Abstract
Renal and urogenital disease is a prevalent finding of extrapulmonary Mycobacterium tuberculosis. Patients can present with unusual complaints not immediately suspicious for tuberculosis. We describe a 38-year-old man who presented with vomiting and an acute kidney injury. Imaging studies showed nodules throughout the lungs, retroperitoneum, abdominal viscera, and kidneys. Asymmetrical hydronephrosis was found on renal imaging. A classic beaded ureteral appearance (ureteritis cystica) was found during retrograde pyelography. The patient was screened and found to have a negative purified protein derivative skin test, negative acid-fast bacilli of sputum in three samples, and an indeterminate QuantiFERON Gold test. Evaluation of the urine for acid-fast bacilli was negative in four separate samples. A fifth urine sample for acid-fast bacilli was positive. A renal biopsy showed granulomatous interstitial nephritis with multiple areas of caseous necrosis. The patient was diagnosed with active tuberculosis and started on traditional four-drug antituberculous medical therapy. Kidney function remained marginal but did mildly improve with volume expansion and urinary drainage.
A 38-year-old Mexican man presented with progressive nausea and vomiting, explaining that he had had dry mouth, nausea, and nonbloody emesis for several weeks. He had noticed a 10-pound weight loss, but he was not anorectic. Urinary hesitancy without dysuria had been an increasing problem for several months. He denied fever, chills, cough, dizziness, syncope, chest pain, shortness of breath, swelling, rash, or leg weakness; he was not taking any medications and had no allergies; and he denied use of tobacco, alcohol, or street drugs. He had undergone a left-sided orchiectomy for a nonhealing abscess in Mexico over 20 years earlier. He was born in Mexico and moved to Houston as a young adult, living there 13 years before moving to Dallas several years ago. He worked in construction and described no recent travel, known sick contacts, or prior exposure to tuberculosis.
His initial vital signs were normal (temperature, 98.2°F; blood pressure, 124/87 mm Hg; heart rate, 105 beats per minute; respirations, 16 breaths per minute with 97% oxygen saturation), and his lung auscultation was clear. His abdomen was diffusely tender to palpation with no guarding or rigidity. The left testicle was missing with a well-healed scrotal scar and round soft right testicle. There was no skin rash or edema.
His pertinent laboratory results were as follows: white blood cell count, 14.4 K/uL, with 90% neutrophils, 2% lymphocytes, and no bands; hemoglobin, 11.4 g/dL; hematocrit, 34.1%; sodium, 125 mEq/L; potassium, 5.0 mEq/L; chloride, 80 mEq/L; bicarbonate, 19 mEq/L; anion gap, 26 mEq/L; blood urea nitrogen, 190 mg/dL; creatinine, 19.7 mg/dL; glucose, 151 mg/dL; total protein, 9.7 g/dL; albumin, 4.5 g/dL; and lactate, 1.3 mEq/L. Urinalysis results revealed a specific gravity of 1.015, pH of 6, 2+ protein, 2+ blood, 4 red blood cells per high-power field, 50 to 100 white blood cells per high-power field, and positive dipstick for leukocyte esterase and nitrite. In spot urine chemistries, the fractional excretion of sodium was 12.5%. Timed urine collection revealed a urinary volume of 2250 mL/day, a creatinine clearance of 6 mL/min, a urea clearance of 4 mL/min, and urine protein of 1.5 g/day.
Several imaging studies were completed. Renal sonography revealed a 13.0 × 7.0 cm right kidney with a 2.9 × 3.7 cm anechoic lesion in the superior pole and mild hydronephrosis, and a 13.4 × 8.5 cm left kidney with numerous echogenic lesions in the posterior pole up to 1.3 cm and severe hydronephrosis (Figure 1). No ureteral jets were visualized. Computed tomography (CT) of the abdomen and pelvis without contrast revealed multilocular renal cysts, 7.6 cm in the left kidney and 3.7 cm in the right kidney, multiple calcified retroperitoneal lymph nodes up to 13 mm in size, and calcific deposits in the prostate and both seminal vesicles. CT of the chest (Figure 2) revealed innumerable 1 to 2 mm nodules in both lungs, predominantly in the upper lobes. A chest radiograph revealed increased density in the medial left lung apex.
