Abstract
Background:
The osteoporosis and lumbar canal stenosis, in elderly patients are under diagnosed and under reported. We report a cross sectional study to demonstrate the osteoporotic profile in patients with lumbar spinal stenosis (LSS) and to determine the proportion of patients with LSS who need to be treated for osteoporosis.
Materials and Methods:
One hundred and six postmenopausal patients with symptomatic LSS were evaluated for osteoporotic profile, which included lumbar and hip bone mineral density (BMD), serum vitamin D concentration, bone resorption and formation markers. Demographic and disease related variables were analyzed to identify the association with the risk of osteoporosis or osteopenia. Statistical analysis used were multivariate logistic regression with a forward stepwise procedure.
Results:
Twenty-four patients (22.6%) had osteoporosis and 60 (56.6%) had osteopenia. Overall, 84 patients (79.2%) with symptomatic LSS had osteoporosis or osteopenia. Fifty-nine patients (55.6%) had hypovitaminosis D. All bone turnover makers [alkaline phosphatase, osteocalcin, urinary-N-terminal telopeptide (u-NTx)] were demonstrated to be within normal range. Only age was associated with the risk of osteoporosis or osteopenia in the hip region. In the lumbar spine, all variables were not associated with osteoporosis or osteopenia. 44 patients (41.5%) required treatment for osteoporosis as per risk factors for osteoporosis. According to the guidelines from the Health Insurance Review Agency, however, only 20 patients (18.8% required) qualified for reimbursement for osteoporosis medications.
Conclusions:
LSS is associated with osteopenia, osteoporosis, and hypovitaminosis D, which should prompt careful screening and treatment in cases of osteoporosis and osteoarthritis.
Keywords: Bone mineral density, bone turnover marker, hypovitaminosis D, lumbar spinal stenosis, osteoporosis
INTRODUCTION
Osteoporosis and lumbar spinal stenosis (LSS) are the most common musculoskeletal conditions in the elderly population and also are one of the most common indications for lumbar spinal surgery at an advanced age.1–8 Nevertheless, under-diagnosis and under-treatment of osteoporosis in the general population and in elderly patients with musculoskeletal conditions are worldwide phenomena.9,10
The objectives of the current study are to demonstrate the prevalence of osteoporosis and osteopenia in patients with LSS and to determine the proportion of patients with LSS who need to be treated for treatment. Accordingly, the osteoporotic profile was analyzed in patients with symptomatic LSS in a prospective manner. Additionally, coexisting knee osteoarthritis (OA) was also investigated to have a correlation with osteoporotic condition.
MATERIALS AND METHODS
One hundred and six female patients with symptomatic LSS who presented between January 2009 and December 2009 were enrolled in this study. The inclusion criteria were being postmenopausal, having walking difficulty due to neurogenic claudication caused by LSS, and a stenotic lesion in the lumbar spine confirmed by magnetic resonance imaging (MRI). We defined stenotic lesion as midsagittal diameter being smaller than 12 mm in MRI.11 The exclusion criteria were having a history of chronic systemic disease or peripheral vascular disease, showing evidence of metabolic bone diseases such as hypo- or hyperparathyroidism and chronic renal disease, or using bone-specific medications (hormone replacement, bisphosphonates, and corticosteroids), and having concurrent serious medical conditions affecting bone metabolism, including sepsis or neoplasia.
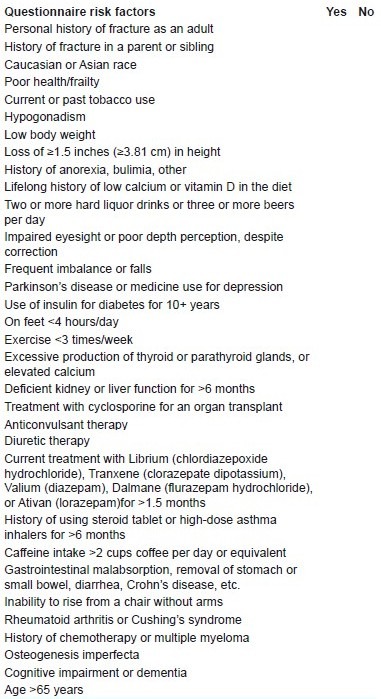
All patients were scheduled to be treated with lumbar spinal decompression and/or posterior spinal fusion. Patients’ demographic characteristics were obtained and each completed a questionnaire assessing for osteoporotic risk factors [Appendix 1].12 Preoperative imaging studies included plain radiographs of the lumbar and thoracic spine, including dynamogram of designated level and both knee joints. MRI of the lumbosacral spine was performed to confirm LSS and subsequently plan lumbar spinal surgery was done. For the assessment of functional disability due to spinal stenosis, the Oswestry Disability Index (ODI) was recorded before the surgical treatment. Osteoporotic profiles included measurement of lumbar and hip bone mineral density (BMD) (Hologic, Waltham, MA, USA), serum vitamin D concentration, urinary-N-terminal telopeptide (u-NTx) as a bone resorption marker, and serum osteocalcin and serum alkaline phosphatase as bone formation markers.
