astrocytes can be exploited to aid in the development of better treatment strategies for brain and spinal cord trauma.
RESULTS
Taking advantage of a novel, highly potent specific inhibitor of GSK-3, Ro3303544, the authors show that inhibition of GSK-3 stimulates astrocyte migration by regulating the expression of β1-integrin both in vitro and in vivo. Administration of Ro3303544 for the first 5 days after contusive SCI resulted in faster migration of reactive astrocytes, preservation of more myelinated fibres, and significantly improved recovery of movement. Labelling for CSPG and collagen IV confirmed that Ro3303544 treatment reduced the size of the lesion scar.
IMPACT
Here, for the first time, the authors were able to pharmacologically stimulate the beneficial effect of reactive astrocytes on immune cell restriction and scar formation. These findings reveal a novel effect of GSK-3 inhibition that could improve recovery from SCI and suggest that the pharmacological stimulation of reactive astrocyte migration holds promise as a new therapeutic strategy for the treatment of SCI.
Cell culture
See Supporting Information for detailed cell culture methods. Briefly, astrocytes in primary cultures were prepared as previously described (Araujo et al, 1993) from 1-day postnatal wild-type C57BL/6J mice.
Modified Boyden's chamber assay (transwell assay)
Polyethelene terephthalate (PET) filters with 8-µm pores (BD Biocoat), used to separate the upper and lower chambers, were coated with 10 µg/ml fibronectin (Sigma) or various concentrations of laminin for 3 h at 37°C. Astrocytes were trypsinized on day 13 in vitro, stained with trypan blue, and counted using a hemocytometre. A total of 3 × 104 cells were resuspended in MEM-F12 complete medium containing 1% FBS, 10 µg/ml aphidicolin (Sigma), and the indicated Ro3303544 concentrations, and the cells were allowed to migrate towards the same culture medium supplemented with 10% FBS in the lower chamber. After 15 h at 37°C, the filters were fixed for 20 min at room temperature with 4% paraformaldehyde in PBS (pH 7.5), and the non-migrated cells were removed by wiping the upper side of the membranes with cotton swabs. Cell nuclei were stained using Hoechst 33258, and the filters were mounted on slides in Fluoromount medium (Diagnostic Biosystems). Using Axiovision® software connected to an epifluorescence microscope (Zeiss, Axioplan 2), 12 fields per membrane were captured, and the cells were counted manually.
SCI model
All surgical and animal-care procedures were in accordance with the Laboratory Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, USA), and the Guidelines and Policies for Animal Surgery provided by the Animal Study Committee of Murayama Medical Center, and they were approved by the ethics committee of Murayama Medical Center. Adult female C57BL/6J mice (8 weeks of age) were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). The dorsal surface of the dura mater was exposed through a laminectomy at the 10th thoracic vertebra, and SCI was induced using an Infinite Horizon impactor (60 kDyn; Precision Systems & Instrumentation, Lexington KY) as previously described (Scheff et al, 2003). Actual impact forces did not differ between groups (control, 62.83 ± 2.55 kDyn; Ro3303544, 62.61 ± 1.98 kDyn). The mice were injected intraperitoneally with 0.5 ml Ro3303544 at 500 µM, dissolved in saline or saline containing an equivalent concentration of DMSO (0.1%) twice daily for 5 days, beginning immediately after SCI. The mice were returned to their cages and given free access to water and food.
Behavioural analysis
Hindlimb motor function was evaluated 1, 3, 7, 14, 21, 28, 35 and 42 days after the injury using the locomotor rating of the BMS (Basso et al, 2006). A team of three experienced examiners evaluated each animal for 4 min and assigned an operationally defined score for each hindlimb, which were then averaged. Animals with incomplete paralysis at 1 DPI (BMS score not equal to zero) were excluded from the study. Data were analysed with two-way repeated measures ANOVA followed by Bonferroni post-hoc test (n = 12 and 13 mice in the control and Ro3303544 groups, respectively).
Immunohistochemistry
Techniques and protocols for immunohistochemistry-based analyses are detailed in Supporting Information.
Gene microarray analysis
Microarray processing was performed by the Core Instrumentation Facility of Keio University School of Medicine. Detailed procedures are described in Supporting Information. The data set has been deposited in the NCBI Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) and is accessible through GEO series accession number GSE25770.
Statistical analysis
Data were analysed with one-way ANOVA and Bonferroni's multiple comparison test, unless otherwise specified in the figure legends, using GraphPad Prism software version 5.00 for Windows (GraphPad Software, San Diego, CA, USA).
Acknowledgments
We are grateful to Gary Peltz for providing the Ro3303544 and to Shizue Ohsawa for the gift of the HRE-luc constructs. We thank Mari Fujiwara (Core Instrumentation Facility, Keio University School of Medicine) for gene expression microarray processing, Yumi Matsuzaki for advice in flow cytometry, and Tokuko Harada for mouse care. We thank all the members of Dr. Okano's laboratory for helpful discussions and support. This study was supported by the Project for the Realization of Regenerative Medicine and Support for the Core Institutes for iPS Cell Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan, a grant-in-aid for the Global COE Program from MEXT to Keio University, a research grant from Takeda Foundation, by grants-in-aid for scientific research from MEXT (FRM, AI, and MN), fellowships from JST-SORST and the Japan Society for Promotion of Science (JSPS), as well as grants-in-aid from Keio University, JSPS, and the Naito Foundation to FRM.
