Abstract
That the renin–angiotensin system (RAS) is involved in regulation of blood pressure, vasoconstriction, sodium intake and potassium excretion is well established. Studies in the last few years have however documented new roles for this molecule as a pro-inflammatory molecule and more recently as a possible pro-fibrotic agent that contributes to progressive deterioration of organ function in disease. Binding of Ang II to its receptors (in particular AT1) mediates intracellular free radical generation that contributes to tissue damage by promoting mitochondrial dysfunction. Blocking Ang II signalling protects against neurodegenerative processes and promotes longevity in rodents. Altogether these findings open the unanticipated perspective for exploring Ang II signalling in therapeutic interventions in inflammatory diseases and aging-related tissue injury. This review extends from the discovery of Ang II and its implications in renal and cardiovascular physiology to cover the roles of the system in inflammation, tissue injury, autoimmunity, oxidative stress and aging.
Keywords: angiotensin II, inflammation, autoimmune disease, Alzheimer's disease, longevity
Introduction
To control body fluid volume homeostasis is essential to life and the complex system devoted to this task in humans is the result of an evolutionary process that was shaped by survival responses (Yun et al, 2006). To respond to trauma for example, activation of the sympathetic nervous system promotes the release of renin, angiotensin, aldosterone, catecholamine and natriuretic peptide whose effects maintain fluid pressure in the circulation despite volume loss due to haemorrhage (Helwig et al, 1956; Yun et al, 2006). The maintenance of volume homeostasis allowed animals to migrate from the sea's salty water to fresh water and dry lands (Smith, 1953). In human evolution, the renin–angiotensin system (RAS) played an important role given its ability to control salt intake and stimulate thirst. The study of contemporary primitive tribes suggests that hunting–gathering ancestors survived on little salt possibly thanks to RAS activation without developing hypertension (Lev-Ran & Porta, 2005). As eating habits changed, salt intake also increased, turning blood pressure increase mediated by RAS activation into a negative factor. Thus, natural selection was strongly influenced by the development of hypertension, whose incidence varied widely among populations with different geographic and ethnic origins (Wooding, 2004). In ancient human populations residing in hot and humid areas like the tropics, the tendency to retain salt as an adaptation to low salt availability and the risk of electrolyte imbalance evolved (Gleibermann, 1973). By contrast, populations in cooler, drier climates such as the temperate zones, adapted to conditions of greater sodium availability and less sodium loss (Gleibermann, 1973). In this regard, variations in the human RAS genes might account for some of the regional differences in hypertension susceptibility (Nakajima et al, 2004). Beside salt retention and hypertension, recent studies have unravelled roles of RAS and particularly, its main effector molecule angiotensin II (Ang II) in inflammation, autoimmunity and aging. The aim of the present review is to highlight such novel roles of Ang II and their possible clinical implications.
The Effects of the Renin–Angiotensin System Components
In 1898, Robert Tigerstedt and his student Per Gunnar Bergman discovered the presence of a pressor compound in the renal cortex extracts of the rabbit that they named renin (Tigerstedt & Bergman, 1898). The interest in the nature of the pressor substance released by the kidney was renewed when in 1934, Henry Goldblatt demonstrated that constriction of dog renal arteries with silver clamps produced chronic hypertension (Goldblatt et al, 1934). Using the same technique, Braun-Menéndez et al, as well as Page and Helmer later demonstrated the renal secretion of yet another compound with a quick pressor action of very short duration, angiotensin (Braun-Menéndez et al, 1940; Braun-Menéndez & Page, 1958; Page & Helmer, 1940). Since these early studies our understanding of Ang II in physiology and pathophysiology has improved considerably.
Glossary
Aldosterone
Aldosterone is a steroid hormone that acts on the distal tubules and collecting ducts of the nephron causing sodium retention, potassium secretion, water retention and blood pressure increase.
Alzheimer's disease
Alzheimer's disease is the most common form of dementia with advancing age. It is associated with a deterioration of memory and other cognitive domains.