APPENDIX 1.
Total lumbar (lumbar 2nd to 4th), total hip, and femoral neck BMD were measured. The BMD of the Ward triangle was not counted in this study. Patients with a T score below –2.5 in at least one of the three sites were diagnosed with osteoporosis. Patients with a T score between –2.5 and –1.0 in at least one of the three sites were diagnosed with osteopenia. Patients with prevalent vertebral fractures and a T score below –2.5 were diagnosed with severe osteoporosis.13 OA of the knee and hip joint was graded using the Kellgren–Lawrence method.14 Patients with Kellgren–Lawrence grade II, III, and IV radiographic findings in the knee and hip joint were regarded as having OA.
u-NTx was measured from a morning urine sample using a chemiluminescent-based method (Ortho ECi, Ortho-Clinical Diagnostics, Raritan, NJ, USA). The coefficient of variation was 2.42%. Alkaline phosphatase was measured in a fasting early morning venous blood sample using an enzymatic method (Hitachi 7600, Hitachi Co., Tokyo, Japan). The coefficient of variation was 2.88%.
Osteocalcin was measured in a fasting morning blood sample by electrochemiluminescence immunoassay (ECLIA) (Modular E170, Roche Diagnostics, Mannheim, Germany). The coefficient of variation was 2.26%. 25-hydroxyvitamin D [25(OH)D] was measured by radioimmunoassay (DiaSorin Inc., Stillwater, OK, USA), and a serum vitamin D level below 20 ng/ml was classified as hypovitaminosis D. A seasonal correction was not made; however, all blood and urine specimens were collected in the morning to correct for diurnal variation.
The demographic data and disease-related variables such as ODI and symptom duration were analyzed by logistic regression analysis. Odds ratio for osteoporosis or osteopenia was evaluated by stepwise multivariate logistic regression with a forward stepwise procedure. All statistical analyses were performed using the SPSS 12.0.1 statistical package (SPSS, Inc., Chicago, IL, USA). A value of P<0.05 was accepted as significant.
Treatment guidelines for osteoporosis were defined as a subject having 1) a T score below –1.5 with positive risk factors for osteoporosis, 2) a T score below –2.0 without risk factors for osteoporosis, and 3) any fragility fractures as cited by the National Osteoporosis Foundation (NOF).15
These NOF-designated risk factors are: (1) history of adult fracture, (2) adult fracture in a first-degree relative, (3) current cigarette smoking, and (4) weight <58 kg.
RESULTS
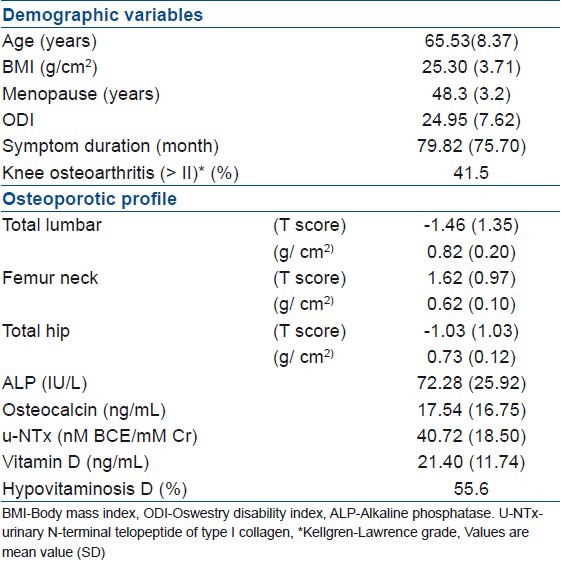
One hundred and six patients with LSS received osteoporotic profile measurements. The study population consisted of postmenopausal female patients with LSS, with mean symptom duration of 79.82 months. The average (SD) of ODI score was 24.95 (7.62) [Table 1]. Forty-four patients (41.5%) had OA of the knee joint (Kellgren–Lawrence grade II≤) and only four patients (3.7%) had hip OA (Kellgren–Lawrence grade II≤). Fifty-nine patients (55.6%) had hypovitaminosis D. All bone turnover markers (alkaline phosphatase, osteocalcin, u-NTx) were found to be within normal range [Table 1].
