Abstract
Mucormycosis is one among the aggressive, invasive fungal infections usually seen in immunocompromised patients. Mucormycosis osteomyelitis is very rare. We present a patient with acute myeloid leukemia who complained of pain over the right proximal thigh. Plain radiograph revealed ill defined osteolytic lesion of proximal femur. MRI showed altered signal in proximal femur with focal collection and cortical breach. Biopsy and tissue culture diagnosed mucormycosis both histologically and microbiologically. He was treated with aggressive debridement, skeletal stabilization, and amphotericin antifungal cement beads. He recovered with no residual pain, minimal limb shortening, and no clinical or radiological evidence of recurrence at 3 years followup. The high index of suspicion, early diagnosis, aggressive surgical debridement, and adequate antifungal therapy play a significant role in the treatment of musculoskeletal mucormycosis.
Keywords: Skeletal mucormycosis, amphotericin B, cement beads
INTRODUCTION
Mucormycosis is an aggressive invasive opportunistic fungal infection belonging to the order mucorales, usually found in immunocompromised individuals. It is a life and limb threatening fungal infection with high mortality rate of 40%.1 Though mucormycosis presents as a spectrum of disease, it is very rare in the musculoskeletal system. Almost all the immunocompromised patients with osteomyelitis of long bones due to mucormycosis require aggressive debridement and may sometimes need amputation.2 We present a patient with acute myeloid leukemia, who was diagnosed to have mucormycosis osteomyelitis of right proximal femur and was treated with limb salvage surgery successfully.
CASE REPORT
A 41-year-old gentleman presented with sudden onset of pain and swelling over his right groin and upper thigh for a period of 10 days. He was a known patient with Type II diabetes mellitus and acute myeloid leukemia M2 diagnosed on the basis of bone marrow morphology and immunophenotyping 2 years ago. He received induction chemotherapy with cytosine and daunorubicin, followed by consolidation chemotherapy and peripheral autologous bone marrow transplant. Fourteen months post bone marrow transplantation, he had remission and developed the above complaints. Examination revealed mild tenderness over the right proximal femur with no significant restriction of movements. Plain radiographs of the right proximal femur and hip joint were normal. Magnetic resonance imaging (MRI) of the right hip revealed altered signal intensity in the right proximal femur with no cortical breach or evidence of abscess. There was edema in the surrounding muscles, especially the illiopsoas. He was treated conservatively with analgesics for the same.
His complaints gradually increased in the next 3 weeks and he had difficulty in weight bearing and walking. On clinical examination, he had severe tenderness over the right proximal femur with painful restriction of movements at the hip joint. Plain radiographs revealed a 6 × 3 cm, ill-defined osteolytic lesion in the proximal shaft of right femur extending into the greater trochanter with wide zone of transition and adjacent soft tissue swelling [Figure 1a]. MRI showed altered signal intensity in proximal metadiaphysis of right femur, including greater trochanter, with focal collection of 5 × 2.6 cm with cortical breach. There were two small abscesses measuring 1.5 and 0.7 cm, respectively, in the soft tissues [Figure 1b].
Figure 1.
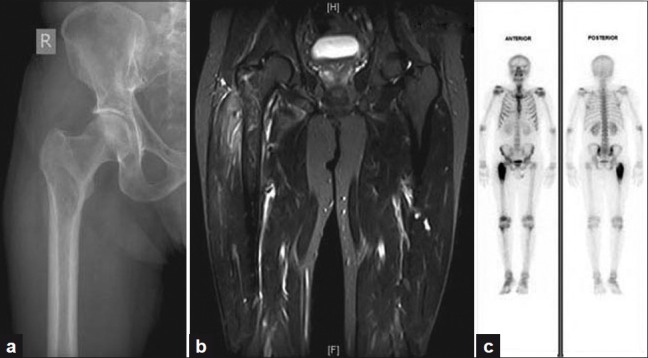
(a) Plain radiograph revealing an osteolytic lesion involving the right proximal femur (b) MRI showing altered signal intensity in the proximal metadiaphysis of right femur with abscesses and cortical breach (c) Bone scan demonstrating increased tracer uptake in the metadiaphyseal region of right proximal femur
Bone scintigraphy with Technetium 99 m methylene diphosphonate (Tc 99 m MDP) demonstrated intense increase in tracer uptake in the proximal third of right femur with normal tracer distribution in rest of the skeleton, suggestive of an infective pathology [Figure 1c].
An open biopsy of the right proximal femur was done. Intraoperatively, black discoloration of the right proximal femoral metaphysis with foul smelling discharge was noticed. The tissue was smeared with 10% KOH solution and calcofluor white solution initially and then cultured in Sabraoud's Dextrose Agar (SDA) and lactophenol cotton blue (LPCB) mount which revealed broad aseptate fungal hyphae suggestive of mucormycosis. Histopathology of the cancellous bone and soft tissue exhibited large areas of necrosis and hemorrhage, replaced by inflammatory granulation tissue with dense infiltrates of lymphocytes, plasma cells, histiocytes, neutrophils, and few multinucleate giant cells. There were many broad, twisted, and few branching fungal filaments suggestive of mucormycosis [Figure 2].