Figure 1.
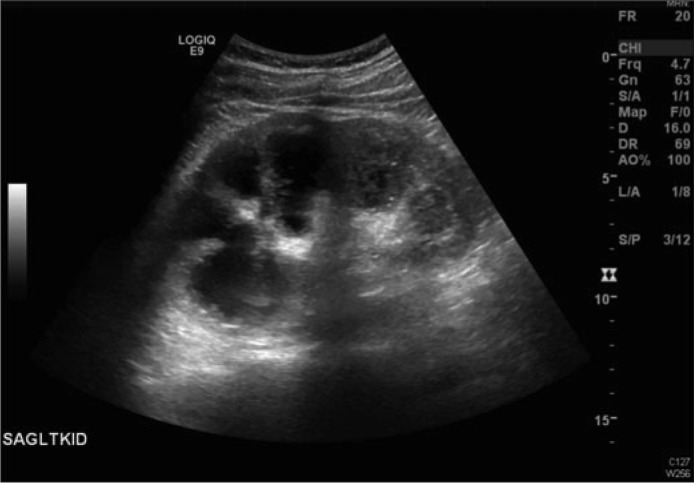
Renal sonogram of the left kidney, which was 13.4 cm in length, showing severe hydronephrosis and posterior acoustic shadowing compatible with debris.
Figure 2.
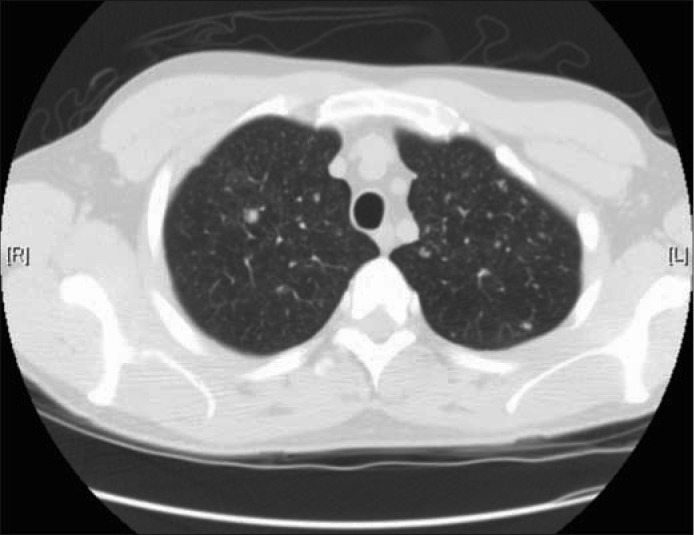
Chest CT showing innumerable diffuse small nodules throughout the lungs and a 6 mm nodule in the right upper lobe.
On Day 2, the patient's renal function was unchanged. Because of difficulties placing the Foley catheter, urology was consulted. The catheter was placed with difficulty during cystoscopy because of a rigid “leadpipe” urethra. The bladder contained a large amount of white debris, which was adherent to the bladder mucosa but easily irrigated off the mucosa. The bladder debris was sent for bacterial, fungal, and mycobacterial culture. Retrograde pyelography revealed an irregular beaded appearance of both ureters consistent with ureteritis cystica (Figure 3). Bilateral ureteral stents were placed, and the Foley catheter was left in place. Renal biopsy was performed, which showed several areas of granulomatous interstitial nephritis with multiple areas of caseous necrosis (Figure 4). There was evidence of extensive tubular atrophy. Visualized glomeruli were normal with no glomerulosclerosis. Fungal and mycobacterial stains were negative. All initial tuberculosis tests were negative, including a purified protein derivative skin test, two sputum acid-fast bacilli tests, and three urine stains for acid-fast bacilli. In addition, a QuantiFERON TB Gold test was indeterminate.
