Abstract
Protein phosphatase 2B (PP2B) is one of the major brain phosphatases and can dephosphorylate tau at several phosphorylation sites in vitro. Previous studies that measured PP2B activity in human brain crude extracts showed that PP2B activity was either unchanged or decreased in AD brain. These results led to the speculation that PP2B might regulate tau phosphorylation and that a down-regulation of PP2B might contribute to abnormal hyperphosphorylation of tau. In this study, we immunoprecipitated PP2B from brains of six AD subjects and seven postmortem- and age-matched controls and then measured the phosphatase activity. We found a three-fold increase in PP2B activity in AD brain as compared with control brains. The activation was due to the partial cleavage of PP2B by calpain I that was activated in AD brain. The truncation of PP2B appeared to alter its intracellular distribution in the brain. In human brains, PP2B activity correlated positively, rather than negatively, to the levels of tau phosphorylation at several sites that can be dephosphorylated by PP2B in vitro. Truncation of PP2B in the frontal cortex was more than in the temporal cortex, and tau phosphorylation was also more in the frontal cortex. Taken together, these results indicate that truncation of PP2B by calpain I elevates its activity but does not counteract the abnormal hyperphosphorylation tau in AD brain.
Keywords: Alzheimer's disease, calpain, hyperphosphorylation, protein phosphatase 2B, tau
INTRODUCTION
Neurofibrillary degeneration characterized by the intracellular deposition of abnormally hyperphosphorylated tau as neurofibrillary tangles (NFTs) correlates directly to the severity of dementia symptoms [1,2] and therefore is crucial to the pathogenesis of Alzheimer's disease (AD). The abnormal hyperphosphorylation of tau appears to be responsible for the loss of its biological activity, gain of its toxicity, and aggregation into NFTs [3,4]. In order to understand the mechanism by which tau becomes abnormally hyperphosphorylated in AD brain, many studies have focused on identification of tau phosphatases, which regulate tau phosphorylation level, and their dysregulation. Among the major brain protein phosphatases (PP), PP1, PP2A, PP2B, and PP5, but not PP2C, have been shown to dephosphorylate the abnormal hyperphosphorylated tau in vitro [5-13]. PP2A and PP5 have also been found to be down-regulated in AD brain [14-19], which probably partially contributes to the abnormal hyperphosphorylation of tau. Studies of whether PP2B is dysregulated in AD brain yielded inconsistent results. When using 32p-labelled phosphorylase kinase as a protein substrate for measuring PP2B activity in brain extracts, we previously found no difference in the activity between AD and control brains [14]. However, by using p-nitrophenyl phosphate (pNPP) as a substrate for assaying brain extract PP activity, it was reported that the nickel- and manganese-stimulated PP activity (that mainly represents PP2B activity) is decreased in some areas of AD brain as compared with controls [20,21]. Therefore, whether PP2B is dysregulated and whether the dysregulation contributes to the abnormal hyperphosphorylation of tau require further investigation.
PP2B, also known as calcineurin in the brain, is a Ca2+/calmodulin-dependent protein phosphatase. The holoenzyme is a heterodimer, consisting of a 60-kDa catalytic subunit (A subunit) and an 18-kDa regulatory subunit (B subunit) [22]. The catalytic subunit has a calmodulin-binding domain and an autoinhibitory domain in the C-terminal part, which normally mask the catalytic domain and keep the enzyme inactive. PP2B is activated by binding of Ca2+/calmodulin to the calmodulin-binding domain, which triggers the release of the autoinhibitory domain from the catalytic site [23,24]. PP2B can also be activated by proteolytic cleavage of the autoinhibitory domains, resulting in a Ca2+/calmodulin-independent, active phosphatase [23,25,26]. It has been reported that calpain I, a major Ca2+-activated protease in the brain, cleaves and activates PP2B [24,26,27].
To elucidate whether dysregulated PP2B has an impact on the abnormal hyperphosphorylation of tau in AD brain, and if so, how, we immunoprecipitated the catalytic subunit of PP2B from AD and control brains and then measured the phosphatase activity toward phospho-tau. We found that PP2B was activated due to cleavage by activated calpain I in AD brain. The truncated PP2B was more active than the full-length PP2B. Truncation of PP2B did not correlate to tau phosphorylation at any phosphorylation sites studied in human brains.
MATERIALS AND METHODS
Materials
The longest isoform of human tau (tau441) and cyclin-dependent kinase 5 and its activator p25 (cdk5/p25) were cloned, expressed, and purified as described previously [28,29]. The catalytic subunit of cAMP-dependent protein kinase (PKA) and calpain I were purchased from Sigma (St. Louis, MO). PP2B was purified as described previously [18]. Protein G-agarose bead was purchased from Pierce (Rockford, IL). Monoclonal antibodies against catalytic subunit of PP2B and calpain I were from Sigma (St. Louis, MO). Polyclonal antibody against catalytic subunit of PP2B was raised in rabbits as described previously [30]. Peroxidase-conjugated anti-mouse and anti-rabbit IgG were from Jackson ImmunoResearch Labratories (West Grove, PA). ECL kit was from Amersham Pharmacia Biotech (Piscataway, NJ). N-acetyl-Leu-Leu-Nle-CHO (ALLN) was obtained from Calbiochem (La Jolla, CA). [γ-32P]ATP was purchased from ICN Biomedicals (Costa Mesa, CA). Bradford protein assay reagent was from Bio-Rad Laboratories, Inc. (Hercules, CA).
Brain tissue
The medial temporal or frontal cortices of six AD and seven age-matched normal human brains (Table 1) used for this study were obtained from the Sun Health Research Institute Donation Program (Sun City, AZ). All brain samples were pathologically confirmed and stored at -70°C until used. The use of frozen human brain tissue was in accordance with the U.S. National Institutes of Health guidelines and approved by our institutional review board.
Table 1.