Angiotensin II
Angiotensin II is the main effector molecule of the RAS. It causes increases in blood pressure, influences renal tubuli to retain sodium and water, and stimulates aldosterone release from adrenal gland. Besides being a potent vasoconstrictor, Ang II also exerts proliferative, pro-inflammatory and pro-fibrotic activities.
Bradykinin
Bradykinin is a potent endothelium-dependent vasodilator, causes dilation of non-vascular smooth cells, increases vascular permeability, promotes natriuresis and reduces blood pressure.
Catecholamine
Catecholamines are neuromodulators released by the adrenal glands in response to stress causing increases in heart rate, blood pressure and blood glucose levels.
Dendritic cells
Dendritic cells are “professional” antigen-presenting cells, which capture and present the antigen to the T cells.
Macrophage
Macrophages are white blood cells that phagocyte cellular debris and pathogens and stimulate lymphocytes and other immune cells to respond to the pathogen.
Multiple sclerosis
Multiple sclerosis is an autoimmune disease in which the fatty myelin sheaths around the axons of the brain and spinal cord are damaged, leading to demyelization and scarring as well as a broad spectrum of signs and symptoms.
Natriuretic peptide
Natriuretic peptides (NPs) are peptide hormones that are synthesized by the heart, brain and other organs and are involved in the long-term regulation of sodium and water balance, blood volume and arterial pressure.
Pre-eclampsia
Pre-eclampsia is a pregnancy condition in which high blood pressure and proteinuria develop after the 20th week of pregnancy.
Regulatory T cells
A specialized subpopulation of T cells that act to suppress activation of the immune system and thereby maintain immune system homeostasis and tolerance to antigens.
Renin
Renin is secreted by the kidney from specialized cells called granular cells of the juxtaglomerular apparatus. Renin activates the RAS by cleaving angiotensinogen, produced by the liver, to produce angiotensin I.
Sirtuins
Sirtuins are nicotinamide adenine dinucleotide (NAD)-dependent deacetylases associated with mitochondrial and cell cycle regulation, apoptosis, DNA damage repair and longevity.
Systemic lupus erythematosus
Systemic lupus erythematosus is an autoimmune disease with numerous immunological and clinical manifestations. In particular, acute or chronic renal impairment may develop with lupus nephritis leading to acute or end-stage renal failure.
Th1
A CD4+ helper T-cell that produces the cytokines IFN-γ and IL-2.
Th2
A CD4+ helper T-cell that produces the cytokines IL-4, IL-5 and IL-13.
Th17
A recently discovered helper T-cell that produces IL-17.
Toll-like receptors
Proteins that act as sensors for the presence of microorganisms and tissue damage and activate immune cell responses.
Angiotensin peptides
Ang II is an octapeptide produced from the substrate angiotensinogen through sequential enzymatic cleavages by renin and angiotensin converting enzyme (ACE). Specifically, renin cleaves angiotensinogen, forming Ang I that in turn is converted to Ang II by ACE. The angiotensinogen substrate is produced in the liver, while renin is produced in the kidney and Ang II in the vascular tissue (Timmermans et al, 1993). ACE is a circulating enzyme that also degrades bradykinin to inactive fragments, reducing the serum levels of endogenous vasodilators (Brewster & Perazella, 2004; Fleming, 2006). The genetic analysis of ACE has revealed an important insertion (I)/deletion (D) polymorphism, a 287 bp DNA sequence in the intron 16 of chromosome 17 in the ACE gene, a major locus that accounts for approximately 50% of the total phenotypic variance of circulating and tissue ACE (Rigat et al, 1990). The ACE I/D polymorphism is a reliable tool to identify patients at risk to renal disease progression and to elect those who may benefit the most from treatment with Ang II blockers (Ruggenenti et al, 2008a). It is conceivable that the same applies to patients with cardiovascular disease or progressive deterioration of brain function including senile dementia and Alzheimer, but studies of this kind are not available at the moment.
