See related article in Nature Medicine http://dx.doi.org/10.1038/nm.2368
HOST-PATHOGEN COMPETITION FOR IRON
The survival of higher organisms requires constant vigilance against encroachment by potentially pathogenic microorganisms. Every portal of entry, and every potential niche, must be defended simultaneously against a wide spectrum of invaders. Each of these invaders – whether symbiotic or potentially pathogenic – has adopted their own cunning strategy to avoid detection and elimination, and to hijack the host's nutritional resources and metabolic apparatus to multiply and spread. In response to these threats humans have evolved a bewildering array of innate and adaptive defenses of which our knowledge is still only in its infancy. Among the innate defenses are mechanisms designed to withhold nutritional resources from the invaders and, of all the nutrients, iron is by far the most critical. It lies at the epicenter of most host-pathogen battles for resource control; it is the equivalent of crude oil in global geopolitics.
But 32% of the earth's crust is composed of iron (and 88% of its core) making it the most abundant element on the planet, so why should it be the subject of such blatant avarice? And what is the evidence that it is the focus of a fierce arms race between host and pathogen?
The avarice is generally explained by the very low solubility of iron compounds in their usual oxidized ferric state and by the usefulness of iron's redox potentials, which make it a suitable co-factor for numerous enzymes and pathways.
The evidence for its centrality in host-pathogen competition comes, inter alia, from the following sources: (i) most bacteria have made a disproportionate genetic investment in processes for iron acquisition; (ii) many of these genes are concentrated in islands of high pathogenicity, are important in defining niche specificity, and knock-downs are out-competed by wild types; (iii) there are over 500 known bacterial siderophores some with iron binding capacities that elude the best synthetic chemists (e.g. 10−52 M for enterobactin); (iv) genetic detective work shows that horizontal capture of iron-acquiring genes has been essential to niche transitions as enteric organisms find ways to colonize the low-iron systemic environment; (v) in response to these threats, and also to avoid potential iron-mediated oxidative damage, the human host has developed a suite of chaperone and storage proteins (transferrin, lactoferrin, ferritin, hemopexin, haptoglobin, etc.) and complex mechanisms for regulating dietary iron absorption and distribution; and (vi) the negative acute phase response, in which plasma iron levels can be decreased by fourfold or more within hours of an infection has long been hypothesized to be an element of the innate immune system.
»…hepcidin, turns out to be the master regulator of these iron-mediated mammalian defenses«
The circulating hormone hepcidin, discovered in 2000, turns out to be the master regulator of these iron-mediated mammalian defenses (Ganz, 2011). Hepcidin is a 25 amino acid peptide hormone produced primarily in the liver and functions by binding to the cellular iron exporter, ferroportin, causing its loss of function by internalization. Down regulation of ferroportin on the basolateral membrane of enterocytes causes an intracellular build up of iron which blocks further absorption at the apical surface, thus blocking the entry of dietary iron. Ferroportin inhibition in macrophages, the primary medium for iron re-cycling, blocks this process and results in its (relatively) safe sequestration for long periods if necessary. Through this process hepcidin orchestrates a major redistribution of body iron at times of infection and inflammation.
IRON AND MALARIA: NEW EVIDENCE THAT IRON IS CRITICAL
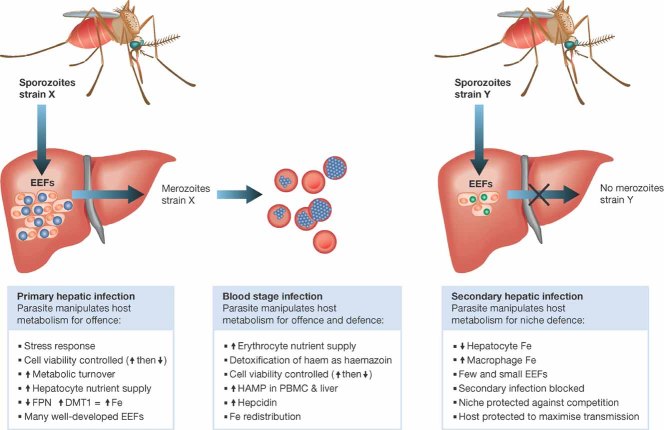
A recent paper in Nature Medicine has brought these themes together and provides strong evidence that hepcidin-mediated iron redistribution represents an important component of innate immune defence, at least against malaria (Portugal et al, 2011). Portugal and colleagues showed that mice carrying a Plasmodium berghei blood stage infection above a certain threshold were profoundly resistant to a new liver stage infection when inoculated with sporozoites, the mosquito invasive form of the parasite (Fig 1). This effect was reproducible across different rodent Plasmodium species and strains. Examination of liver sections 40–48 h after inoculation showed a reduction in both number and size of exoerythrocytic forms (EEFs) in infected mice compared to controls. These reductions matched qRT-PCR measures of infection in the same livers. No EEFs were observed 72 h after infection in the mice carrying a blood-stage infection.
Figure 1. Inter-kingdom signalling by Plasmodium parasites diverts host iron (Fe) supply for both offence and defence.