Human brain tissue used in this study
| Case | Age at death (years) | Gender | PMIa (h) | Braak stageb | Tangle scorec |
|---|---|---|---|---|---|
| AD 1 | 89 | F | 3 | V | 14.5 |
| AD 2 | 80 | F | 2.25 | VI | 14.5 |
| AD 3 | 78 | F | 1.83 | VI | 15.0 |
| AD 4 | 95 | F | 3.16 | VI | 10.0 |
| AD 5 | 86 | M | 2.25 | VI | 13.5 |
| AD 6 | 91 | F | 3 | V | 8.50 |
| Mean ± SD | 86.5 ± 6.5 | 2.58 ± 0.54 | 12.67 ± 2.73 | ||
| Con 1 | 85 | M | 25 | II | 4.25 |
| Con 2 | 86 | F | 2.5 | III | 5.00 |
| Con 3 | 81 | M | 2.75 | III | 6.41 |
| Con 4 | 88 | F | 3 | II | 2.00 |
| Con 5 | 90 | F | 3 | III | 4.50 |
| Con 6 | 88 | F | 3.5 | III | 2.50 |
| Con 7 | 88 | F | 3 | IV | 4.50 |
| Mean ± SD | 86.6 ± 2.9 | 2.89 ± 0.39 | 4.17 ± 1.50 |
PMI, postmortem interval.
Neurofibrillary pathology was staged according to Braak and Braak (1995).
Tangle score is a density estimate and was designated as none, sparse, moderate, or frequent (0, 1, 2, or 3, respectively, for statistics), as defined according to CERAD AD criteria (Mirra et al. 1991). Five areas (frontal, temporal, parietal, hippocampal, and entorhinal) were examined, and the scores were added up for a maximum of 15.
Immunoprecipitation of PP2B catalytic subunit
Human brain tissue was homogenized with 9×vol. of buffer containing 50 mM Tris-HCl, pH 7.0, 8.5% sucrose, 10 mM β-mercaptoethanol, 2 mM EDTA, 2 mM Benzamidine, and 2.0 μg/ml each of aprotinin, leupeptin, and pepstatinin. The 16,000μg extracts were prepared from the homogenates, and the protein concentrations were measured by the Bradford method [31]. The extracts were then incubated with monoclonal antibody against the catalytic subunit of PP2B, which was pre-coupled to protein G-agarose beads for 4 h at 4°C. The negative control was prepared with protein G-agarose beads without pre-coupling with the antibody. The immunoprecipitated complex was washed with Tris-buffered saline three times and with 50 mM Tris-HCl (pH 7.4) twice, and then used for Western blots and PP2B activity assays. The success of the immunoprecipitation was examined by Western blot analyses, as described [18].
Preparation of phosphorylated tau (32P-tau)
Recombinant human brain tau441 was phosphorylated in vitro with PKA and cdk5, as described previously [18]. Under these conditions, ~3 moles of phosphates were incorporated to each mole of tau441, and many sites were phosphorylated.
PP2B activity assays
PP2B activity was assayed in a reaction mixture (20 μl) containing 50 mM Tris-HCl, pH 7.4, 10 mM β-mercaptoethanol, 0.1 mg/ml 32P-tau, 1.5 mM CaCl2, 1.5 μM CaM, and immunoprecipitated PP2B complex. When crude brain extracts were used for PP2B activity assays, 100 nM okadaic acid was included in the reaction mixture to inhibit other major brain phosphatases, including PP1, PP2A, and PP5. After incubation at 30°C for 20 min, the reaction was terminated, and the released 32Pi was determined by Cerenkov counting after separation from 32P-tau by ascending paper chromatography, as described previously [32].
Immuno-dot-blot assays
The levels of PP2B catalytic subunit and tau phosphorylation at specific sites in the brain extracts were determined by immuno-dot-blot assays using monoclonal antibody to PP2B catalytic subunit and to phosphorylated tau at specific sites as primary antibody, as described previously [33].
Determination of tau phosphorylation at various phosphorylation sites
Human brain tissue was homogenized with buffer containing 50 mM Tris-HCl, pH 7.0, 8.5% sucrose, 10 mM β-mercaptoethanol, 2 mM EDTA, 50 mM NaF, 100 mM acetyl-glucosamine, 2 mM benzamidine, and 2.0 μg/ml each of aprotinin, leupeptin, and pepstatin. The phosphorylation state of tau in these homogenate samples was analyzed by Western blotting, as described previously [34].
Transfection of HEK293T cells and subcellular fractionation
HEK293T cells were cultured and transiently transfected with pCDNA3.1/PP2B60 or pCDNA3.1/PP2B57 by using FuGENE 6 (Roche), according to the manufacturer's instructions. The cells were harvested and lysed in SDS-PAGE sample buffer 48 h after transfection, and the lysates were analyzed by Western blots.
For subcellular fractionation, cells after 48-h transfection were homogenized in the buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, 10 mM β-mercaptoethanol, 50 mM NaF, 1 mM NaVO4, and 2.0 μg/ml each of aprotinin, leupeptin and pepstatin. After being centrifuged at 900 g for 10 min, the pellet was homogenized in the same buffer and centrifuged again. The pellets (nuclear fraction) and the combined supernatants (cytosolic fraction) were subjected to Western blots.
Localization of PP2B and truncated PP2B
HeLa cells were transfected with pCDNA3.1/PP2B60 or pCDNA3.1/PP2B57, as described above. After 48-h transfection, the cells were washed with PBS and fixed with 4% paraformaldehyde in PBS for 30 min at room temperature. After washing with PBS, the cells were blocked with 10% goat serum in 0.2% Triton X-100-PBS for 2 h at 37°C and incubated with mouse anti-myc antibody (1:1000) overnight at 4°C. The cells were then washed and incubated for 1 h with secondary antibodies (FITC-conjugated goat anti-mouse IgG, 1:200), and then incubated with 5 μg/ml Hoechst for 15 min at room temperature. After washing with PBS, the cells were mounted with Fluoromount-G and observed with a Leica TCS-SP2 laser-scanning confocal microscope.
Immunohistochemistry
Human brain tissue was first fixed in 10% phosphate-buffered, saline-buffered formalin and embedded in paraffin. Thin (6-μm-thick) sections were cut, and immunohistochemical staining was carried out by using the avidin–biotin-peroxidase complex system (Vector Labs, Inc., Burlingame, CA, USA) and visualized by diaminobenzidine staining.
