Abstract
X-linked adrenoleukodystrophy (X-ALD) is caused by mutations in the ABCD1 gene encoding the peroxisomal ABC transporter adrenoleukodystrophy protein (ALDP). X-ALD is characterized by the accumulation of very long-chain fatty acids (VLCFA; ≥C24) in plasma and tissues. In this manuscript we provide insight into the pathway underlying the elevated levels of C26:0 in X-ALD. ALDP transports VLCFacyl-CoA across the peroxisomal membrane. A deficiency in ALDP impairs peroxisomal β-oxidation of VLCFA but also raises cytosolic levels of VLCFacyl-CoA which are substrate for further elongation. We identify ELOVL1 (elongation of very-long-chain-fatty acids) as the single elongase catalysing the synthesis of both saturated VLCFA (C26:0) and mono-unsaturated VLCFA (C26:1). ELOVL1 expression is not increased in X-ALD fibroblasts suggesting that increased levels of C26:0 result from increased substrate availability due to the primary deficiency in ALDP. Importantly, ELOVL1 knockdown reduces elongation of C22:0 to C26:0 and lowers C26:0 levels in X-ALD fibroblasts. Given the likely pathogenic effects of high C26:0 levels, our findings highlight the potential of modulating ELOVL1 activity in the treatment of X-ALD.
Keywords: adrenoleukodystrophy, elongase, ELOVL, peroxisome, VLCFA
INTRODUCTION
X-linked adrenoleukodystrophy (X-ALD) has several clinically distinct phenotypes including childhood cerebral ALD (CCALD), adrenomyeloneuropathy (AMN) and primary adrenocortical insufficiency (Moser et al, 2001). All X-ALD patients have mutations in the ABCD1 gene (Mosser et al, 1993) encoding the peroxisomal ABC transporter ALDP. Mutations in ALDP impair peroxisomal β-oxidation of very long-chain fatty acids (VLCFA) (Fourcade et al, 2009; Singh et al, 1984) resulting in elevated levels of VLCFA in plasma and tissues (Moser et al, 1999).
Plasma VLCFA levels do not correlate with phenotype. However, myelin from CCALD patients contains higher levels of C26:0, C28:0 and C30:0 compared to myelin from AMN patients (Asheuer et al, 2005), suggesting a correlation between VLCFA levels in the cerebral white matter and expression of the cerebral phenotype. Interestingly, C26:0 decreases the response of adrenocortical cells to adrenocorticotropic hormone (ACTH) stimulation (Whitcomb et al, 1988). A pathogenic role for C26:0 is further supported by its disruptive effects on the structure, stability and function of cell membranes (Ho et al, 1995; Knazek et al, 1983) and by its possible contribution to oxidative stress (Fourcade et al, 2008; Powers et al, 2005).
VLCFA accumulating in X-ALD are partly absorbed from the diet (Kishimoto et al, 1980) but mostly result from endogenous synthesis through elongation of LCFA (Tsuji et al, 1981).
Treatment options for X-ALD are limited. Lorenzo's oil reduces plasma C26:0 but does not halt progression of the disease (Aubourg et al, 1993). Lovastatin was considered a possible treatment (Singh et al, 1998), but a recent placebo-controlled trial revealed that it has no effect on VLCFA in blood cells or low-density lipoprotein (LDL)-lipoprotein particles (Engelen et al, 2010). Haematopoietic stem cell transplantation can halt or reverse clinical deterioration (Peters et al, 2004). However, it is only effective in patients with the earliest stage of CCALD. The recent breakthrough in gene therapy is currently only applied to CCALD (Cartier et al, 2009). We hypothesize that preventing or reducing the formation of C26:0 could generate new treatment options for X-ALD.
The synthesis of saturated VLCFA, mono-unsaturated VLCFA and poly-unsaturated fatty acids (PUFA) involves four sequential reactions. The first, rate-limiting step, is carried out by one of seven mammalian elongases (designated ELOVL1-7) each with different fatty acid substrate preferences (Jakobsson et al, 2006; Leonard et al, 2004). We set out to (1) resolve the pathway underlying the elevated levels of C26:0 in X-ALD, (2) identify the human C26:0-specific elongase and (3) investigate whether inhibition of this elongase reduces C26:0 levels.
RESULTS AND DISCUSSION
D3-VLCFA levels in control and X-ALD fibroblasts
VLCFA levels are determined by the balance between degradation via peroxisomal β-oxidation and synthesis through elongation of LCFA. X-ALD fibroblasts have residual degradation capacities of 25% for C22:0 and C24:0 and 15% for C26:0 (McGuinness et al, 2001). Surprisingly, only C26:0 levels are increased in these cells, whereas C22:0 levels are decreased and C24:0 levels are near-normal (Valianpour et al, 2003). These data suggest elongation of C22:0 and C24:0 to C26:0. We previously confirmed elongation of C24:0 to C26:0 in X-ALD fibroblasts by measuring D3-C26:0 at 3 days of treatment with D3-C24:0 (Kemp et al, 2005). In order to investigate the pathway underlying the elevated levels of C26:0, we now measured D3-C26:0 in control and X-ALD fibroblasts at 3 days of treatment with D3-C16:0, an LCFA. X-ALD fibroblasts had 20% lower D3-C22:0 levels, 7-fold higher D3-C26:0 levels and a 9-fold increase in D3-C26:0/D3-C22:0 ratio (Fig 1). No differences were found for D3-C18:0, D3-C20:0 and D3-C24:0 (data not shown). The differences observed in D3-VLCFA profiles between control and X-ALD fibroblasts match the endogenous VLCFA profiles of control and X-ALD fibroblasts (Valianpour et al, 2003) thus validating this method for the study of VLCFA homeostasis in whole cells.