Table 1.
Demographic, clinical, osteoporotic profile of the 106 patients who have LSS
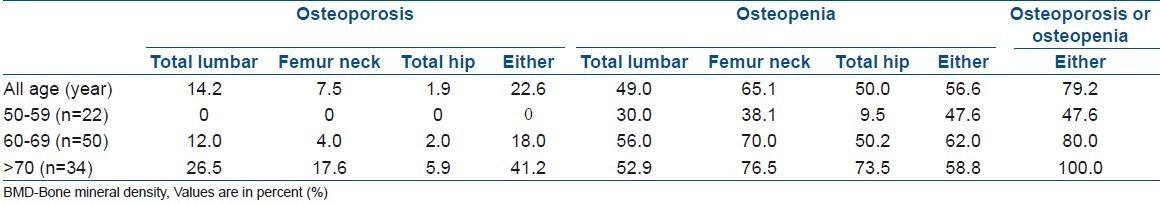
Fifteen patients (14.2%) had osteoporosis in the lumbar spine, and eight patients (7.5%) and two patients (1.9%) had osteoporosis in the femur neck and hip, respectively. Fifty-two patients (49.0%) had osteopenia in the lumbar spine, and 69 patients (65.1%) and 53 patients (50.0%) had osteopenia in the femur neck and hip, respectively. Among the patients with LSS, 24 (22.6%) had osteoporosis and 60 (56.6%) had osteopenia. Overall, 84 patients (79.2%) with symptomatic LSS had osteoporosis or osteopenia. Eight patients (7.5%), who had one or more vertebral fractures and a lumbar and/or hip BMD below a T score of -2.5, were diagnosed with severe osteoporosis. Only 22 patients (20.8%) with LSS showed normal BMD in the spine and hip region [Table 2].
Table 2.
Prevalence of osteoporosis and reduced BMD in the spine and hip
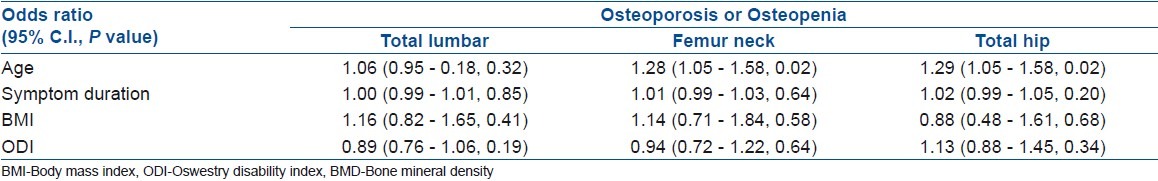
Logistic regression analysis showed that only age was associated with the risk of osteoporosis or osteopenia in the femur neck and total hip. In the lumbar spine, all variables were not associated with osteoporosis or osteopenia. Variables related with spinal stenosis, such as symptom duration of spinal stenosis and ODI, were not associated with the risk of osteoporosis or osteopenia in both the lumbar spine and hip region [Table 3].
Table 3.
Regression analysis between osteoporosis and reduced BMD in the spine and the hip and independent variables
Surveying the risk factors for osteoporosis revealed that 44 patients (41.5%) were indicated for treatment (T score<–1.5 with positive risk factor, T score<–2.0 without risk factor, with fragility fracture).15 According to the guidelines from the Health Insurance Review Agency, only 20 patients (18.8%) qualified for reimbursement for osteoporosis medications. In reality, 18 patients (17.0%) were prescribed for bone active osteoporosis medication. Therefore, there is a significant gap between the osteoporosis treatment indications suggested by NOF and those of the Health Insurance Review Agency. Twenty-four patients (22.6%) with LSS, who were eligible for osteoporosis treatment, were not covered by health insurance due to strict national guidelines.
DISCUSSION
The prevalence of osteoporosis in association with specific disease entities provides valuable information in screening for osteoporosis before definitive treatment of specific diseases. Prevalence of osteoporosis was found to be high in patients with rheumatoid arthritis or organ and cell transplantation or osteoarthritis or in those receiving corticosteroid therapy.16–19 Randomized clinical trials involving postmenopausal women demonstrated that alendronate, risedronate, zoledronate, and teriparatite were effective in reducing the incidence of vertebral and nonvertebral fractures.20–23 OA and degenerative conditions of the lumbar spine are musculoskeletal conditions of the elderly.16,24 Patients with symptomatic OA of the hip, who were scheduled to undergo total hip arthroplasty, demonstrated osteoporosis (T score<–2.5) in 28% and osteopenia (T score<–1.0) in 45% of cases.16 Thus, there is an urgent need to screen patients with LSS for osteoporosis since both the conditions share similar at risk elderly populations and may result in significant morbidity and mortality if not properly treated.