RESULTS
PP2B activity is increased in AD brain
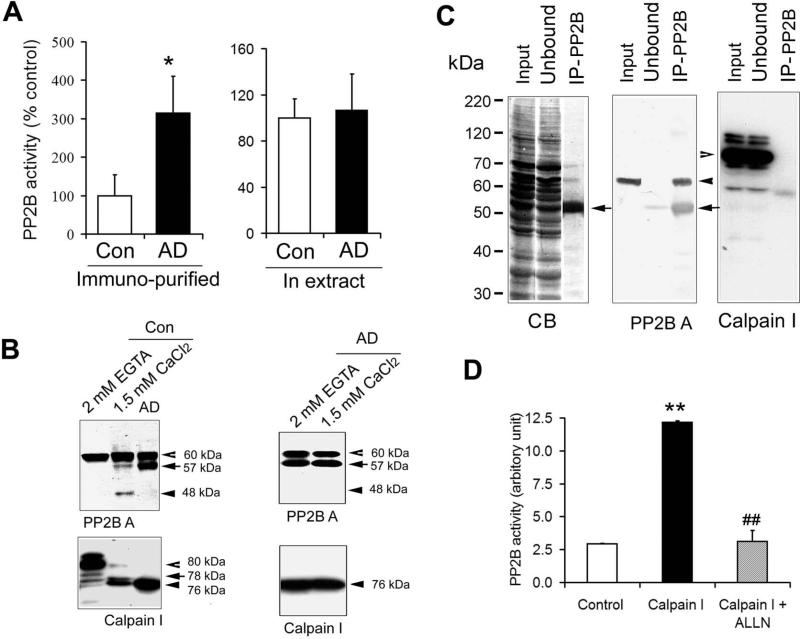
The previously reported inconsistent PP2B activity in AD brain might result from the limited specificity of the assay, because the substrates used were not specific. To overcome this potential weakness, we immunoprecipitated PP2B catalytic subunit from human brain crude extracts with monoclonal anti-CaNA antibody and then determined the phosphatase activity toward 32P-tau as a substrate. Western blots of the unbound fraction and the immunocomplex indicated that the monoclonal antibody precipitated PP2B completely and did not co-precipitate PP1, PP2A, or PP5 [18]. To our surprise, we found that PP2B activity was three-fold higher in AD brain than in control brain (Fig. 1A).
Fig. 1.
Truncation and activation of PP2B by calpain I in Alzheimer's brain. (A) PP2B activity assayed using immunoprecipitated PP2B (left) or crude extracts of the temporal cortexes from six AD and seven control brains. The assay was carried out by using 32P-tau as a substrate and in the presence of 1.5 mM Ca2+ and 1.5 μM calmodulin. (B) Western blots of the PP2B assay mixtures of crude extracts after incubation in the presence or absence of Ca2+/calmodulin (CaM) for 20 min. Control incubation was carried out in the presence of 2.0 mM EGTA to chelate endogenous Ca2+. The blots were developed with antibodies to the catalytic subunit of PP2B (PP2B A) and calpain I, respectively. (C) PP2B A was immunoprecipitated from control brain extracts. The original extract (Input), the unbound, and the immunoprecipitate (IP) were then analyzed by SDS-PAGE stained with Coomassie blue (CB) and Western blots developed with antibodies to PP2B A or calpain I. Closed arrowhead indicates PP2B catalytic subunit. Open arrowhead indicates calpain I band. Closed arrows indicate the heavy chain of IgG. (D) The phosphatase activity of the immunoprecipitated PP2B from control brain extracts was also assayed in the presence of 2.14 μM calpain I alone or together with 20 μM ALLN. **, p<0.01 vs. Control; ##, p<0.01 vs. Calpain I.
To understand why the previous studies, in which crude brain extracts were used directly for PP2B activity assays, did not observe such an increase in PP2B activity in AD brain [14,20,21], we also assayed PP2B activity of the human brain extracts without immunoprecipitation and found no difference in PP2B activity toward 32P -tau between AD and control extract samples (Fig. 1A). Thus, we examined PP2B in the assay mixture after 20 min of assay reaction and found that the catalytic subunit of PP2B (PP2B A) in the control brain extracts was partially cleaved into 57-kDa and 48-kDa truncated forms (Fig. 1B, left) after incubation with Ca2+/calmodulin that were included in the reaction mixtures of the present and the previous studies. Removal of Ca2+/calmodulin from the reaction mixture eliminated the partial cleavage. In AD brain, the PP2B catalytic subunit was already partially cleared to the 57-kDa form, and no further cleavage was seen during incubation in the assay mixture (Fig. 1B, right). These results suggest that when brain extracts were used for assaying PP2B activity, the PP2B A in the control brains may be activated by partial cleavage to the level seen in AD brain during activity assay incubation.
It has been reported that the catalytic subunit of PP2B can be cleaved and activated by calpain I [24,26,27] and that calpain I is over-activated in AD brains [35,27]. Thus, we examined the truncation of calpain I that is present in the control brain extracts during assay incubation and found that it was indeed cleaved and activated to a similar level as in AD brain (Fig. 1B, lower panel). Exclusion of Ca2+ with EGTA from the incubation mixture eliminated the cleavage of both PP2B and calpain I during incubation (Fig. 1B, the first lane of the left panel).
In contrast, when the immunoprecipitated PP2B catalytic subunit was used for the activity assay, PP2B from control brains was not truncated, because of the lack of calpain I in the assay mixture (Fig. 1C). To confirm the partial cleavage and activation of PP2B-A by calpain I, we studied the in vitro proteolysis and activation of PP2B with calpain I. We first immunoprecipitated PP2B from control human brain extracts to eliminate calpain I (Fig. 1C). Incubation of the immunoprecipitated PP2B in the phosphatase assay buffer, in which calpain I and Ca2+/calmomodulin were also added, resulted in a three-fold activation of the phosphatase activity, and this activation was prohibited when ALLN, a calpain inhibitor, was also included (Fig. 1D). These results suggest that when normal brain extracts were used for PP2B assay, the addition of Ca2+ in the PP2B assay mixture activated endogenous calpain I, which in turn, cleaved and activated PP2B to the same level as seen in AD brain extracts. In AD brain, truncation of PP2B to 57 kDa by calpain I elevated its activity.