Figure 1. D3-VLCFA levels in control and X-ALD fibroblasts.
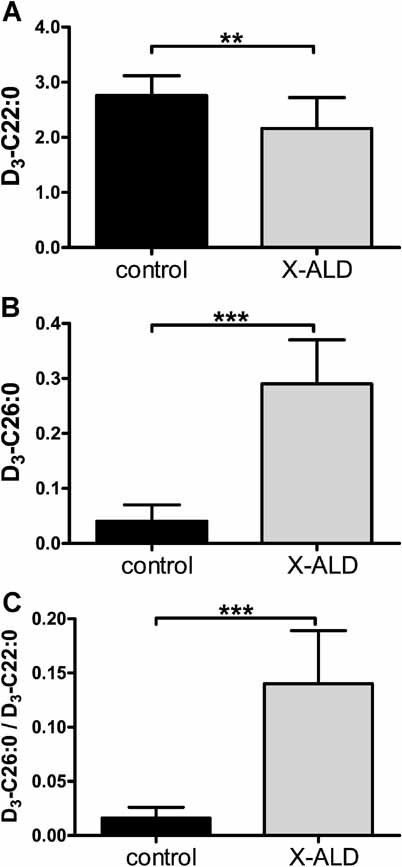
- D3-C22:0 levels in control versus X-ALD cell lines.
- D3-C26:0 levels in control versus X-ALD cell lines.
-
D3-C26:0/D3-C22:0 ratio in control versus X-ALD cell lines.Eight control and 25 X-ALD cell lines were cultured for 72 h with 50 µM D3-C16:0. Fatty acids are in nmol/mg protein. Data are mean ± SD. **p < 0.01, ***p < 0.001 by unpaired student's t-test.
C24:0-CoA and C26:0-CoA levels are elevated in X-ALD fibroblasts
ALDP transports fatty acid-CoA across the peroxisomal membrane in yeast (van Roermund et al, 2008). In order to investigate whether ALDP deficiency raises cytosolic VLCFacyl-CoA levels, we measured VLCFacyl-CoA in control and X-ALD fibroblasts cultured with C24:0 for 24 h. As a control, we incubated the same cell lines with pristanic acid. No differences were observed in pristanoyl-CoA levels between control and X-ALD fibroblasts (Fig 2A) as expected based on the normal pristanic acid oxidation in X-ALD (Kemp et al, 1998). After incubation with C24:0, C24:0-CoA and C26:0-CoA levels were, respectively, 8- and 14-fold higher in X-ALD fibroblasts (Fig 2B, C). These data indicate that (1) ALDP is involved in the transport of VLCFacyl-CoA into peroxisomes, (2) a defect in ALDP results in increased levels of C24:0-CoA in the cytosol, which then become available as a substrate for further elongation to C26:0-CoA.
Figure 2. VLCFacyl-CoA levels are increased in X-ALD fibroblasts.
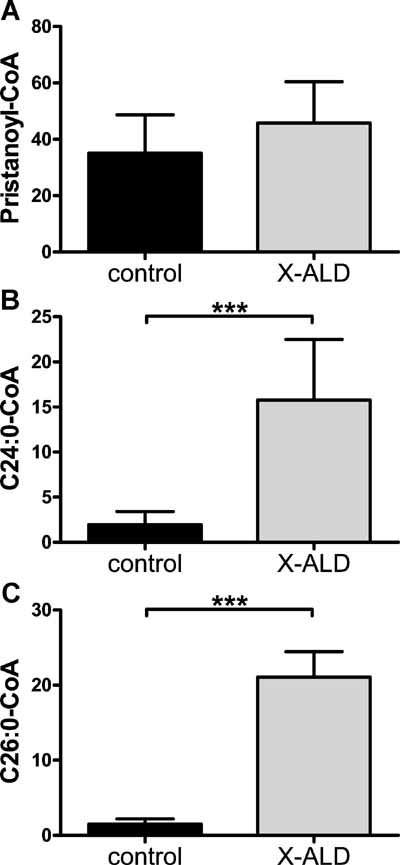
- Pristanoyl-CoA levels in control versus X-ALD cell lines.
- C24:0-CoA levels in control versus X-ALD cell lines.
- C26:0-CoA levels in control versus X-ALD cell lines.
- Fatty acyl-CoAs are in pmol/mg protein. Data are mean ± SD in 3 control and 6 X-ALD cell lines. ***p < 0.001 by unpaired student's t-test.
Human ELOVL1 is the functional orthologue of yeast Elo3p
In yeast, there are three elongases: Elo1p, Elo2p and Elo3p (Oh et al, 1997; Toke & Martin, 1996). We confirmed that Elo3p is the elongase primarily involved in the synthesis of C26:0 from C22:0 (Oh et al, 1997) (Supporting Information Fig S1). To identify the human ELOVL involved in C26:0 synthesis, we expressed the seven human ELOVL proteins in the yeast Elo3p deletion strain. Protein expression was confirmed by Western blot analysis (Supporting Information Fig S2A). No significant changes in fatty acid levels were observed in transformants expressing either ELOVL2 or ELOVL7 (data not shown). Transformants expressing ELOVL3 had increased C22:1 and C24:1 (Table 1). Expression of ELOVL4 increased C22:0 (Table 1). Transformants expressing ELOVL5 had increased C18:1, C20:1 and C22:1 (Table 1). Expression of ELOVL6 decreased C16:0 and increased C18:1, C18:0 and C20:0 (Table 1). Only transformants expressing human ELOVL1 or yeast Elo3p had a 10-fold increase in C26:0 and C26:1 and a strong reduction in C22:0 (Table 1). Based on these results, we conclude that human ELOVL1 is the functional orthologue of yeast Elo3p. This is in line with previous findings on mouse ELOVL1 (Tvrdik et al, 2000).