ACE2 is another carboxypeptidase that cleaves one aminoacid from Ang II leading to the production of the heptapeptide vasodilatory Ang 1–7 (Crackower et al, 2002; Ferrario & Chappell, 2004) and the balance between ACE and ACE2 is crucial for controlling Ang II levels (Danilczyk & Penninger, 2006). Ang II levels can also be regulated by chymase, an enzyme expressed in the heart by mast cells, endothelial and mesenchymal interstitial cells (Urata et al, 1993) and in the kidney by mesangial and vascular smooth muscle cells (Huang et al, 2003). Chymase-mediated Ang II production has emerged as an alternative pathway to ACE in cardiac, vascular and renal tissue, particularly in disease conditions (Bacani & Frishman, 2006; Huang et al, 2003; Miyazaki & Takai, 2006) (Fig 1). Finally, Ang II can also be cleaved in the circulation by other aminopeptidases generating Ang (2–8) (Ang III) and Ang (3–8) (Ang IV). Ang III has similar effects to Ang II, although with lower potency, of enhancing blood pressure and vasopressin release (Cesari et al, 2002; Reaux et al, 2001) and stimulating the expression of pro-inflammatory mediators in cultured renal cells (Ruiz-Ortega et al, 2000). Ang IV exerts a protective role by increasing blood flow in the kidney (Hamilton et al, 2001) and brain (Kramar et al, 1997).
Figure 1. The renin–angiotensin system (RAS).
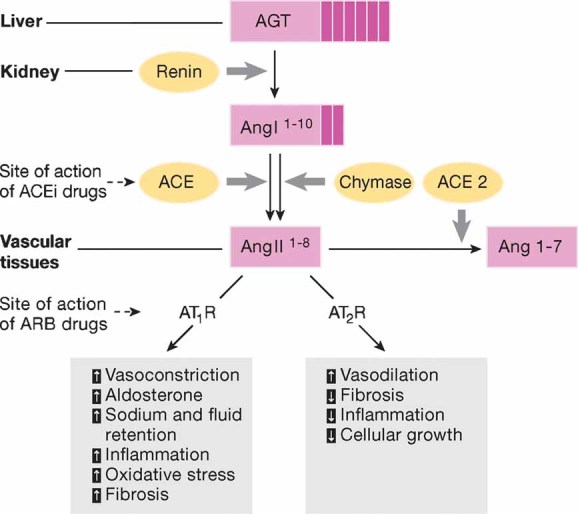
Renin, a protease produced in the kidney, cleaves the AGT to produce the inactive decapeptide angiotensin I. Cleavage of angiotensin I by ACE or alternatively by chymase produces the active octapeptide Ang II that acts via AT1 and AT2 receptors. Ang II levels are also regulated by ACE2 that cleaves Ang II to produce the heptapetide vasodilatory Ang 1–7. AGT, angiotensinogen; ACE, angiotensin converting enzyme; AT1R, angiotensin type 1 receptor; AT2R, angiotensin type 2 receptor; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Circulating Ang II contributes to increased blood pressure and influences renal tubuli to retain sodium and water (Brewster & Perazella, 2004; Kim & Hiroshi, 2003). One of the most significant advances in the field, in the past two decades has been the discovery of local or tissue RAS. The local system is characterized by the presence of RAS components in several organs including the heart (Van Kats et al, 1998), kidney (Kobori et al, 2004), brain (Moulik et al, 2002) and pancreas (Ghiani & Masini, 1995), as well as reproductive (Thomas & Sernia, 1990), lymphatic (Iwai et al, 1996) and adipose tissues (Karlsson et al, 1998). The local RAS exerts different functions in each organ and it can operate independently, as in the adrenal glands and brain, or in close interaction with circulating RAS as in the heart and kidney. In addition a functional intracellular RAS has been identified (De Mello, 2003; Re & Cook, 2006). The discovery of local and intracellular RAS highlights several prominent non-haemodynamic effects of Ang II including pro-inflammatory, proliferative and pro-fibrotic activities. Ang II promotes reactive oxygen species (ROS) production, cell growth, apoptosis, cell migration and differentiation, extracellular matrix remodelling, regulates gene expression and can activate multiple intracellular signalling pathways leading to tissue injury (Ruster & Wolf, 2006). In tissues such as kidney, heart and vasculature, Ang II induces an inflammatory response by fostering the expression of pro-inflammatory chemokines, responsible for tissue accumulation of immunocompetent cells (Suzuki et al, 2003). In hypertension, a noxious amplification mechanism occurs in the kidney, in which Ang II induces renal angiotensinogen expression and thereby its own synthesis (Kobori et al, 2001).