The truncated PP2B has more phosphatase activity than full-length PP2B in cultured cells
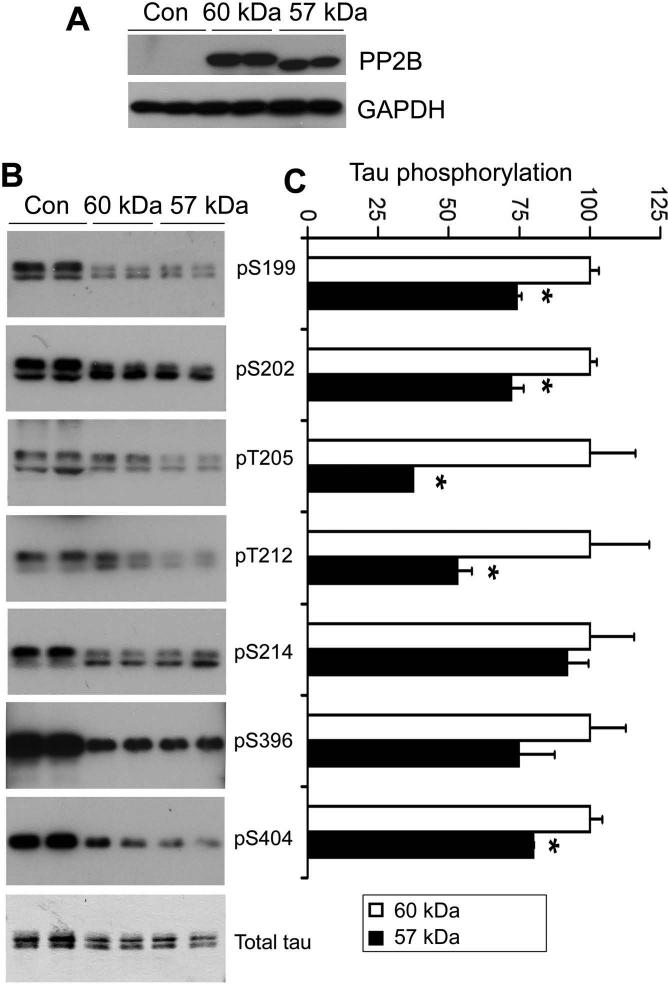
To confirm that the truncation of PP2B catalytic subunit increases its phosphatase activity in live cells, we overexpressed PP2B60 or PP2B57 together with human tau441 in HEK-293T cells. The cells were lysed 2 days after transfection, and the cell lysates were analyzed by Western blots for tau phosphorylation. We observed that overexpression of both the full-length and the truncated PP2B significantly decreased tau phosphorylation at all the phosphorylation sites examined (Fig. 2). Compared with the full-length PP2B, the truncated PP2B induced dephosphorylation of tau at Ser199, Ser202, Thr205, Thr212, and Ser404 to larger degrees (Fig. 2B), despite the fact that expression of the truncated PP2B was similar or even less than that of the full-length PP2B (Fig. 2A). These results suggest that the truncated PP2B is more active in its phosphatase activity than the full-length PP2B in cultured cells.
Fig. 2.
Dephosphorylation of tau by overexpression of full-length and truncated PP2B in HEK293T cells. The full-length (60 kD) or truncated (57 kD) PP2B catalytic subunit was transfected together with human tau441 into HEK293T cells. After 48-h transfection, cell lysates were subjected to Western blot analysis for determination of the level of PP2B expression (A) and tau phosphorylation at individual phosphorylation sites recognized by the indicated phosphorylation-dependent and site-specific tau antibodies (B). Quantification of the blots is shown in (C), where the immunoreactivities of cell lysates with the full-length PP2B overexpression were defined as 100.
PP2B truncation alters its subcellular distribution
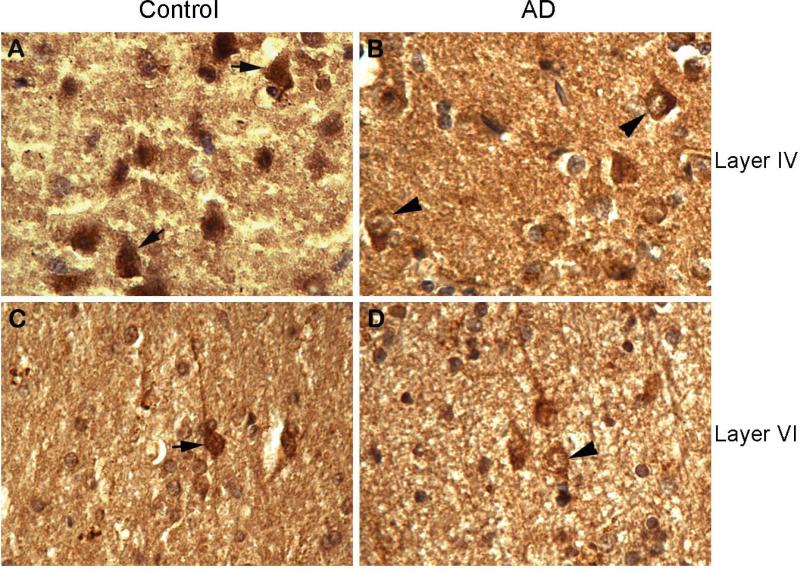
PP2B is involved in many facets of neuronal physiology via dephosphorylation of its substrate proteins locally inside the cell. Thus, we studied the impact of the truncation on the intracellular distribution of PP2B by immunohistochemistry. We observed that in normal control human brains, PP2B was distributed over the cytoplasm, the nucleus, and the processes (Fig. 3A, C). In AD brains in which PP2B was partially truncated, the immunostaining of PP2B was stronger in the cytoplasm than in the nucleus in many neurons (Fig. 3B, D). These observations suggest that the truncation may alter PP2B distribution.
Fig. 3.
Immunohistochemical staining of PP2B A in human mid frontal cortex. Tissue sections from control and AD brains were immunostained with anti-PP2B A. Arrows indicate neurons with typical PP2B localization in control brains. Arrowheads indicate neurons showing a shift of PP2B from the nucleus into cytoplasm in AD brains.