Table 1.
Fatty acid levels in Elo3p mutant yeast transformed with human ELOVL proteins and yeast Elo3p
| ELOVL1 | ELOVL3 | ELOVL4 | ELOVL5 | ELOVL6 | ScELO3 | |
|---|---|---|---|---|---|---|
| C18:1 | — | — | — | 170%** | 170%*** | — |
| C20:1 | — | — | — | 380%*** | — | — |
| C22:1 | — | 340%*** | — | 220%* | — | — |
| C24:1 | 810%*** | >1000%*** | — | — | — | — |
| C26:1 | 830%*** | — | — | — | — | >1000%*** |
| C16:0 | — | — | — | — | 51%*** | — |
| C18:0 | — | — | — | — | 160%** | — |
| C20:0 | — | — | — | — | 160%* | 20%* |
| C22:0 | 45%** | — | 140%* | — | — | 15%*** |
| C24:0 | >1000%*** | — | — | — | — | — |
| C26:0 | >1000%*** | — | — | — | — | >1000%*** |
| C28:0 | — | — | — | — | — | >1000%** |
Data are presented only for those transformants for which the ANOVA p-value was <0.05. No significant changes were observed in transformants expressing either ELOVL2 or ELOVL7. For each elongase, at least three independent clones were analysed. Results are expressed as percentage relative to the amount measured in BY4742Δelo3 yeast. *p < 0.05, **p < 0.01 and ***p < 0.001 by ANOVA followed by Dunnett's multiple comparison test compared with BY4742Δelo3.
ELOVL1 expression in CHO cells increases VLCFA levels
To further investigate the function of the seven human ELOVL proteins in VLCFA biosynthesis in higher eukaryotes, we stably expressed them in Chinese hamster ovary (CHO) cells. Expression was confirmed at the cDNA level by RT-PCR (data not shown). CHO cells expressing ELOVL3, ELOVL4 or ELOVL7 had the same fatty acid profiles as mock-transfected cells (data not shown). CHO cells expressing ELOVL2 had reduced C22:5 and increased C24:4, C24:5 and C24:6 (Table 2), confirming ELOVL2's role in PUFA metabolism (Kobayashi et al, 2007). CHO cells expressing ELOVL5 had increased C20:1 and C22:1 (Table 2). CHO cells expressing ELOVL6 had reduced C14:0 and C16:0 and increased C18:0 (Table 2), confirming ELOVL6's role of LCFA elongase (Moon et al, 2001). CHO cells expressing ELOVL1 had markedly increased C26:1, C24:0, C26:0 and C28:0 along with reduced C20:1, C22:1 and C24:1 (Table 2). This further supports our conclusion that ELOVL1 is involved in C26:0 synthesis and extrapolates our findings in yeast to mammalian cells.
Table 2.
Fatty acid levels in CHO clones expressing human ELOVL proteins
| ELOVL1 | ELOVL2 | ELOVL5 | ELOVL6 | |
|---|---|---|---|---|
| C22:5 | — | 28%** | — | — |
| C24:4 | — | >1000%*** | — | — |
| C24:5 | — | >1000%*** | — | — |
| C24:6 | — | 510%*** | — | — |
| C20:1 | 39%*** | — | 150%** | — |
| C22:1 | 56%*** | — | 129%* | — |
| C24:1 | 62%*** | — | — | — |
| C26:1 | >1000%*** | — | — | — |
| C14:0 | — | — | — | 22%*** |
| C16:0 | 164%*** | — | — | 38%*** |
| C18:0 | 147%** | — | — | 135%* |
| C24:0 | 239%*** | — | — | — |
| C26:0 | >1000%*** | — | — | — |
| C28:0 | >1000%*** | — | — | — |
Data are presented only for those transfectants for which the ANOVA p-value was <0.05. CHO cells expressing ELOVL3, ELOVL4 or ELOVL7 had the same fatty acid profiles as the mock-transfected CHO cells. For each elongase, at least three independent clones were analysed. Results are expressed as percentage relative to the amount measured in mock-transfected CHO cells. *p < 0.05, **p < 0.01 and ***p < 0.001 by ANOVA followed by Dunnett's multiple comparison test compared with mock-transfected CHO cells.
Increased C26:0 synthesis in CHO cells expressing ELOVL1
To establish whether human ELOVL1 is the only elongase synthesizing C26 fatty acids, we assessed VLCFA synthesis using D3-C16:0. No differences with mock-transfected CHO cells were observed for ELOVL2, ELOVL4 and ELOVL7 (data not shown). CHO cells expressing ELOVL3 had increased D3-C20:0 and D3-C22:0 (Table 3), in line with previous findings for mouse Elovl3 (Westerberg et al, 2004). CHO cells expressing ELOVL5 had increased D3-C20:1 and D3-C22:1 (Table 3), in line with the results presented in Tables 1 and 2. CHO cells expressing ELOVL6 had increased D3-C18 fatty acids (Table 3), confirming ELOVL6's role of LCFA elongase (Moon et al, 2001). Interestingly, CHO cells expressing ELOVL6 also had significantly increased D3-C20 and D3-C22 fatty acids (Table 3), a previously unreported finding. Our data thus indicate a broader substrate range for ELOVL6 than previously recognized.
Table 3.