Figure 3.
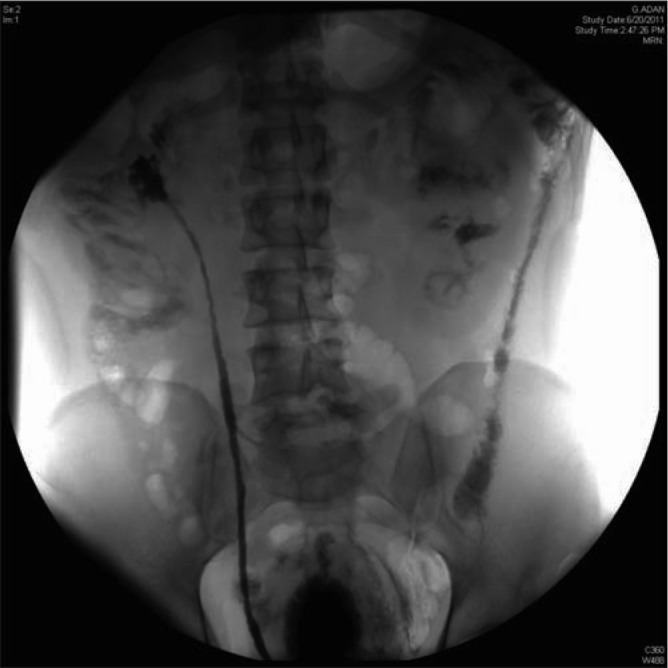
A pyelogram showing an irregular beaded appearance of the right ureter consistent with ureteritis cystica.
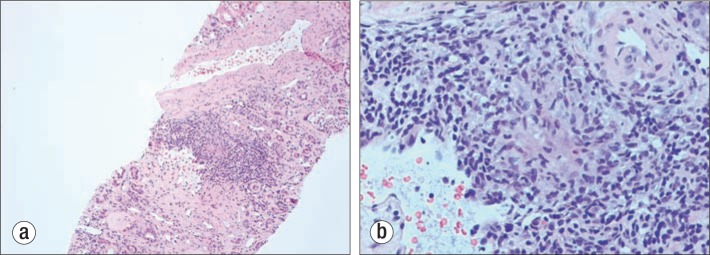
Figure 4.
Renal biopsy with hematoxylin-eosin stain. (a) A low-power view (10×) showing granulomatous interstitial nephritis. (b) A high-power view (100×) showing a classic granuloma.
The patient's renal function improved throughout his hospital stay with volume expansion and continued urinary drainage. Repeat timed urine collection revealed urinary volume of 2800 mL/day, creatinine clearance of 10 mL/min, urea clearance of 7 mL/min, and urinary protein of 1.5 g/day. No hemodialysis was needed. Lung nodules and retroperitoneal lymph nodes were felt to be too small for successful biopsy.
A fifth urine test for acid-fast bacilli on day 11 yielded a positive result. Culture of the bladder debris ultimately returned positive for Mycobacterium tuberculosis after a 7-week incubation. It was concluded that the patient had disseminated M. tuberculosis with both pulmonary involvement and extrapulmonary spread. The patient was started on rifampin, isoniazid, pyrazinamide, and ethambutol, with plans for 9 months of directly observed therapy.
At discharge, renal chemistries had stabilized with a blood urea nitrogen of 134 mg/dL and creatinine of 9.9 mg/dL. It was felt that the patient would likely need hemodialysis within the next several weeks or months. An arteriovenous fistula was placed in preparation for hemodialysis. His volume status was stable, with urine output matching oral fluid intake. He had no uremic symptoms.
DISCUSSION
Tuberculosis is the second most common cause of infectious disease–related mortality in the world behind HIV (1). Data from the World Health Organization in 2010 indicated that one third of the world population has been exposed to tuberculosis and has at least latent disease (2). The world incidence of active tuberculosis ranges from 30 to 340 cases (mean 140) per 100,000 population, with a total of 9.4 million cases. The world prevalence of active tuberculosis ranges from 40 to 500 (mean 165) per 100,000 population, with a total of 14 million cases. The annual mortality from tuberculosis ranges from 2 to 50 (mean 19) per 100,000 population, with a total death rate of 1.3 million. Tuberculosis in the USA is usually confined to patients with malignancy, immunosuppression, and HIV infection.