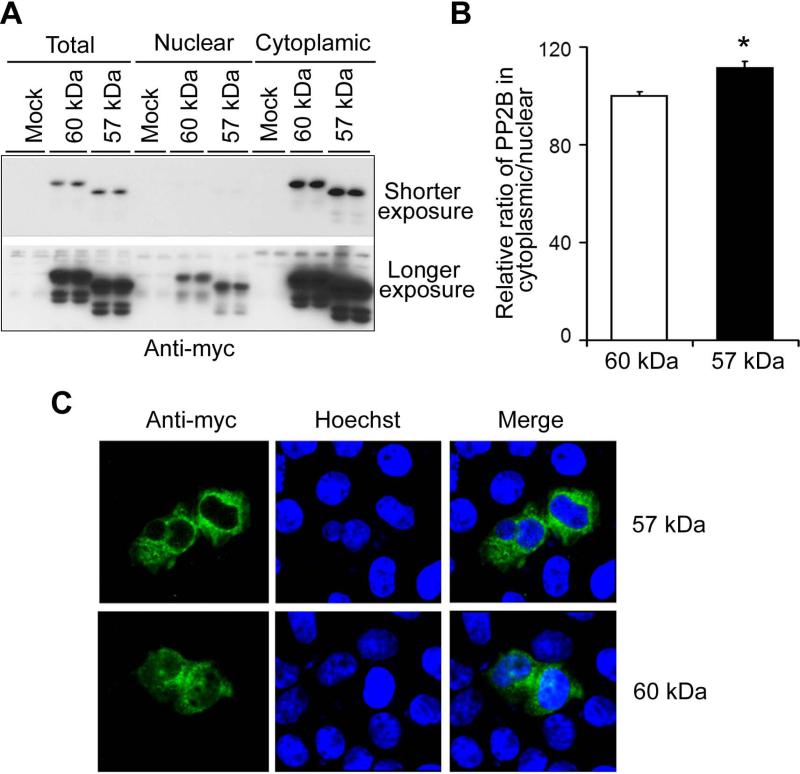
To confirm the effects of the PP2B truncation on its intracellular localization, we over-expressed the full-length (60 kDa) and the truncated (57 kDa) PP2B tagged with myc in HEK-293T cells and then determined the level of PP2B in the nuclear and cytosal fractions separately, 48 h after transfection. We observed that both the full-length and the truncated PP2B were mostly present in the cytosal fraction in HEK-293T cells (Fig. 4A). However, the ratio of the truncated PP2B in the cytosal over the nucleus was larger than that of the full-length PP2B (Fig. 4B), suggesting that the truncated PP2B is distributed more in the cytoplasm than the full-length PP2B. In HeLa cells, localization of the truncated PP2B (57 kDa) was almost exclusively in the cytoplasm (Fig. 4C), but significant amounts of the full-length PP2B were also localized in the nucleus (Fig. 4C). These results confirmed that truncation of PP2B leads to an increased localization to the cytoplasm.
Fig. 4.
Localization of the full-length and truncated PP2B. (A) The full-length (60 kDa) and truncated (57 kDa) PP2B catalytic subunits tagged with myc were transfected into HEK293T cells. After 48-h transfection, the cells were harvested, and the nuclear and cytosal fractions were isolated. The equal amount of proteins of the cytosal and the nuclear fractions were subjected to Western blots with anti-myc antibody. (B) The blots shown in (A) were quantitated densitometrically, and the relative ratio of PP2B in the cytoplasm and the nucleus was calculated. * p <0.05 for 57-kDa vs. 60-kDa PP2B. (C) The full-length (60 kDa) and the truncated (57 kDa) PP2B catalytic subunits tagged with myc were transfected into HeLa cells. After 48-h transfection, the cells were immunostained with anti-myc antibody for PP2B (green) and counter-stained with Hoechst staining for nuclear staining (blue).
Activation of PP2B cannot counteract hyperphosphorylation of tau in AD brain
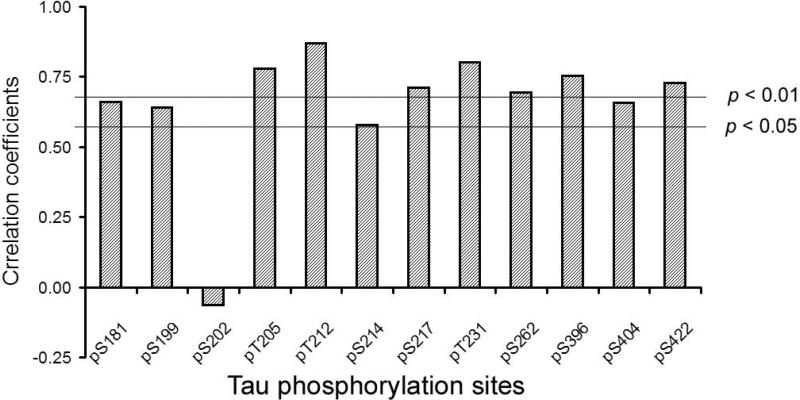
As PP2B dephosphorylated tau at multiple sites in vitro [18] and in cultured cells, its activation due to truncation would have led to hypophosphorylation, rather than hyperphosphorylation, of tau in AD brain, if PP2B were the major tau phosphatase in the brain. The fact that tau is abnormally hyperphosphorylated in AD brain suggests that PP2B may not be a major tau phosphatase in vivo, and its activation does not counteract the hyperphosphorylation of tau resulting from other mechanisms. To investigate whether PP2B activation has any role in counteracting tau hyperphosphorylation in AD, we measured tau phosphorylation levels at individual phosphorylation sites by immuno-dot blots and analyzed the correlation between PP2B truncation and tau phosphorylation in 13 human brains, including both AD and controls. We found no negative correlation between PP2B truncation/activation and tau phosphorylation (Fig. 5), which would be otherwise expected if PP2B dephosphorylated tau in the human brain. Instead, tau phosphorylation at all of these sites except Ser202 correlated positively or had a tendency to correlate positively to PP2B truncation. These results suggest that PP2B is not a major phosphatase regulating tau phosphorylation in the brain, and thus, the increased PP2B truncation/activity cannot counteract tau hyperphosphorylation in AD brain.
Fig. 5.
Correlation analyses between phosphorylation levels of tau at individual phosphorylation sites and PP2B truncation in human brains (six AD cases and seven controls). Tau phosphorylation levels were determined by immuno-dot blots developed with phosphorylation-dependent and site-specific tau antibodies. PP2B truncation was determined as in Fig. 2A. Two lines indicate statistically significant coefficient p = 0.05 or p = 0.01.