Fatty acid synthesis in CHO clones expressing human ELOVL proteins
| ELOVL1 | ELOVL3 | ELOVL5 | ELOVL6 | |
|---|---|---|---|---|
| D3-C18:1 | — | — | — | 145%*** |
| D3-C20:1 | 29%*** | — | 180%*** | 150%** |
| D3-C22:1 | 33%** | — | 160%** | 170%*** |
| D3-C24:1 | 35%** | — | — | — |
| D3-C26:1 | >1000%*** | — | — | — |
| D3-C18:0 | — | — | — | 170%* |
| D3-C20:0 | 200%*** | 220%*** | — | 180%*** |
| D3-C22:0 | — | 150%** | — | 170%*** |
| D3-C24:0 | 230%*** | — | — | — |
| D3-C26:0 | >1000%*** | — | — | — |
| D3-C28:0 | >1000%*** | — | — | — |
Data are presented only for those transfectants for which the ANOVA p-value was <0.05. No differences with mock-transfected CHO cells were observed for ELOVL2, ELOVL4 and ELOVL7. For each elongase, at least three independent clones were analysed. Results are expressed as percentage relative to the amount measured in mock-transfected CHO cells. *p < 0.05, **p < 0.01 and ***p < 0.001 by ANOVA followed by Dunnett's multiple comparison test compared with mock-transfected CHO cells.
CHO cells expressing ELOVL1 had a 2-fold increase in D3-C24:0 and a 10-fold increase in D3-C26:0, D3-C28:0 and D3-C26:1 (Table 3). These data provide convincing evidence in favour of ELOVL1 as the sole elongase involved in the biosynthesis of both C26:0 and C26:1.
ELOVL3 expression is restricted to brown adipose tissue, liver and skin (Tvrdik et al, 2000). ELOVL4 is involved in the synthesis of >C28 fatty acids and C28-C38 VLC-PUFAs (Agbaga et al, 2008; Vasireddy et al, 2007). It is expressed predominantly in retina, brain and sperm (Tvrdik et al, 2000; Vasireddy et al, 2007; Zhang et al, 2001). Considering the omnipresence of saturated and mono-unsaturated VLCFA and the ubiquitous expression pattern of both ELOVL1 (Tvrdik et al, 2000) and ELOVL6 (Moon et al, 2001), we conclude that VLCFA are synthesized from LCFA via the concerted action of ELOVL6 (C18:0–C22:0) and ELOVL1 (C24:0–C26:0).
ELOVL1 expression is not elevated in X-ALD fibroblasts
To rule out that the increase in C26:0 is caused by elevated expression of ELOVL1, we analysed ELOVL1 expression in control and X-ALD fibroblasts at mRNA and protein level. ELOVL1 mRNA was 20% lower in X-ALD fibroblasts (Fig 3A). However, this was not statistically significant. Overall ELOVL1 protein was not increased in X-ALD fibroblasts (Fig 3B). Some X-ALD cell lines even had markedly lower ELOVL1 expression.
Figure 3. ELOVL1 expression is not increased in X-ALD fibroblasts.
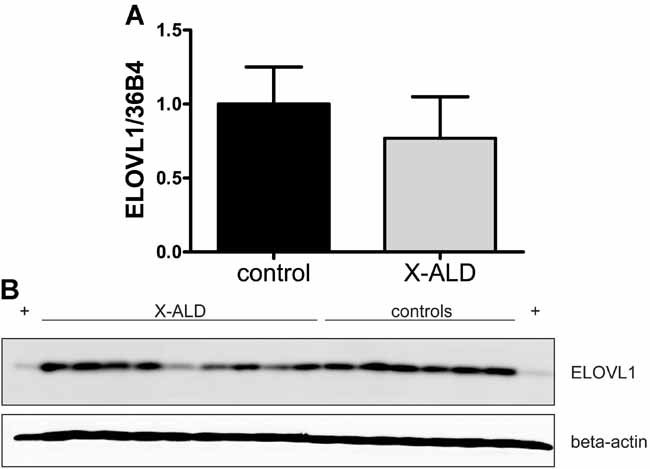
- ELOVL1 mRNA expression in 6 control and 9 X-ALD cell lines. Data are mean ± SD.
- ELOVL1 protein expression in control and X-ALD cell lines. (+) positive control: CHO cells expressing ELOVL1.
ELOVL1 knockdown reduces C26:0 levels in X-ALD fibroblasts
We investigated the effect of ELOVL1 knockdown on C26:0 levels using siRNA. The efficacy of ELOVL1 siRNA was assessed by Western blot analysis. ELOVL1 protein expression was reduced by 80% at 6 days of treatment (Fig 4A). Treatment with control siRNA did not affect ELOVL1 expression (Fig 4B). The potential cytotoxic effect of ELOVL1 knockdown was analysed by comparing the effect of control and ELOVL1 siRNA on cell proliferation. ELOVL1 knockdown reduced cell proliferation by 10% at 6 days of treatment (Fig 4C). The effect of ELOVL1 knockdown on C26:0 synthesis was determined by culturing 3 different X-ALD cell lines for 7 days with ELOVL1 siRNA, adding D3-C22:0 at day 5. ELOVL1 knockdown reduced D3-C26:0 levels by, respectively, 40, 32 and 35% (Fig 4D). Finally, the effect of ELOVL1 knockdown on endogenous C26:0 levels was determined at 10 days of ELOVL1 siRNA treatment. C26:0 levels were reduced by 36, 25 and 38% in the three X-ALD cell lines transfected with ELOVL1 siRNA (Fig 4E). These experiments demonstrate that ELOVL1 knockdown reduces elongation from C22:0 to C26:0 and lowers C26:0 levels in X-ALD fibroblasts. However, it should be noted that these levels are still twice as high as in untransfected control fibroblasts (Fig 4E).