Angiotensin receptors
Angiotensin II acts through two pharmacologically distinct G protein-coupled receptors, angiotensin type 1 and the type 2 (AT1 and AT2) receptors (Hunyady & Catt, 2006; Porrello et al, 2009). Human cells express a single AT1 receptor, while two isoforms, AT1A and AT1B with 95% of aminoacid sequence identity, can be found in rat and mouse. The AT1A receptor, the closest murine homologue to the human AT1 receptor, is expressed in the kidney, heart, brain adrenal gland, vascular smooth muscle, liver and several other tissues (Burson et al, 1994). AT1B on the other hand is predominantly expressed in anterior pituitary gland and adrenal zona glomerulosa (Oliverio & Coffman, 2000). AT1A confers most of classical actions of Ang II such as blood pressure increase (Ito et al, 1995), aldosterone release from the adrenal zona glomerulosa (Aguilera, 1992), salt retention in proximal tubular cells (Thekkumkara et al, 1998) and stimulation of the sympathetic nervous system via receptors in the brain (Davisson et al, 2000). AT1B regulates blood pressure when AT1A receptor is absent (Oliverio & Coffman, 2000). Angiotensin type II receptor is ubiquitously expressed in developing foetal tissues, it decreases after birth remaining low in various adult tissues including adrenal medulla, uterus, ovary, vascular endothelium and distinct brain areas (Steckelings et al, 2005). AT1 and AT2 receptor have counter-regulatory actions in the cardiovascular and renal system (reviewed by Schulman and Raij (2008)). Ang II binding to the AT2 receptor induces vasodilation in resistance and conduit arteries and improves artery remodelling in humans and mice. The AT2 receptor is upregulated in conditions associated with cardiovascular injury and it exerts a cardio-protective role against ischemia-reperfusion injury and acute myocardial infarction (Schulman & Raij, 2008). Furthermore, this receptor is protective against kidney fibrosis and ischemic renal injury as lack of AT2 receptor aggravates renal injury and reduces survival in a mouse renal ablation model (Benndorf et al, 2009). A pro-inflammatory role of the AT2 receptor, through activation of the NF-κB pathway has also been proposed in response to Ang II (Esteban et al, 2004; Ruiz-Ortega et al, 2003; Wolf et al, 2002).
Finally, the AT1 and AT2 receptors are also binding site for Ang III, while the AT4 receptor is specific for Ang IV and is expressed in brain, kidney, heart and vessels (de Gasparo et al, 2000).