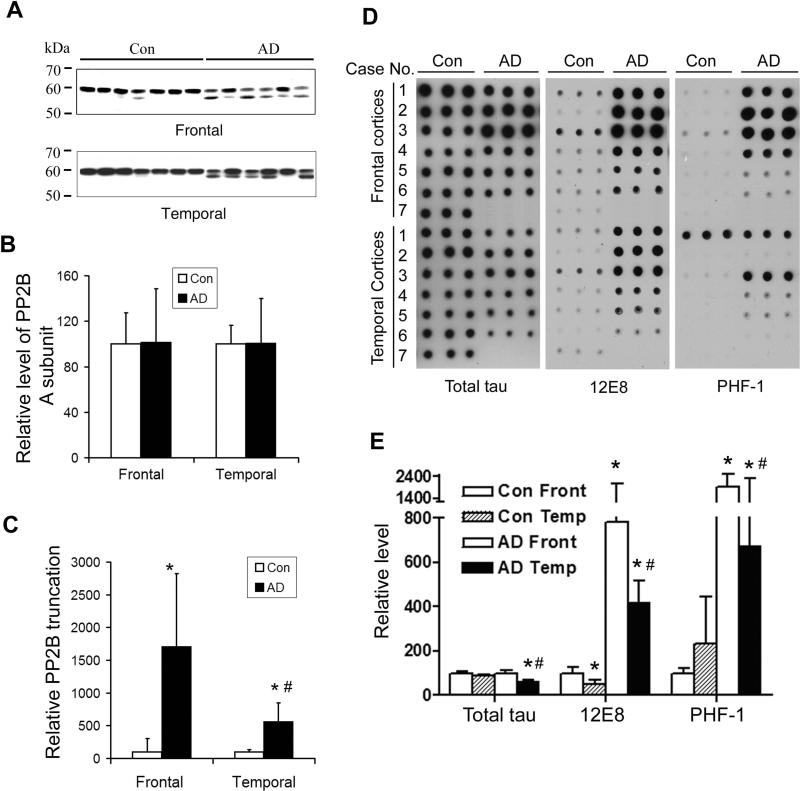
In addition to the temporal cortices, we also analyzed the truncation of the PP2B catalytic subunit and the level of tau phosphorylation in the frontal cortices. Western blots indicated that the PP2B catalytic subunit was truncated into 57 kDa in the frontal cortices, and the degree of the truncation was larger than that in the temporal cortices, although the total level of PP2B was unchanged in both areas of the brain (Fig. 6A, 6B). Phosphorylation of tau at 12E8 sites (Ser262/Ser356) and PHF-1 (Ser396/Ser404) in the frontal cortices was also found to be more, rather than less, than in the temporal cortices (Fig. 6C, 6D). These results further suggest that increased PP2B activation due to truncation cannot counteract the hyperphosphorylation of tau in AD brain.
Fig. 6.
Truncation and tau phosphorylation in the frontal and temporal cortices. (A) Western blots of the frontal and temporal cerebral cortical homogenates from six AD and seven control brains, which were developed with antibody to the catalytic subunit of PP2B. (B) The protein level of PP2B catalytic subunits of the same samples was measured by immuno-dot-blots assay. (C) The blots in panel A were quantitated densitometrically, and the relative ratios of the truncated (57-kDa) over the full-length (60-kDa) catalytic subunits of PP2B are presented. (D) Levels of phosphorylation of tau in frontal and temporal cerebral cortical homogenates from six AD and seven control brains were measured by immuno-dot-blots developed with antibodies against tau phosphorylated at Ser262/Ser356 (12E8) and Ser396/Ser404 (PHF-1), respectively. Dot blots with antibody R134d were included to detect the total tau levels in the samples. (E) The blots in panel D were quantitated densitometrically. * p < 0.05 for AD vs. control; # p < 0.05 for frontal vs. temporal cortices.
DISCUSSION
PP2B activity was previously measured in human brain extracts by using selective PP2B inhibitors or activators and found to be the same or slightly decreased in AD brain as compared to controls [14,20,21]. On the basis of these observations and the studies showing efficient dephosphorylation of tau at multiple sites by PP2B in vitro and in cultured cells, it was hypothesized that PP2B might regulate tau phosphorylation in the brain. In the present study, we determined PP2B activity in AD and control brains after immunoprecipitation of PP2B from brain extracts. To our surprise, we found a three-fold higher PP2B activity in AD brain than in controls. Further investigations demonstrated that the increased activity was due to partial cleavage of PP2B by calpain I, which activates PP2B, in AD brain. The present study also explains the reason why previous studies using brain extracts for PP2B assay failed to observe the elevated PP2B activity in AD brain. That was most likely because Ca2+/calmodulin included in the assay mixture activated endogenous calpain I of the brain extracts, which in turn cleaved and activated PP2B activity to an extent similar to that seen in AD brain. Calcium homeostasis is disturbed and calpain I is over-activated in AD brain [35,36,27], which appears to cleave and activate the PP2B already in AD brain, and therefore, there is no further activation of PP2B during PP2B assay incubation. When we compared PP2B activity between AD and control brains by measuring the activity of PP2B immunoprecipitated from brain extracts, because the assay mixture did not contaminate calpain I and, hence, PP2B from control brains was not activated during assay incubation, we were able to observe activated PP2B in AD brain as compared to controls brains.
Tau is abnormally hyperphosphorylated rather than hypophosphorylated in AD brain at multiple phosphorylation sites, including those that can be dephosphorylated by PP2B in vitro [37]. Truncated PP2B A was more active in the dephosphorylation of tau at multiple sites in cultured cells. These data suggest that the activated PP2B could not override the hyperphosphorylation of tau in AD brain. Otherwise, the elevated PP2B activity in AD brain might have resulted in decreased phosphorylation, rather than hyperphosphorylation, of tau at PP2B-relevant phosphorylation sites. Our conclusion is consistent with previous studies in which inhibition of PP2A, a major tau phosphatase [10,12,38,39,18], induced a marked increase in tau phosphorylation in animal brains [40], whereas inhibition of PP2B did not induce a significant increase in tau phosphorylation in animal brains, except for a mild increase in tau phosphorylation restricted to the mossy fibers of the hippocampus [39,41].