Figure 4. ELOVL1 knockdown reduces C26:0 levels in X-ALD fibroblasts.
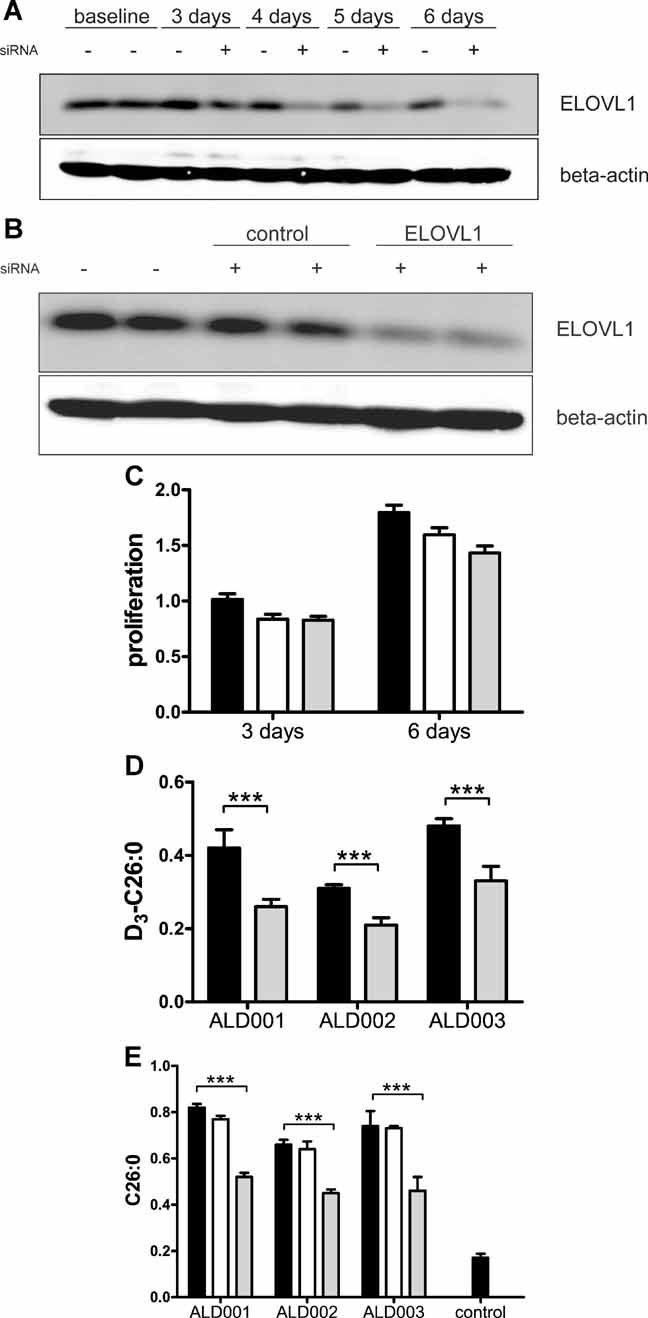
- ELOVL1 expression in X-ALD fibroblasts cultured for 3, 4, 5 or 6 days in medium devoid of (−) or supplemented with (+) ELOVL1 siRNA.
- ELOVL1 expression in X-ALD fibroblasts which were untransfected (−) or transfected (+) with siRNA targeting either cyclophilin-B (control) or ELOVL1.
- Effect of control siRNA (white bars) or ELOVL1 siRNA (grey bars) on cell proliferation in comparison with untransfected cells (black bars).
- D3-C26:0 levels in ELOVL1 siRNA transfected (grey bars) versus untransfected (black bars) X-ALD fibroblasts.
- C26:0 levels in control and X-ALD fibroblasts which were untransfected (black bars) and in X-ALD fibroblasts which were transfected with either control siRNA (white bars) or ELOVL1 siRNA (grey bars). Fatty acids are in nmol/mg protein. Data are mean ± SD. ***p < 0.001 by unpaired student's t-test.
This indicates that low levels of ELOVL1 can still account for considerable C26:0 synthesis. It remains to be determined whether pharmacological inhibition of ELOVL1 could be more effective. Hence, future research should focus on the identification of a specific ELOVL1 inhibitor. Further experiments in X-ALD mice will be necessary to investigate the in vivo potential of ELOVL1 inhibition on VLCFA levels in tissues.
Concluding remarks
ALDP transports VLCFacyl-CoA across the peroxisomal membrane. A deficiency in ALDP impairs peroxisomal β-oxidation of VLCFA but also raises cytosolic levels of VLCFacyl-CoA which are then further elongated by ELOVL1, the human C26-specific elongase. ELOVL1 expression is not increased in X-ALD fibroblasts. Thus, increased substrate availability caused by the primary deficiency in ALDP appears to underlie the elevated C26 levels in X-ALD. Importantly, ELOVL1 knockdown reduces elongation of C22:0 to C26:0 and lowers C26:0 levels in X-ALD fibroblasts. Given the apparent harmful effects of high C26:0 levels, further research is now warranted to investigate ELOVL1's therapeutic potential for X-ALD.
MATERIALS AND METHODS
Chemicals
A polyclonal antibody against C-terminal amino acids of human ELOVL1 (LQQNGAPGIAKVKAN) was generated by Eurogentec (Liege, Belgium).