FPN, ferroportin, DMT1, divalent metal transporter, HAMP, hepcidin gene, PBMC, peripheral blood mononuclear cells, EEF, exoerythrocytic forms of parasite.
Up-regulation of immune related genes in both the blood stage infected mice and after secondary sporozoite challenge suggested that immune mechanisms could have been responsible for the impaired liver stage development. The challenge experiments were repeated in splenectomized animals, and mice genetically deficient for T and B cells, natural killer cells, γδ T cells, IFN-γ, nitric oxide synthase, interleukin-10, and myeloid differentiation factor-88 signalling, C5a-complement factor or mast cells, in mice depleted of tumor necrosis factor-α or IL-6, and in mice treated with the general anti-inflammatory compound N-acetyl-cysteine. None of these conditions prevented the protection against a liver-stage infection in mice that had ongoing blood stage infection; liver-stage re-infections were not even partially allowed. The same was true of immunosuppressed mice (severe combined immunodeficiency) upon re-infection. This series of experiments strongly suggested that classic components of adaptive or innate immunity where not involved. Hepatocyte apoptosis was also excluded.
The reduction in number and size of EEFs suggested that a nutrient or growth factor might be limiting development. Hamp mRNA, encoding hepcidin, was found up-regulated (average fivefold) in mice with a blood-stage infection, confirming previous observations (Armitage et al, 2009). Hepcidin mRNA levels correlated with blood-stage infection level and, above a threshold, were inversely correlated to P. berghei liver infection. These increased hepcidin levels caused a redistribution of iron with increased levels in macrophages and the spleen, but reduced levels in hepatocytes. Previous studies on Plasmodium-infected hepatoma cells had revealed a reduction in ferroportin (regulating iron efflux) and an increase in divalent metal transporter-1 (regulating influx) suggesting that the parasite was re-engineering the intra-cellular milieu to benefit its own growth (Albuquerque et al, 2009), and strongly implying that iron acquisition might be essential for the parasite complete development. This was further confirmed by demonstrating that addition of iron chelators markedly inhibited EEF development in a dose-dependent manner, whereas supplementation with iron markedly stimulated development.
Final confirmation that the inhibition of super-infection was mediated by the hepcidin-iron axis was obtained in mice with no ongoing blood-stage infection but over-expressing hepcidin or following hepcidin peptide administration.
Together these results provide the best demonstration to date of what has been suspected for some decades, namely that host-pathogen competition for iron can be a critical determinant of infectivity. The ability of the blood-stage parasite to protect its niche by out-competing another plasmodial population through host metabolism manipulation represents a beautiful illustration of inter-kingdom signalling and of the importance of nutrient supply to microorganism virulence. The fact that iron is the critical nutrient should come as no surprise.
SIGNIFICANCE FOR GLOBAL HEALTH
It is rare for a basic-science study on molecular mechanisms of disease in a mouse model to catch the attention of the global public health community – but this one should. In 2005 a very large iron and zinc intervention trial in Pemba, Tanzania was prematurely terminated by its data safety monitoring board because the children receiving iron had significantly more serious adverse events (deaths and hospitalizations) than those receiving zinc or placebo (Sazawal et al, 2006). The result has been ascribed to a malign interaction between iron and malaria, and caused WHO to recommend suspension of iron supplementation programmes in malarious regions. This has created a policy vacuum leaving tens of millions of children vulnerable to the short- and longer-term consequences of iron deficiency.
»…nutritional immunity with potentially major consequences for global health.««
By demonstrating how crucial the iron-hepcidin axis is in malaria, the proof-of-principle experiments by Portugal et al (Portugal et al, 2011) will add new impetus to discovery science in nutritional immunity with potentially major consequences for global health. Does the redistribution of iron to macrophages elevate the risk of bacteraemia in malaria? Does iron accelerate disease progression in HIV? Does elevated iron status increase susceptibility to TB? Do other hepcidin-inducing infections exacerbate these effects? There is epidemiological evidence pointing to each of these possibilities, but solid proof at the molecular level would greatly enhance the development of preventative and therapeutic interventions. There may then be a significant role for hepcidin agonists, antagonists or mimetics.
The author declares he has no conflict of interest.
References
- Albuquerque SS, Carret C, Grosso AR, Tarun AS, Peng X, Kappe SH, Prudêncio M, Mota MM. Host cell transcriptional profiling during malaria liver stage infection reveals a coordinated and sequential set of biological events. BMC Genomics. 2009;10:270. doi: 10.1186/1471-2164-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage AE, Pinches R, Eddowes LA, Newbold CI, Drakesmith H. Plasmodium falciparum infected erythrocytes induce hepcidin (HAMP) mRNA synthesis by peripheral blood mononuclear cells. Br J Haematol. 2009;147:769–771. doi: 10.1111/j.1365-2141.2009.07880.x. [DOI] [PubMed] [Google Scholar]
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal S, Carret C, Recker M, Armitage AE, Gonçalves LA, Epiphanio S, Sullivan D, Roy C, Newbold CI, Drakesmith H, et al. Host-mediated regulation of superinfection in malaria. Nat Med. 2011;17:732–737. doi: 10.1038/nm.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]