PP2B is highly expressed in the central nervous system [23,42] and dephosphorylates tau in vitro at several phosphorylation sites [9,11,18]. The reason why such a phosphatase does not regulate tau phosphorylation in the brain may be explained by our findings that PP2B has a five-fold larger Km (~50 μM) than do PP1, PP2A, and PP5 (~10 μM) toward tau [18], suggesting that PP2B has much less affinity to tau than do other major brain PPs. The intraneuronal tau concentration is estimated to be 5–10 μM on the basis of its concentration as measured in brain homogenates [43]. The much larger Km of PP2B than the intraneuronal tau concentration also suggests that PP2B is unlikely to act on tau in vivo. Indeed, when we compared the relative contributions of major brain PPs to dephosphorylation of tau in the total human brain crude extract, we found that PP2B only accounts for 7% of the total tau phosphatase activity in human brain [18]. It is not clear at present what is the pathophysiological significance of the partial cleavage and activation of PP2B in the pathogenesis of AD. Altered calcium homeostasis in AD brain has been well documented [44,45]. We found that the Ca2+-activated calpain I is cleaved and activated in AD brain [27]. Hence, the cleavage and activation of PP2B and the resulting downstream cascade may underlie the role of calcium dysregulation and calpain activation in the molecular mechanism of AD. A recent study in cultured hippocampal neurons has shown that calpain-catalyzed cleavage and activation of PP2B catalytic subunit mediate glutamate- and kainite-induced excitotoxicity of the cells [26,46]. Another recent study reported that Aβ secreted from cultured neurons of Tg2576 mouse causes the elevation of intracellular calcium and activation PP2B, which in turn activates the transcriptional factor NFAT4 (nuclear factor of activated T cells 4) and leads to dystrophic neurites, dendritic simplification, and dendritic spine loss [47].
In summary, we have demonstrated that PP2B dephosphorylated tau at many sites in vitro. The catalytic subunit of PP2B was partially truncated into 57 kDa in AD brains. The truncation increased its activity to dephosphorylate tau in vitro and in cultured cells. PP2B truncation in human brains was positively, rather than negatively, correlated to the phosphorylation of tau at multiple phosphorylation sites. Taken together, our studies indicated that PP2B does not regulate phosphorylation of tau in the brain significantly, although it dephosphorylates tau at several phosphorylation sites in vitro.
ACKNOWLEDGMENTS
This work was supported in part by Nantong University, the New York State Office of Mental Retardation and Developmental Disabilities, and grants from the National Natural Science Foundation of China (30770468 and 30973143 to FL), the Natural Science Foundation of Jiangsu Province, China (BK2009159 to FL), and the US National Institutes of Health (AG027429 to CXG, and AG019158 to KI). We thank Ms. J. Murphy for secretarial assistance and Ms. M. Marlow for editorial assistance. We are also grateful to the Sun Health Research Institute Brain Donation Program of Sun City, Arizona, USA, for the provision of post-mortem human brain tissue. The Brain Donation Program is partially supported by a National Institute on Aging grant (P30 AG19610, Arizona Alzheimer's Disease Core Center).
REFERENCES
- 1.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 2.Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: Findings from the nun study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 3.Iqbal K, Grundke-Iqbal I. Alzheimer neurofibrillary degeneration: Significance, etiopathogenesis, therapeutics and prevention. J Cell Mol Med. 2008;12:38–55. doi: 10.1111/j.1582-4934.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei JJ, Sjogren M, Winblad B. Neurofibrillary degeneration in alzheimer's disease: From molecular mechanisms to identification of drug targets. Curr Opin Psychiatry. 2008;21:555–561. doi: 10.1097/YCO.0b013e328314b78b. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto H, Saitoh Y, Fukunaga K, Nishimura H, Miyamoto E. Dephosphorylation of microtubule proteins by brain protein phosphatases 1 and 2a, and its effect on microtubule assembly. J Neurochem. 1988;50:1614–1623. doi: 10.1111/j.1471-4159.1988.tb03051.x. [DOI] [PubMed] [Google Scholar]
- 6.Drewes G, Mandelkow EM, Baumann K, Goris J, Merlevede W, Mandelkow E. Dephosphorylation of tau protein and alzheimer paired helical filaments by calcineurin and phosphatase-2a. FEBS Lett. 1993;336:425–432. doi: 10.1016/0014-5793(93)80850-t. [DOI] [PubMed] [Google Scholar]
- 7.Gong CX, Grundke-Iqbal I, Damuni Z, Iqbal K. Dephosphorylation of microtubule-associated protein tau by protein phosphatase-1 and -2c and its implication in alzheimer disease. FEBS Lett. 1994;341:94–98. doi: 10.1016/0014-5793(94)80247-5. [DOI] [PubMed] [Google Scholar]
- 8.Gong CX, Grundke-Iqbal I, Iqbal K. Dephosphorylation of alzheimer's disease abnormally phosphorylated tau by protein phosphatase-2a. Neuroscience. 1994;61:765–772. doi: 10.1016/0306-4522(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 9.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Alzheimer's disease abnormally phosphorylated tau is dephosphorylated by protein phosphatase-2b (calcineurin). J Neurochem. 1994;62:803–806. doi: 10.1046/j.1471-4159.1994.62020803.x. [DOI] [PubMed] [Google Scholar]
- 10.Goedert M, Jakes R, Qi Z, Wang JH, Cohen P. Protein phosphatase 2a is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic amp-dependent protein kinase. J Neurochem. 1995;65:2804–2807. doi: 10.1046/j.1471-4159.1995.65062804.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal K. Dephosphorylation of alzheimer paired helical filaments by protein phosphatase-2a and -2b. J Biol Chem. 1995;270:4854–4860. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- 12.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of tau by protein phosphatase 2a. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Iqbal K, Grundke-Iqbal I, Rossie S, Gong CX. Dephosphorylation of tau by protein phosphatase 5: Impairment in alzheimer's disease. J Biol Chem. 2005;280:1790–1796. doi: 10.1074/jbc.M410775200. [DOI] [PubMed] [Google Scholar]
- 14.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in alzheimer disease brain. J Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 15.Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: Decrease in alzheimer disease brain. J Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- 16.Loring JF, Wen X, Lee JM, Seilhamer J, Somogyi R. A gene expression profile of alzheimer's disease. DNA Cell Biol. 2001;20:683–695. doi: 10.1089/10445490152717541. [DOI] [PubMed] [Google Scholar]