The offending microorganism is M. tuberculosis. The tuberculosis bacilli are inhaled into the lung, where they are locally contained by leukocytes and macrophages. Some organisms cause active pulmonary disease, and others spread to distant locations as metastatic foci. Most patients with active tuberculosis present with pulmonary involvement. Extrapulmonary involvement occurs in only 20% of cases (3). After lymph node involvement, the most common site of extrapulmonary tuberculosis infection is the genitourinary system. Genitourinary tuberculosis accounts for 15% to 30% of all cases of extrapulmonary tuberculosis (4–9).
Renal infection with tuberculosis occurs by hematogenous spread of previously dormant organisms from a prior lung infection at the time of marginal cellular immunity (10). The tuberculosis organism has strict growth requirements, which makes the kidney, with its high oxygen tension, a prime site for new growth. The initial renal involvement is multiple cortical granulomas. As host defenses decline, the granulomas grow, coalesce, and ultimately result in caseous necrosis. An intense interstitial nephritis with severe calcification develops as the renal response to the now active infection. Contiguous spread of the infection may lead to ureteral and bladder involvement. Hematogenous-lymphatic spread can lead to involvement of the male (prostate-epididymis-testis) and female (uterus-fallopian tube) reproductive sites.
Renal involvement of tuberculosis often does not present with classic symptoms of fever, weight loss, or night sweats. Patients may present with usual lower urinary tract symptoms suggesting simple bacterial cystitis. Symptoms of renal colic are unusual. Initial urinalysis may show pyuria and hematuria, but no bacteria. Urine culture is often negative for usual bacterial organisms. Tuberculosis is one of the causes of sterile pyuria.
Often, renal imaging procedures will raise the suspicion of renal tuberculosis (11). Calyceal distortion and papillary necrosis may be seen on intravenous pyelogram studies. A markedly abnormal renal contour with multiple intrarenal calcifications on ultrasonography will suggest renal tuberculosis. Cystoscopy and retrograde pyelography can reveal a markedly abnormal urinary bladder and classic changes of ureteritis cystica. Hydronephrosis can be seen on any renal imaging procedure because of bladder or ureteral narrowing.
A definitive microbiologic diagnosis is made by isolating tuberculosis organisms from urine or tissue biopsy specimens. Acid-fast bacilli may be seen on microscopy of centrifuged urine. Renal histology reveals tubulointerstitial nephritis with granuloma formation, often with caseous necrosis (3, 12). Urine culture for acid-fast bacilli is the gold standard for diagnosis of renal tuberculosis; its specificity is 100%, but its sensitivity ranges from 30% to 90% depending on the number of samples tested (13–15). It may take 6 to 8 weeks before a positive urine culture becomes available.
Renal outcomes in renal tuberculosis are extremely variable. Diffuse renal scarring can lead to an autonephrectomy state with no function on the affected side. Spread of intrarenal infection to the renal pelvis can progress to pyonephrosis with a cement or “putty” kidney involving the entire renal pelvis. Ureteral involvement can lead to diffuse ureteral strictures with subsequent urinary obstruction on that side. Bladder scarring can lead to urinary obstruction and compromise of renal function as well. Progression to end-stage renal disease is distinctly unusual. Tuberculosis causes <1% of all cases of end-stage renal disease.
Treatment of renal tuberculosis involves multidrug therapy. This has evolved over the years to the current four-drug regimen involving initial 2-month intensive treatment with rifampin, isoniazid, pyrazinamide, and ethambutol (3, 16). This is usually followed with a 4-month maintenance regimen involving rifampin and isoniazid. Urologic intervention may be indicated for unilateral disease because of pain or hemorrhage or for bladder augmentation (17, 18). Relief of ureteral obstruction by stenting or percutaneous nephrostomy may be indicated, especially if underlying parenchymal renal disease is mild and residual renal function is >15 mL/min, where escape from the dialysis requirement might occur.