Measurement of C24:0-CoA, C26:0-CoA and pristanoyl-CoA levels
Analysis of VLCFacyl-CoA esters was performed using MS/MS. Control and X-ALD fibroblasts were cultured for 24 h with either 15 µM C24:0 or 25 µM pristanic acid. For each CoA measurement, cells from 4× T162 flasks were harvested, pooled, washed with cold PBS, snap frozen and stored at −80°C. Acyl-CoA measurements were done as described (van Roermund et al, 2008). Quantification was based on monitoring typical MS/MS fragment ions: m/z 508.5 → m/z 79.0 for C17-CoA (internal standard), m/z 529.5 → 79.0 for pristanoyl-CoA, m/z 557.5 → m/z 79.0 for C24:0-CoA and m/z 571.5 → m/z 79.0 for C26:0-CoA.
The paper explained
PROBLEM
X-linked adrenoleukodystrophy (X-ALD) is a demyelinating disease caused by mutations in the ABCD1 gene encoding the peroxisomal membrane protein ALDP. Peroxisomal beta-oxidation of very long-chain fatty acids (VLCFA, ≥22 carbons) is thereby impaired in these patients resulting in high VLCFA levels in plasma and tissues. Treatment options are limited.
RESULTS
The authors postulate that preventing or reducing the formation of VLCFAs (e.g. C26:0) could represent an interesting therapeutic approach to this disease. They demonstrate that C24:0-CoA and C26:0-CoA are elevated in patient cells and identify ELOVL1 as the single elongase catalysing the synthesis of both saturated VLCFA (C26:0) and mono-unsaturated VLCFA (C26:1). Finally, they show that ELOVL1 knockdown reduces elongation of C22:0 to C26:0 and lowers C26:0 levels in X-ALD fibroblasts.
IMPACT
As the authors discuss, ALDP deficiency in X-ALD has two major effects: it impairs peroxisomal beta-oxidation of VLCFA and at the same time provides the substrate for further elongation. Given the apparent harmful effects of high C26:0 levels, further research is now warranted to investigate ELOVL1's therapeutic potential for X-ALD.
Cloning of the seven human elongases and yeast ELO3
The complete open reading frame (ORF) of the seven human elongases were PCR amplified from cDNA from HepG2 cells (ELOVL1, 2, 3, 5 and 6), fibroblasts (ELOVL4) or NCI-H295R cells (ELOVL7, kindly provided by Dr A. Pujol). Yeast ELO3 ORF was amplified from BY4247 cDNA. Restriction site-tagged primers were used for sub-cloning of the ORFs (Supporting Information Table S1). Amplicons purified from agarose gel were cloned in pGEM-T and sequenced. N-terminal and C-terminal FLAG-tagged ELOVL proteins were generated using specific primers (Supporting Information Table S1).
Expression of human ELOVL in yeast
Wild type yeast (BY4742), Elo1p (BY4742Δelo1), Elop2 (BY4742Δelo2) and Elo3p (BY4742Δelo3) deletion strains were from the EUROSCARF library. The seven human ELOVL ORFs were cloned into the yeast pYC2/CT expression vector and transformed in the BY4742Δelo3 strain using the lithium acetate procedure. In pYC2/CT, expression is under the control of the GAL1 promoter: inactive under glucose conditions and activated when galactose is added to the medium. After 24 h, the OD600 of the different cultures was determined. For VLCFA analysis an amount of cells equivalent to an OD600 of 1.0 was used.
Expression of human ELOVL in CHO cells
CHO cells stably expressing human ELOVL proteins were generated with the Flp-In™ system (Invitrogen). ORFs were cloned into the pcDNA5/FRT vector. Transfection and selection of three independent clones per ELOVL were performed according to the manufacturer's manual.
Fibroblasts and culture conditions
Human skin fibroblasts were obtained from X-ALD patients (aged 23–63) through the Neurology Outpatient Clinic of the Academic Medical Center. Written informed consent was received from each patient. X-ALD diagnosis was confirmed by VLCFA and ABCD1 mutation analysis. Control fibroblasts were from male anonymous volunteers (aged 20–50). Cells were grown in HAMF10 supplemented with 10% foetal calf serum, 25 mM HEPES, 100 U/ml penicillin, 100 U/ml streptomycin and 2 mM glutamine. Cell lines were used between passage numbers 6 and 20.
Synthesis and analysis of VLCFA
Synthesis of D3-VLCFA in whole cells was measured using D3-palmitate (D3-C16:0; Cambridge Isotope Laboratories). A 12.5 mM stock solution in dimethyl sulfoxide (DMSO) was prepared. Cells were seeded at 40% confluency in T75 flasks. The next day, medium was refreshed and 50 µM D3-C16:0 was added. After 72 h, cells were harvested and VLCFA analysed as described (Valianpour et al, 2003).
Quantitative RT-PCR of ELOVL1 mRNA levels
ELOVL1 mRNA levels in control and X-ALD fibroblasts growing in log phase were determined as described (Engelen et al, 2008) with primer sets (ELOVL1-31F and 330R) and (36B4-F and 36B4-R) (Supporting Information Table S1).
ELOVL1 knockdown using siRNA
Target sequences for the human-specific ELOVL1 Accell SMARTpool siRNA mixture (Dharmacon) were GCAUCAUGGCUAAUCGGAA, CGUGGCUUCAUGAUUGUCU, CUAUGAGUUCCUGAUGUCG and CGUGCAUGUCAUAAUGUAC. siRNA targeting cyclophilin B was used as a control. Fibroblasts were transfected with siRNA (1 µM) following the manufacturer's instructions.
Assessment of cell proliferation
Fibroblasts were seeded at 5000 cells per well in a 48-well plate. The next day, siRNA was added to the medium. Cells were cultured with siRNA for 3 and 6 days. Proliferation was measured with a CellTiter 96 AQ assay kit (Promega).