In this cross-sectional study, we focused on LSS which is a common spinal condition occurring at an advanced age – a known risk factor for osteoporosis and osteopenia. The results showed 79.2% of all patients with LSS had osteoporosis or osteopenia (T score below -1.0) and 22.6% of the patients had osteoporosis in either the spine or the hip. Since the prevalence of osteoporosis or osteopenia depends on many population-specific factors such as genetic factors, race, age, and sex, it is important to compare our data on the prevalence of osteoporosis or osteopenia with those of general population in our country. A previous study reported the prevalence of osteoporosis or osteopenia in postmenopausal women over 50 years of age to be up to 61.4% in the general population of our country.25 Therefore, these different results might suggest that the patients with LSS have relatively decreased BMD compared with the general population; however, comparison of this population should be done with caution due to differences in demographic characteristics, especially age. This higher prevalence could be explained by the fact that walking difficulty or physical inactivity due to claudication is associated with decreased BMD. This association between physical inactivity and decreased BMD has been reported in the case of claudication originating from peripheral arterial occlusive disease.26 We also found that patients with symptomatic LSS had increased bone metabolic rate due to physical inactivity and hypovitaminosis D,27 and their bone turnover rate restored after decompressive surgery.28 But we failed to show any correlation between decreased BMD and LSS because of the confusing effect of osteophytosis on BMD.27,28 After surveying the risk factors for osteoporosis, there is a significant gap in treatment guidelines and reimbursement guidelines. Only 20 patients (18.8%) with LSS were covered by the national health insurance for osteoporosis medication. The remaining 24 patients (22.6%) who were eligible for osteoporosis medication were not covered by the national health insurance system. Hence, the patients, health care providers, policy makers, and health care managers should come to a consensus and treat osteoporosis cost-effectively. Furthermore, knowing the prevalence of osteoporosis in the patients with LSS renders valuable clinical information to spinal surgeons (i.e. regarding the postoperative medical treatment of osteoporosis, the decision between instrumented fusion and decompression alone procedure, and the decision to use bone cement augmentation in cases of weak pedicular screw purchase). Although the patients with LSS had relatively higher prevalence of osteoporosis or osteopenia, the disease-related variables such as ODI and symptom duration were not associated with the risk of osteoporosis or osteopenia. This might suggest that ODI or symptom duration cannot reflect the patient's inactivity, and in order to access the amount of influence of physical inactivity caused by LSS on BMD, a precise measure about physical activity is necessary. Hypovitaminosis D is a silent condition that commonly occurs in the elderly population.29,30 A low level of vitamin D is associated with a poor response to bisphosphonate therapy, an increased fracture risk, poor musculoskeletal coordination, and poor muscle tone.31–36 Therefore, the serum vitamin D level and vitamin D supplementation are important diagnostic and therapeutic procedures, respectively, in the elderly population. In this study, 55.6% of patients with LSS demonstrated hypovitaminosis D which mandates supplementation in any form. The effect of vitamin D on musculoskeletal coordination may be an important clue for spine surgeons on how to cope up with postoperative walking disability, poor coordination, and a decrease in skeletal tone after definitive surgery for LSS. Even with sufficient decompression of the spine, some patients with LSS still complain of poor walking capability. Screening for hypovitaminosis D and sufficient supplementation with vitamin D may be an ideal remedy for postoperative poor walking condition. Bone turnover markers are well known tools used to assess the current status of osteoporosis (i.e. dynamic or non-dynamic state, response to antiresorptive treatment, and patients’ compliance with medication). In this study, alkaline phosphatase, osteocalcin, and