References
- 1.World Health Organization Tuberculosis [Fact sheet no. 104], March 2012. Available at http://www.who.int/mediacentre/factsheets/fs104/en/
- 2.World Health Organization . Global Tuberculosis Control 2011. Geneva, Switzerland: WHO; 2011. Available at http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf. [Google Scholar]
- 3.Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307–1314. doi: 10.1681/ASN.V1261307. [DOI] [PubMed] [Google Scholar]
- 4.Kretschmer HL. Tuberculosis of the kidney, a critical review based on a series of 221 cases. N Engl J Med. 1930;202:660–671. [Google Scholar]
- 5.Jacobs A. Renal tuberculosis. Br Med J. 1934;1(3818):420–424. doi: 10.1136/bmj.1.3818.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morse HD, Scott DL. Renal tuberculosis. Can Med Assoc J. 1940;42(5):424–427. [PMC free article] [PubMed] [Google Scholar]
- 7.Peacock AH. Renal tuberculosis: diagnosis and management. Chest. 1939;5:24–28. [Google Scholar]
- 8.Lattimer JK. Renal tuberculosis. N Engl J Med. 1965;273:208–211. doi: 10.1056/NEJM196507222730407. [DOI] [PubMed] [Google Scholar]
- 9.Christensen WI. Genitourinary tuberculosis: review of 102 cases. Medicine. 1974;53(5):377–390. doi: 10.1097/00005792-197409000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Chijioke A. Current concepts on pathogenesis of renal tuberculosis. West Afr J Med. 2001;20(2):107–110. [PubMed] [Google Scholar]
- 11.Gibson MS, Puckett ML, Shelly ME. Renal tuberculosis. Radiographics. 2004;24(1):251–256. doi: 10.1148/rg.241035071. [DOI] [PubMed] [Google Scholar]
- 12.Chapagain A, Dobbie H, Sheaff M, Yaqoob MM. Presentation, diagnosis, and treatment outcome of tuberculous-mediated tubulointerstitial nephritis. Kidney Int. 2011;79(6):671–677. doi: 10.1038/ki.2010.482. [DOI] [PubMed] [Google Scholar]
- 13.Chun HM, Hale B. Renal tuberculosis: three cases and a review of the literature. Infect Dis Clin Prac. 2004;12(2):117–122. [Google Scholar]
- 14.Najar MS, Bhat MA, Wani IA, Banday KA, Reshi AR, Daga BA, Fazili TH. Profile of renal tuberculosis in 63 patients. Ind J Nephrol. 2003;13:104–107. [Google Scholar]
- 15.Chaudhari AP, Ranganath R, Pavan M. Unusual presentation of renal tuberculosis. Iran J Kidney Dis. 2011;5(3):207–209. [PubMed] [Google Scholar]
- 16.Lattimer JK, Wechsler H. Spirito AL, Whittle GT. Treatment of renal tuberculosis with triple-drug therapy; use of a combination of streptomycin, isoniazid, and sodium aminosalicylic acid. J Am Med Assoc. 1956;160(7):544–546. doi: 10.1001/jama.1956.02960420024007. [DOI] [PubMed] [Google Scholar]
- 17.Cek M, Lenk S, Naber KG, Bishop MC, Johansen TE, Botto H, Grabe M, Lobel B, Redorta JP, Tenke P, Members of the Urinary Tract Infection (UTI) Working Group of the European Association of Urology (EAU) Guidelines Office EAU guidelines for the management of genitourinary tuberculosis. Eur Urol. 2005;48(3):353–362. doi: 10.1016/j.eururo.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamoorthy S, Gopalakrishnan G. Surgical management of renal tuberculosis. Indian J Urol. 2008;24(3):369–375. doi: 10.4103/0970-1591.42620. [DOI] [PMC free article] [PubMed] [Google Scholar]