Figure 2.
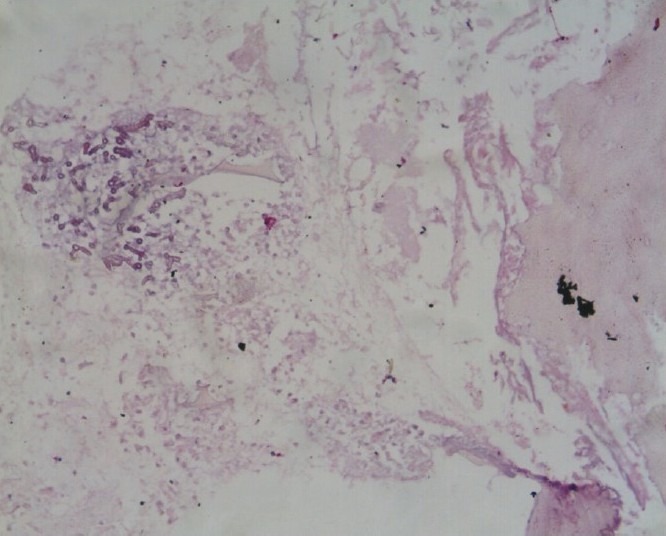
Histopathology with broad, twisted, and few branching fungal elements
After the diagnosis of mucormycosis was confirmed, patient was evaluated for primary infection. He had no cutaneous lesions. ENT evaluation ruled out infection of nasal sinuses. Computed tomography scan of paranasal sinus did not reveal any signs of active infection. There was no history of gastrointestinal infections and previous biopsy from gastric mucosa was negative. There were no pre-existing wounds or deterioration of the same. Hence, primary source of infection could not be traced.
In the immediate postoperative period, the patient developed pathological fracture at the same site and was put on skeletal traction for 19 days before definitive fixation. He was treated with liposomal amphotericin 3 mg/kg/day for 19 days, following which radical debridement of the lesion proceeded by skeletal stabilization with 95° condylar blade plate for the right proximal femur. Considering the location of the pathological fracture (proximal femur) and the need for aggressive debridement and stable fixation, a 95° condylar blade plate fixation was achieved after adequate debridement. To prevent the usual complications of external fixation instead of infection such as pin tract infection, difficulty in mobilization and also considering adequate preoperative antifungal therapy and aggressive debridement, internal fixation was decided.
Surgery was supplemented with amphotericin mixed with polymethylmethacrylate (PMMA) beads. 300 mg of fungitericin (amphotericin B) in powdered form was mixed with 40 g of simplex cement polymer and the mixture was then added with monomer, and while setting, cement beads were made into chains using stainless steel wire (two strings with 15 beads each) [Figure 3a].
Figure 3.
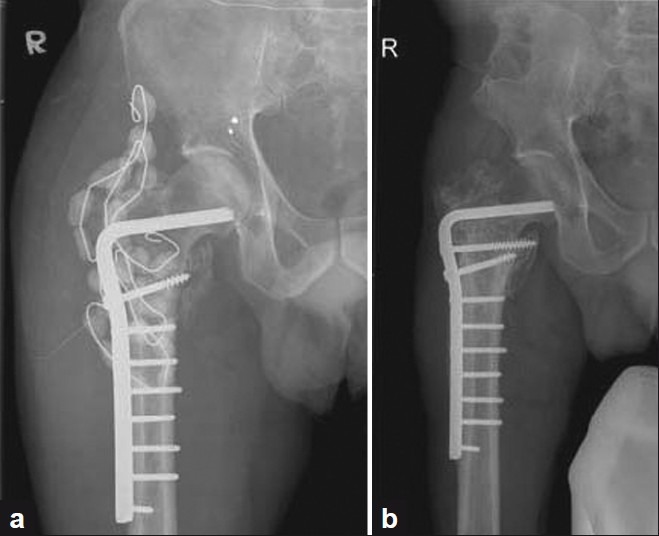
(a) Postoperative plain radiograph with proximal femur stabilized with condylar blade plate and augmented with antifungal cement beads (b) Three years followup plain radiograph with no evidence of osseous lesion with abundant callus
Intraoperatively, he had torrential blood loss amounting to 2.5–3 litres and went into hypovolemic shock. He was resuscitated with five units of packed red cells, eight units of cryoprecipitate, and eight units of fresh frozen plasma. Radical debridement, inability to use the tourniquet, and difficulty in differentiating infected and uninfected marrow were the causes for unexpected blood loss. After aggressive debridement, bone to bone cortical contact was achieved for healing which led to loss of metaphyseal bone loss of 3 cm.