u-NTx were within normal limits. Bone turnover markers usually have a wide normal range with poor reproducibility and diurnal and/or seasonal variation. Thus, more patients need to be enrolled to find clear, significant correlations between bone turnover markers and other factors (i.e. BMD, severity of LSS). The present study could have benefited from a control group consisting of age-matched women without LSS. This would have allowed us to detect any differences in bone turnover and/or BMD in patients with or without LSS. The strength of our current study is its well selected homogenous patient population with symptomatic LSS. Compared with the data of the general population, the current study suggests the necessity of screening test for BMD in the patients with LSS. In conclusion, LSS could be associated with osteopenia, osteoporosis, and hypovitaminosis D, which should prompt careful screening and treatment in cases of osteoporosis. However a large longitudinal study will be necessary in order to endorse the higher prevalence of osteoporosis in patients with LSS.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States 2005-2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.Haussler B, Gothe H, Gol D, Glaeske G, Pientka L, Felsenberg D. Epidemiology, treatment and costs of osteoporosis in Germany–the BoneEVA Study. Osteoporos Int. 2007;18:77–84. doi: 10.1007/s00198-006-0206-y. [DOI] [PubMed] [Google Scholar]
- 3.Caliri A, De Filippis L, Bagnato GL, Bagnato GF. Osteoporotic fractures: Mortality and quality of life. Panminerva Med. 2007;49:21–7. [PubMed] [Google Scholar]
- 4.Fierens J, Broos PL. Quality of life after hip fracture surgery in the elderly. Acta Chir Belg. 2006;106:393–6. doi: 10.1080/00015458.2006.11679913. [DOI] [PubMed] [Google Scholar]
- 5.Pande I, Scott DL, O’Neill TW, Pritchard C, Woolf AD, Davis MJ. Quality of life, morbidity, and mortality after low trauma hip fracture in men. Ann Rheum Dis. 2006;65:87–92. doi: 10.1136/ard.2004.034611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson CW, Merrilees MJ, Wilkinson TJ, McKie JS, Gilchrist NL. Hip fracture mortality and morbidity-can we do better? N Z Med J. 2001;114:329–32. [PubMed] [Google Scholar]
- 7.Kaptan H, Kasimcan O, Cakiroglu K, Ilhan MN, Kilic C. Lumbar spinal stenosis in elderly patients. Ann N Y Acad Sci. 2007;1100:173–8. doi: 10.1196/annals.1395.015. [DOI] [PubMed] [Google Scholar]
- 8.Szpalski M, Gunzburg R. Lumbar spinal stenosis in the elderly: An overview. Eur Spine J. 2003;12(Suppl 2):S170–5. doi: 10.1007/s00586-003-0612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delmas PD, van de Langerijt L, Watts NB, Eastell R, Genant H, Grauer A, et al. Underdiagnosis of vertebral fractures is a worldwide problem: The IMPACT study. J Bone Miner Res. 2005;20:557–63. doi: 10.1359/JBMR.041214. [DOI] [PubMed] [Google Scholar]
- 10.Makinen TJ, Alm JJ, Laine H, Svedstrom E, Aro HT. The incidence of osteopenia and osteoporosis in women with hip osteoarthritis scheduled for cementless total joint replacement. Bone. 2007;40:1041–7. doi: 10.1016/j.bone.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Verbiest H. The significance and principles of computerized axial tomography in idiopathic developmental stenosis of the bony lumbar vertebral canal. Spine (Phila Pa 1976) 1979;4:369–78. doi: 10.1097/00007632-197907000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Skedros JG, Sybrowsky CL, Stoddard GJ. The osteoporosis self-assessment screening tool: A useful tool for the orthopaedic surgeon. J Bone Joint Surg Am. 2007;89:765–72. doi: 10.2106/JBJS.F.00347. [DOI] [PubMed] [Google Scholar]
- 13.National Osteoporosis Foundation. Osteoporosis: Review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Executive summary. Osteoporos Int. 1998;8(Suppl 4):S3–6. [PubMed] [Google Scholar]
- 14.Kellgren JH. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;32:1951–7. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Physician's Guide to Prevention and Treatment of Osteoporosis ed. Belle Mead, NJ: Excerpta Medica Inc; 1999. National Osteoporosis Foundation. [Google Scholar]
- 16.Westesson PL, Lee RK, Ketkar MA, Lin EP. Underdiagnosis and undertreatment of osteoporosis. Lancet. 2002;360:1891. doi: 10.1016/S0140-6736(02)11763-6. [DOI] [PubMed] [Google Scholar]