Supporting information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
FRM conducted all in vitro experiments, performed the analysis of lesion volume and demyelination and wrote the paper; HK, TI, AI, AY and SN performed SCI and analysed locomotor function; TI analyzed BrdU incorporation; MMukaino analyzed the lesion scar; KS, MMatsushita and KK provided material; HK, TI, AI, MMukaino, AK, SN, HT, YM, SS and SO analyzed the data and edited the manuscript; YT, MN and HO supervised the study.
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Akiyama SK, Yamada KM. Biosynthesis and acquisition of biological activity of the fibronectin receptor. J Biol Chem. 1987;262:17536–17542. [PubMed] [Google Scholar]
- Alabed YZ, Pool M, Ong Tone S, Sutherland C, Fournier AE. GSK3 beta regulates myelin-dependent axon outgrowth inhibition through CRMP4. J Neurosci. 2010;30:5635–5643. doi: 10.1523/JNEUROSCI.6154-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albiges-Rizo C, Frachet P, Block MR. Down regulation of talin alters cell adhesion and the processing of the alpha 5 beta 1 integrin. J Cell Sci. 1995;108:3317–3329. doi: 10.1242/jcs.108.10.3317. [DOI] [PubMed] [Google Scholar]
- Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- Araujo H, Danziger N, Cordier J, Glowinski J, Chneiweiss H. Characterization of PEA-15, a major substrate for protein kinase C in astrocytes. J Biol Chem. 1993;268:5911–5920. [PubMed] [Google Scholar]
- Azim K, Butt AM. GSK3beta negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia. 2011;59:540–553. doi: 10.1002/glia.21122. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Chico LK, Van Eldik LJ, Watterson DM. Targeting protein kinases in central nervous system disorders. Nat Rev Drug Discov. 2009;8:892–909. doi: 10.1038/nrd2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Collin M, Esposito E, Bramanti P, Thiemermann C. Glycogen synthase kinase-3 beta inhibition reduces secondary damage in experimental spinal cord trauma. J Pharmacol Exp Ther. 2006;318:79–89. doi: 10.1124/jpet.106.102863. [DOI] [PubMed] [Google Scholar]
- Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci. 2008;28:8914–8928. doi: 10.1523/JNEUROSCI.1178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMilla PA, Barbee K, Lauffenburger DA. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991;60:15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin I, Camand E, Etienne-Manneville S. Classical cadherins control nucleus and centrosome position and cell polarity. J Cell Biol. 2009;185:779–786. doi: 10.1083/jcb.200812034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. Activated macrophages and the blood-brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp Neurol. 1997;148:587–603. doi: 10.1006/exnr.1997.6701. [DOI] [PubMed] [Google Scholar]
- Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugel D, Gorlach A, Michiels C, Kietzmann T. Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1alpha and mediates its destabilization in a VHL-independent manner. Mol Cell Biol. 2007;27:3253–3265. doi: 10.1128/MCB.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci. 2007;64:1930–1944. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet C, Marganski WA, Kim S, Brown CT, Gunderia V, Dembo M, Wong JY. Influence of type I collagen surface density on fibroblast spreading, motility, and contractility. Biophys J. 2003;85:3329–3335. doi: 10.1016/S0006-3495(03)74752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti C, Zhang YP, Shields LB, Han Y, Burke DA, Xu XM, Shields CB. Dural repair reduces connective tissue scar invasion and cystic cavity formation after acute spinal cord laceration injury in adult rats. J Neurotrauma. 2006;23:853–865. doi: 10.1089/neu.2006.23.853. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Liu S, Shi-wen X, Huh K, McCann M, Denton CP, Woodgett JR, Abraham DJ, Leask A. GSK-3beta in mouse fibroblasts controls wound healing and fibrosis through an endothelin-1-dependent mechanism. J Clin Invest. 2008;118:3279–3290. doi: 10.1172/JCI35381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Klambt C. Modes and regulation of glial migration in vertebrates and invertebrates. Nat Rev Neurosci. 2009;10:769–779. doi: 10.1038/nrn2720. [DOI] [PubMed] [Google Scholar]
- Klapka N, Muller HW. Collagen matrix in spinal cord injury. J Neurotrauma. 2006;23:422–435. doi: 10.1089/neu.2006.23.422. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, Gerald WL, Massague J. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Okano H. Neural stem cells and strategies for the regeneration of the central nervous system. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:438–450. doi: 10.2183/pjab.86.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Renault-Mihara F, Okada S, Shibata S, Nakamura M, Toyama Y, Okano H. Spinal cord injury: emerging beneficial role of reactive astrocytes' migration. Int J Biochem Cell Biol. 2008;40:1649–1653. doi: 10.1016/j.biocel.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Salicioni AM, Gaultier A, Brownlee C, Cheezum MK, Gonias SL. Low density lipoprotein receptor-related protein-1 promotes beta1 integrin maturation and transport to the cell surface. J Biol Chem. 2004;279:10005–10012. doi: 10.1074/jbc.M306625200. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Hu W, Xu R, Jin J, Szulc ZM, Zhang G, Galadari SH, Obeid LM, Mao C. Alkaline ceramidase 2 regulates beta1 integrin maturation and cell adhesion. FASEB J. 2009;23:656–666. doi: 10.1096/fj.08-115634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci USA. 2010;107:12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE, Jakeman LB. Don't fence me in: harnessing the beneficial roles of astrocytes for spinal cord repair. Restor Neurol Neurosci. 2008;26:197–214. [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Zhao T, Li J, Li L, Hu R, Hu S, Feng H, Lin J. Antisense vimentin cDNA combined with chondroitinase ABC reduces glial scar and cystic cavity formation following spinal cord injury in rats. Biochem Biophys Res Commun. 2008;377:562–566. doi: 10.1016/j.bbrc.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