For more information
X-linked Adrenoleukodystrophy Database: http://www.x-ald.nl/
Acknowledgments
We thank Femke Stet, Janet Koster, Henk van Lenthe and Henk Overmars for expert technical assistance and Dr Vincent de Boer, Dr Sander Houten and Dr Marc Engelen for critical comments on the manuscript. This work was supported by the European Leukodystrophy Association (Grant Nos. 2006-031I4 and 2008-001C4 (S.K.)) and the Netherlands Organization for Scientific Research (VIDI-Grant No. 016.086.328 (S.K.)).
Supporting information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
I.D. and S.K. performed the D3-C16:0 studies and VLCFA analyses. CvR and AvC performed the VLCFacyl-CoA analysis. N.B. and S.K. cloned the elongases. CvR and N.B. generated the ELOVL-expressing yeast clones. R.O. generated and screened the FLAG-tagged elongases. I.D. did the RT-PCR analysis and generated the ELOVL-expressing CHO clones. R.O. and I.D. performed the Western blots. M.T. carried out the transfection studies. R.O., I.D. and S.K. performed the siRNA studies. R.O., CvE, R.W. and S.K. wrote the article. S.K. obtained funding and supervised the project.
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Agbaga MP, Brush RS, Mandal MN, Henry K, Elliott MH, Anderson RE. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. PNAS. 2008;105:12843–12848. doi: 10.1073/pnas.0802607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asheuer M, Bieche I, Laurendeau I, Moser A, Hainque B, Vidaud M, Aubourg P. Decreased expression of ABCD4 and BG1 genes early in the pathogenesis of X-linked adrenoleukodystrophy. Hum Mol Genet. 2005;14:1293–1303. doi: 10.1093/hmg/ddi140. [DOI] [PubMed] [Google Scholar]
- Aubourg P, Adamsbaum C, Lavallard-Rousseau MC, Rocchiccioli F, Cartier N, Jambaque I, Jakobezak C, Lemaitre A, Boureau F, Wolf C. A two-year trial of oleic and erucic acids (“Lorenzo's oil”) as treatment for adrenomyeloneuropathy. N Engl J Med. 1993;329:745–752. doi: 10.1056/NEJM199309093291101. [DOI] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Engelen M, Ofman R, Mooijer PAW, Poll-The BT, Wanders RJA, Kemp S. Cholesterol-deprivation increases mono-unsaturated very long-chain fatty acids in skin fibroblasts from patients with X-linked adrenoleukodystrophy. Biochim Biophys Acta. 2008;1781:105–111. doi: 10.1016/j.bbalip.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Engelen M, Ofman R, Dijkgraaf MGW, Hijzen M, van der Wardt LA, van Geel BM, de Visser M, Wanders RJA, Poll-The BT, Kemp S. Lovastatin in X-linked adrenoleukodystrophy. N Engl J Med. 2010;362:276–277. doi: 10.1056/NEJMc0907735. [DOI] [PubMed] [Google Scholar]
- Fourcade S, Lopez-Erauskin J, Galino J, Duval C, Naudi A, Jove M, Kemp S, Villarroya F, Ferrer I, Pamplona R, et al. Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum Mol Genet. 2008;17:1762–1773. doi: 10.1093/hmg/ddn085. [DOI] [PubMed] [Google Scholar]
- Fourcade S, Ruiz M, Camps C, Schluter A, Houten SM, Mooyer PAW, Pampols T, Dacremont G, Wanders RJA, Giros M, et al. A key role for the peroxisomal ABCD2 transporter in fatty acid homeostasis. Am J Physiol Endocrinol Metab. 2009;296:E211–E221. doi: 10.1152/ajpendo.90736.2008. [DOI] [PubMed] [Google Scholar]
- Ho JK, Moser H, Kishimoto Y, Hamilton JA. Interactions of a very long chain fatty acid with model membranes and serum albumin. Implications for the pathogenesis of adrenoleukodystrophy. J Clin Invest. 1995;96:1455–1463. doi: 10.1172/JCI118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kemp S, Wei HM, Lu JF, Braiterman LT, McGuinness MC, Moser AB, Watkins PA, Smith KD. Gene redundancy and pharmacological gene therapy: implications for X-linked adrenoleukodystrophy. Nat Med. 1998;4:1261–1268. doi: 10.1038/3242. [DOI] [PubMed] [Google Scholar]
- Kemp S, Valianpour F, Denis S, Ofman R, Sanders RJ, Mooyer P, Barth PG, Wanders RJA. Elongation of very long-chain fatty acids is enhanced in X-linked adrenoleukodystrophy. Mol Genet Metab. 2005;84:144–151. doi: 10.1016/j.ymgme.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Moser HW, Kawamura N, Platt M, Pallante SL, Fenselau C. Adrenoleukodystrophy: evidence that abnormal very long chain fatty acids of brain cholesterol esters are of exogenous origin. Biochem Biophys Res Commun. 1980;96:69–76. doi: 10.1016/0006-291x(80)91182-1. [DOI] [PubMed] [Google Scholar]
- Knazek RA, Rizzo WB, Schulman JD, Dave JR. Membrane microviscosity is increased in the erythrocytes of patients with adrenoleukodystrophy and adrenomyeloneuropathy. J Clin Invest. 1983;72:245–248. doi: 10.1172/JCI110963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Zadravec D, Jacobsson A. ELOVL2 overexpression enhances triacylglycerol synthesis in 3T3-L1 and F442A cells. FEBS Lett. 2007;581:3157–3163. doi: 10.1016/j.febslet.2007.05.081. [DOI] [PubMed] [Google Scholar]