- 17.Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL., 3rd Altered expression levels of the protein phosphatase 2a abalphac enzyme are associated with alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases pp1, pp2a, pp2b and pp5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Zhou XW, Tanila H, Bjorkdahl C, Wang JZ, Guan ZZ, Cao Y, Gustafsson JA, Winblad B, Pei JJ. Phosphorylated pp2a (tyrosine 307) is associated with alzheimer neurofibrillary pathology. J Cell Mol Med. 2008;12:241–257. doi: 10.1111/j.1582-4934.2008.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladner CJ, Czech J, Maurice J, Lorens SA, Lee JM. Reduction of calcineurin enzymatic activity in alzheimer's disease: Correlation with neuropathologic changes. J Neuropathol Exp Neurol. 1996;55:924–931. doi: 10.1097/00005072-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Lian Q, Ladner CJ, Magnuson D, Lee JM. Selective changes of calcineurin (protein phosphatase 2b) activity in alzheimer's disease cerebral cortex. Exp Neurol. 2001;167:158–165. doi: 10.1006/exnr.2000.7534. [DOI] [PubMed] [Google Scholar]
- 22.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 23.Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 24.Wang KK, Roufogalis BD, Villalobo A. Characterization of the fragmented forms of calcineurin produced by calpain i. Biochem Cell Biol. 1989;67:703–711. doi: 10.1139/o89-105. [DOI] [PubMed] [Google Scholar]
- 25.Perrino BA, Ng LY, Soderling TR. Calcium regulation of calcineurin phosphatase activity by its b subunit and calmodulin. Role of the autoinhibitory domain. J Biol Chem. 1995;270:7012. doi: 10.1074/jbc.270.12.7012. [DOI] [PubMed] [Google Scholar]
- 26.Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, Li ST, Moriwaki A, Matsui H. Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem. 2004;279:4929–4940. doi: 10.1074/jbc.M309767200. [DOI] [PubMed] [Google Scholar]
- 27.Liu F, Grundke-Iqbal I, Iqbal K, Oda Y, Tomizawa K, Gong CX. Truncation and activation of calcineurin a by calpain i in alzheimer disease brain. J Biol Chem. 2005;280:37755–37762. doi: 10.1074/jbc.M507475200. [DOI] [PubMed] [Google Scholar]
- 28.Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Involvement of aberrant glycosylation in phosphorylation of tau by cdk5 and gsk-3beta. FEBS Lett. 2002;530:209–214. doi: 10.1016/s0014-5793(02)03487-7. [DOI] [PubMed] [Google Scholar]
- 30.Pei JJ, Gong CX, Iqbal K, Grundke-Iqbal I, Wu QL, Winblad B, Cowburn RF. Subcellular distribution of protein phosphatases and abnormally phosphorylated tau in the temporal cortex from alzheimer's disease and control brains. J Neural Transm. 1998;105:69–83. doi: 10.1007/s007020050039. [DOI] [PubMed] [Google Scholar]
- 31.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Gong CX, Shaikh S, Grundke-Iqbal I, Iqbal K. Inhibition of protein phosphatase-2b (calcineurin) activity towards alzheimer abnormally phosphorylated tau by neuroleptics. Brain Res. 1996;741:95–102. doi: 10.1016/s0006-8993(96)00904-3. [DOI] [PubMed] [Google Scholar]
- 33.Liu F, Zaidi T, Iqbal K, Grundke-Iqbal I, Gong CX. Aberrant glycosylation modulates phosphorylation of tau by protein kinase a and dephosphorylation of tau by protein phosphatase 2a and 5. Neuroscience. 2002;115:829–837. doi: 10.1016/s0306-4522(02)00510-9. [DOI] [PubMed] [Google Scholar]
- 34.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-glcnacylation regulates phosphorylation of tau: A mechanism involved in alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:10804–10809. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in alzheimer disease: A potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veeranna, Kaji T, Boland B, Odrljin T, Mohan P, Basavarajappa BS, Peterhoff C, Cataldo A, Rudnicki A, Amin N, Li BS, Pant HC, Hungund BL, Arancio O, Nixon RA. Calpain mediates calcium-induced activation of the erk1,2 mapk pathway and cytoskeletal phosphorylation in neurons: Relevance to alzheimer's disease. Am J Pathol. 2004;165:795–805. doi: 10.1016/S0002-9440(10)63342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85:148–175. doi: 10.1016/j.pneurobio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Sontag E, Nunbhakdi-Craig V, Lee G, Brandt R, Kamibayashi C, Kuret J, White CL, 3rd, Mumby MC, Bloom GS. Molecular interactions among protein phosphatase 2a, tau, and microtubules. Implications for the regulation of tau phosphorylation and the development of tauopathies. J Biol Chem. 1999;274:25490–25498. doi: 10.1074/jbc.274.36.25490. [DOI] [PubMed] [Google Scholar]
- 39.Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2a in mammalian brain. Implications for neurofibrillary degeneration in alzheimer's disease. J Biol Chem. 2000;275:5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- 40.Kins S, Crameri A, Evans DR, Hemmings BA, Nitsch RM, Gotz J. Reduced protein phosphatase 2a activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J Biol Chem. 2001;276:38193–38200. doi: 10.1074/jbc.M102621200. [DOI] [PubMed] [Google Scholar]
- 41.Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K. Role of protein phosphatase-2a and -1 in the regulation of gsk-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 2000;485:87–93. doi: 10.1016/s0014-5793(00)02203-1. [DOI] [PubMed] [Google Scholar]
- 42.Pei JJ, Sersen E, Iqbal K, Grundke-Iqbal I. Expression of protein phosphatases (pp-1, pp-2a, pp-2b and ptp-1b) and protein kinases (map kinase and p34cdc2) in the hippocampus of patients with alzheimer disease and normal aged individuals. Brain Res. 1994;655:70–76. doi: 10.1016/0006-8993(94)91598-9. [DOI] [PubMed] [Google Scholar]
- 43.Khatoon S, Grundke-Iqbal I, Iqbal K. Brain levels of microtubule-associated protein tau are elevated in alzheimer's disease: A radioimmuno-slot-blot assay for nanograms of the protein. J Neurochem. 1992;59:750–753. doi: 10.1111/j.1471-4159.1992.tb09432.x. [DOI] [PubMed] [Google Scholar]
- 44.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in alzheimer's disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 45.Mattson MP, Chan SL. Neuronal and glial calcium signaling in alzheimer's disease. Cell Calcium. 2003;34:385–397. doi: 10.1016/s0143-4160(03)00128-3. [DOI] [PubMed] [Google Scholar]
- 46.Liang Z, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Dysregulation of tau phosphorylation in mouse brain during excitotoxic damage. J Alzheimers Dis. 2009;17:531–539. doi: 10.3233/JAD-2009-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]