- 17.Sinigaglia L, Varenna M, Girasole G, Bianchi G. Epidemiology of osteoporosis in rheumatic diseases. Rheum Dis Clin North Am. 2006;32:631–58. doi: 10.1016/j.rdc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Peshin R, Phelan M. A national survey to assess investigation and management protocols followed in dealing with steroid-induced osteoporosis. Clin Rheumatol. 2007;26:1439–43. doi: 10.1007/s10067-006-0516-7. [DOI] [PubMed] [Google Scholar]
- 19.Savani BN, Donohue T, Kozanas E, Shenoy A, Singh AK, Childs RW, et al. Increased risk of bone loss without fracture risk in long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:517–20. doi: 10.1016/j.bbmt.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 20.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, et al. Fracture risk reduction with alendronate in women with osteoporosis: The Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118–24. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- 21.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 22.Li EC, Davis LE. Zoledronic acid: A new parenteral bisphosphonate. Clin Ther. 2003;25:2669–708. doi: 10.1016/s0149-2918(03)80327-2. [DOI] [PubMed] [Google Scholar]
- 23.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 24.Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. Prevalence and impact of osteoarthritis and osteoporosis on health-related quality of life among active subjects. Aging Clin Exp Res. 2007;19:55–60. doi: 10.1007/BF03325211. [DOI] [PubMed] [Google Scholar]
- 25.Lee WS, Park HM, Bae DH. Prevalence of osteoporosis in korean women. J Korean Soc Menopause. 2003;9:339–46. [Google Scholar]
- 26.Fahrleitner-Pammer A, Obernosterer A, Pilger E, Dobnig H, Dimai HP, Leb G, et al. Hypovitaminosis D, impaired bone turnover and low bone mass are common in patients with peripheral arterial disease. Osteoporos Int. 2005;16:319–24. doi: 10.1007/s00198-004-1693-3. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Lee HM, Kim HS, Park JO, Moon ES, Park H, et al. Bone metabolism in postmenopausal women with lumbar spinal stenosis: Analysis of bone mineral density and bone turnover markers. Spine (Phila Pa 1976) 2008;33:2435–9. doi: 10.1097/BRS.0b013e3181829fca. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Lee HM, Chun HJ, Kang KT, Kim HS, Park JO, et al. Restoration of bone turnover rate after decompression surgery in patients with symptomatic lumbar spinal stenosis: Preliminary report. Spine (Phila Pa 1976) 2009;34:E635–9. doi: 10.1097/BRS.0b013e3181ab3e88. [DOI] [PubMed] [Google Scholar]
- 29.Lata PF, Elliott ME. Patient assessment in the diagnosis, prevention, and treatment of osteoporosis. Nutr Clin Pract. 2007;22:261–75. doi: 10.1177/0115426507022003261. [DOI] [PubMed] [Google Scholar]
- 30.Prince RL. Calcium and vitamin D - for whom and when. Menopause Int. 2007;13:35–7. doi: 10.1258/175404507780456755. [DOI] [PubMed] [Google Scholar]
- 31.Shinchuk L, Holick MF. Vitamin d and rehabilitation: Improving functional outcomes. Nutr Clin Pract. 2007;22:297–304. doi: 10.1177/0115426507022003297. [DOI] [PubMed] [Google Scholar]
- 32.Deane A, Constancio L, Fogelman I, Hampson G. The impact of vitamin D status on changes in bone mineral density during treatment with bisphosphonates and after discontinuation following long-term use in post-menopausal osteoporosis. BMC Musculoskelet Disord. 2007;8:3. doi: 10.1186/1471-2474-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujita T, Nakamura S, Ohue M, Fujii Y, Miyauchi A, Takagi Y, et al. Postural stabilizing effect of alfacalcidol and active absorbable algal calcium (AAA Ca) compared with calcium carbonate assessed by computerized posturography. J Bone Miner Metab. 2007;25:68–73. doi: 10.1007/s00774-006-0729-5. [DOI] [PubMed] [Google Scholar]
- 34.Boonen S, Bischoff-Ferrari HA, Cooper C, Lips P, Ljunggren O, Meunier PJ, et al. Addressing the musculoskeletal components of fracture risk with calcium and vitamin D: A review of the evidence. Calcif Tissue Int. 2006;78:257–70. doi: 10.1007/s00223-005-0009-8. [DOI] [PubMed] [Google Scholar]
- 35.Gaugris S, Heaney RP, Boonen S, Kurth H, Bentkover JD, Sen SS. Vitamin D inadequacy among post-menopausal women: A systematic review. Qjm. 2005;98:667–76. doi: 10.1093/qjmed/hci096. [DOI] [PubMed] [Google Scholar]
- 36.Fain O. Musculoskeletal manifestations of scurvy. Joint Bone Spine. 2005;72:124–8. doi: 10.1016/j.jbspin.2004.01.007. [DOI] [PubMed] [Google Scholar]