- Leonard AE, Pereira SL, Sprecher H, Huang YS. Elongation of long-chain fatty acids. Prog Lipid Res. 2004;43:36–54. doi: 10.1016/s0163-7827(03)00040-7. [DOI] [PubMed] [Google Scholar]
- McGuinness MC, Zhang HP, Smith KD. Evaluation of pharmacological induction of fatty acid beta-oxidation in X-linked adrenoleukodystrophy. Mol Genet Metab. 2001;74:256–263. doi: 10.1006/mgme.2001.3239. [DOI] [PubMed] [Google Scholar]
- Moon YA, Shah NA, Mohapatra S, Warrington JA, Horton JD. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J Biol Chem. 2001;276:45358–45366. doi: 10.1074/jbc.M108413200. [DOI] [PubMed] [Google Scholar]
- Moser AB, Kreiter N, Bezman L, Lu S, Raymond GV, Naidu S, Moser HW. Plasma very long chain fatty acids in 3,000 peroxisome disease patients and 29,000 controls. Ann Neurol. 1999;45:100–110. doi: 10.1002/1531-8249(199901)45:1<100::aid-art16>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Moser HW, Smith KD, Watkins PA, Powers J, Moser AB. X-linked adrenoleukodystrophy. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw Hill; 2001. pp. 3257–3301. [Google Scholar]
- Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, Poustka AM, Mandel JL, Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- Oh CS, Toke DA, Mandala S, Martin CE. ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J Biol Chem. 1997;272:17376–17384. doi: 10.1074/jbc.272.28.17376. [DOI] [PubMed] [Google Scholar]
- Peters C, Charnas LR, Tan Y, Ziegler RS, Shapiro EG, DeFor T, Grewal SS, Orchard PJ, Abel SL, Goldman AI, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104:881–888. doi: 10.1182/blood-2003-10-3402. [DOI] [PubMed] [Google Scholar]
- Powers JM, Pei Z, Heinzer AK, Deering R, Moser AB, Moser HW, Watkins PA, Smith KD. Adreno-leukodystrophy: oxidative stress of mice and men. J Neuropathol Exp Neurol. 2005;64:1067–1079. doi: 10.1097/01.jnen.0000190064.28559.a4. [DOI] [PubMed] [Google Scholar]
- Singh I, Moser AE, Moser HW, Kishimoto Y. Adrenoleukodystrophy: impaired oxidation of very long chain fatty acids in white blood cells, cultured skin fibroblasts, and amniocytes. Pediatr Res. 1984;18:286–290. doi: 10.1203/00006450-198403000-00016. [DOI] [PubMed] [Google Scholar]
- Singh I, Khan M, Key L, Pai S. Lovastatin for X-Linked adrenoleukodystrophy. N Engl J Med. 1998;339:702–703. doi: 10.1056/NEJM199809033391012. [DOI] [PubMed] [Google Scholar]
- Toke DA, Martin CE. Isolation and characterization of a gene affecting fatty acid elongation in Saccharomyces cerevisiae. J Biol Chem. 1996;271:18413–18422. doi: 10.1074/jbc.271.31.18413. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Sano T, Ariga T, Miyatake T. Increased synthesis of hexacosanoic acid (C26:0) by cultured skin fibroblasts from patients with adrenoleukodystrophy (ALD) and adrenomyeloneuropathy (AMN) J Biochem (Tokyo) 1981;90:1233–1236. doi: 10.1093/oxfordjournals.jbchem.a133578. [DOI] [PubMed] [Google Scholar]
- Tvrdik P, Westerberg R, Silve S, Asadi A, Jakobsson A, Cannon B, Loison G, Jacobsson A. Role of a new mammalian gene family in the biosynthesis of very long chain fatty acids and sphingolipids. J Cell Biol. 2000;149:707–718. doi: 10.1083/jcb.149.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valianpour F, Selhorst JJ, van Lint LE, van Gennip AH, Wanders RJ, Kemp S. Analysis of very long-chain fatty acids using electrospray ionization mass spectrometry. Mol Genet Metab. 2003;79:189–196. doi: 10.1016/s1096-7192(03)00098-2. [DOI] [PubMed] [Google Scholar]
- van Roermund CWT, Visser WF, IJlst L, van Cruchten A, Boek M, Kulik W, Waterham HR, Wanders RJA. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22:4201–4208. doi: 10.1096/fj.08-110866. [DOI] [PubMed] [Google Scholar]
- Vasireddy V, Uchida Y, Salem N, Jr, Kim SY, Mandal MN, Reddy GB, Bodepudi R, Alderson NL, Brown JC, Hama H, et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (> or =C28) and the unique omega-O-acylceramides in skin leading to neonatal death. Hum Mol Genet. 2007;16:471–482. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg R, Tvrdik P, Unden AB, Mansson JE, Norlen L, Jakobsson A, Holleran WH, Elias PM, Asadi A, Flodby P, et al. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J Biol Chem. 2004;279:5621–5629. doi: 10.1074/jbc.M310529200. [DOI] [PubMed] [Google Scholar]
- Whitcomb RW, Linehan WM, Knazek RA. Effects of long-chain, saturated fatty acids on membrane microviscosity and adrenocorticotropin responsiveness of human adrenocortical cells in vitro. J Clin Invest. 1988;81:185–188. doi: 10.1172/JCI113292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kniazeva M, Han M, Li W, Yu Z, Yang Z, Li Y, Metzker ML, Allikmets R, Zack DJ, et al. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat Genet. 2001;27:89–93. doi: 10.1038/83817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