Postoperatively, liposomal amphotericin 3 mg/kg/day (180 mg/day) was given for 3 weeks and downgraded to 1 mg/kg/day (60 mg/day) for the next 9 weeks. Patient had received a total dosage of 7560 mg of liposomal amphotericin for a total period of 12 weeks. He was advised postoperatively physiotherapy and toe-touch weight bearing crutch walking for 12 weeks. The beads were removed 12 weeks after bead insertion. Bone grafting was done along with beads removal and the tissue sent during surgery for histopathology and microbiological review was negative for mucormycosis.
At 3-year followup, there was 3 cm limb shortening noticed for which a shoe raise was given. Plain radiographs revealed healed fracture with no residual/recurrent lesion in the metadiaphysis region of right proximal femur [Figure 3b].
DISCUSSION
Mucormycosis is one among the opportunistic fungal infections which occur in immunocompromised patients. The common predisposing factors are uncontrolled diabetes, hematological malignancies like leukemia, lymphoma, etc., organ transplantation, severe burns, diseases like tuberculosis and AIDS, post bone marrow transplantation, neutropenia, renal failure, immunosuppressive medications, etc. Disseminated mucormycosis spreads to the reticuloendothelial system and rarely to the musculoskeletal system. Mucormycosis has been described in various sites like cranium, hands, and feet, humerus, tibiae, femur, vertebrae, and joints.1
The etiopathogenesis of mucormycosis follows after entry into the host via ingestion, inoculation, or inhalation. In the immunocompromised host, the spores undergo angioinvasion leading to local necrosis and necrotizing infection, ultimately leading to systemic inflammatory response syndrome, multiorgan dysfunction, and death.
Treatment modalities include both surgical and medical management in the same order. Surgery includes radical debridement, mostly amputation, followed by high doses of amphotericin B.
Role of antifungal cement beads is limited mainly due to its cement elution properties and high local concentration leading to toxicity. Multiple in vitro studies have reported amphotericin B impregnated PMMA beads to have a successful role in the management of fungal infections.3–7 Goss et al. in their study on elution and mechanical properties of amphotericin concluded that amphotericin was released from cement at a clinically insignificant level.8 In vitro studies on Amphotericin beads have shown to provide adequate release of its concentration (1.75-2.0 microgm/ml) up to 110 days from all bone cements.3 Excessive local concentration of amphotericin B above 100 microgm/ml is lethal and concentration between 5-10microgm/ml causes abnormal morphology and reduced proliferation.4
Of the seven cases of mucormycosis of tibia described in the literature, five were immunocompromised and 60% of those patients underwent amputation.9 The causes of amputation may be related to delayed diagnosis of mucormycosis, compromised immune status, inadequate debridement, or inadequate antifungal therapy.
We present this case in view of the unusual site of presentation, limb-threatening osteomyelitis which was treated with radical debridement, liposomal amphotericin therapy, and using antifungal beads to enable limb salvage.
CONCLUSION
High index of clinical suspicion, early diagnosis, aggressive debridement, and adequate liposomal amphotericin B are the key treatment modalities in the successful management of musculoskeletal mucormycosis. The role of amphotericin antifungal beads needs to be studied in detail.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Bouza E, Munoz P, Guinea J. Mucormycosis: An emerging disease? Clin Microbiol Infect. 2006;12:7–23. [Google Scholar]
- 2.Miller-Crotchett P, Reed RL, 2nd, Johnson PC, Fischer RP. Mucormycosis in trauma patients. Cocanour CS. J Trauma. 1992;32:12–5. doi: 10.1097/00005373-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Sealy PI, Nguyen C, Tucci M, Benghuzzi H, Cleary JD. Delivery of antifungal agents using bioactive and nonbioactive bone cements. Ann Pharmacother. 2009;43:1606–15. doi: 10.1345/aph.1M143. [DOI] [PubMed] [Google Scholar]
- 4.Harmsen S, McLaren AC, Pauken C, McLemore R. Amphotericin B is cytotoxic at locally delivered concentrations. Clin Orthop Related Res. 2011;469:3016–21. doi: 10.1007/s11999-011-1890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverberg D, Kodali P, Dipersio J, Acus R, Askew M. In vitro analysis of antifungal impregnated polymethylmethacrylate bone cement. Clin Orthop Relat Res. 2002;403:228–31. doi: 10.1097/00003086-200210000-00033. [DOI] [PubMed] [Google Scholar]
- 6.Kweon C, McLaren AC, Leon C, McLemore R. Amphotericin B delivery from bone cement increases with porosity but strength decreases. Clin Orthop Relat Res. 2011;469:3002–7. doi: 10.1007/s11999-011-1928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marra F, Robbins GM, Masri BA, Duncan C, Wasan KM, Kwong EH, et al. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can J Surg. 2001;44:383–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Goss B, Lutton C, Weinrauch P, Jabur M, Gillett G, Crawford R. Elution and mechanical properties of antifungal bone cement. J Arthroplasty. 2007;22:902–8. doi: 10.1016/j.arth.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Holtom PD, Obuch AB, Ahlmann ER, Shepherd LE, Patzakis MJ. Mucormycosis of the Tibia: A case report and review of the Literature. Clin Orthop Relat Res. 2000;381:222–8. [PubMed] [Google Scholar]