Beyond Blood Pressure Control, Angiotensin II Promotes Inflammation and Tissue Injury
As discussed above, the roles of Ang II go well beyond controlling circulatory homeostasis. Recently, a large number of experimental studies have shown that Ang II mediates several key events of the inflammatory processes (Fig 2) (Marchesi et al, 2008). Inflammation involves activation of the endothelium of blood vessels and expression of diverse endothelial cell selectins that dictate extravasation of specific leukocyte populations to the site of injury (Medzhitov & Horng, 2009; Pober & Sessa, 2007). In the context of an inflammatory process, local activation of RAS and Ang II synthesis both increased vascular permeability by promoting the expression and secretion of VEGF (vascular endothelial growth factor) (Chua et al, 1998; Kitayama et al, 2006; Suzuki et al, 2003), and induced the expression of endothelial adhesive molecules including selectins (P- and L-selectin), vascular cell adhesion molecules-1 (VCAM-1), intercellular adhesion molecules-1 (ICAM-1) and their ligands, the integrins (Alvarez et al, 2004; Piqueras et al, 2000; Pueyo et al, 2000). Ang II also promotes endothelial dysfunction through COX-2 activation, which generates vasoactive prostaglandins and ROS (Welch, 2008; Wu et al, 2005). Moreover, Ang II favours the recruitment of infiltrating inflammatory cells into tissues by stimulating the production of specific cytokine/chemokines. For example, Ang II induces the production of the potent monocyte chemoattractant MCP-1 in cultured monocytes (Dai et al, 2007). In the aorta of spontaneously hypertensive rats, which bear elevated levels of Ang II, massive macrophage infiltration is accompanied by increased expression of MCP-1 and one of its receptors, the C–C chemokine receptor CCR2. Modulation of MCP-1/CCR2 via AT1 receptor blockade reduces vessel inflammation in these rats (Dai et al, 2007). Furthermore, Ang II-induced macrophage infiltration in the arterial wall is virtually absent in CCR2-deficient mice (Bush et al, 2000). In models of progressive nephropathies, interstitial accumulation of macrophages is accompanied by increased renal expression of MCP-1, and renoprotection afforded by the ACE inhibitor lisinopril limits interstitial inflammation and reduces MCP-1 expression (Donadelli et al, 2000). The pro-inflammatory activity of Ang II is also mediated by activation of dendritic cells (DCs), highly specialized antigen-presenting cells (APCs) responsible for inflammation defence and immune response. Cultured DCs express both Ang II receptors (Lapteva et al, 2001) and Ang II enhances DCs migration, maturation and antigen presenting ability (Lapteva et al, 2002; Muller et al, 2002; Nahmod et al, 2003). In rats with subtotal renal ablation, blockade of Ang II synthesis/biological activity reduces local DC accumulation and attenuates tubulointerstitial damage (Remuzzi et al, 1999; Wu et al, 2006). The beneficial effect of Ang II blockers on tissue inflammation also relies on their ability to block Ang II-mediated activation of Toll-like receptors (TLRs). In cultured mesangial and vascular smooth muscle cells, Ang II via AT1 receptor signalling stimulates TLR-4 expression, which promotes cellular oxidative injury, apoptosis and inflammation (Ji et al, 2009; Lv et al, 2009). These in vitro observations are paralleled by in vivo studies showing protection against myocardial ischemia/reperfusion injury after AT1 blockade through suppression of TLR-4 expression and reduction of cytokine release (Yang et al, 2009). The pro-inflammatory effects of Ang II can also involve T cells. T cells possess an endogenous RAS that modulates T cell proliferation and migration (Jurewicz et al, 2007), NAD(P)H activity and ROS production (Hoch et al, 2009). During inflammation, Ang II acts via its AT1 receptor to stimulate cytoskeletal rearrangements in T cells and to trigger the release of specific cytokines and chemokines that favour T cell recruitment to the sites of inflammation (Crowley et al, 2008; Jurewicz et al, 2007; Kvakan et al, 2009). Tissue infiltration of T cells contributes to the genesis of hypertension as documented by the blunted blood pressure increase to both Ang II infusion and DOCA-salt hypertension in Rag1−/− mice, which lack T and B cells. Adoptive transfer of T, but not B cells, restores the hypertensive response to Ang II in this knockout mouse strain (Guzik et al, 2007). Among T cell subsets, IL-17-producing T cells are critical for the maintenance of Ang II-induced hypertension (Madhur et al, 2010). Ang II can also affect T cell responses in transplantation. In renal-transplant patients, the presence in the serum of activating antibodies targeting the AT1 receptor may contribute to steroid-refractory vascular allograft rejection, and treatment with AT1 receptor antagonist losartan significantly improved allograft survival as compared with untreated patients (Dragun et al, 2005). Furthermore, passive transfer of AT1 receptor activating antibodies in rats receiving kidney transplant promotes vascular rejection and hypertension (Dragun et al, 2005).
Figure 2. The role of Ang II on tissue inflammation.
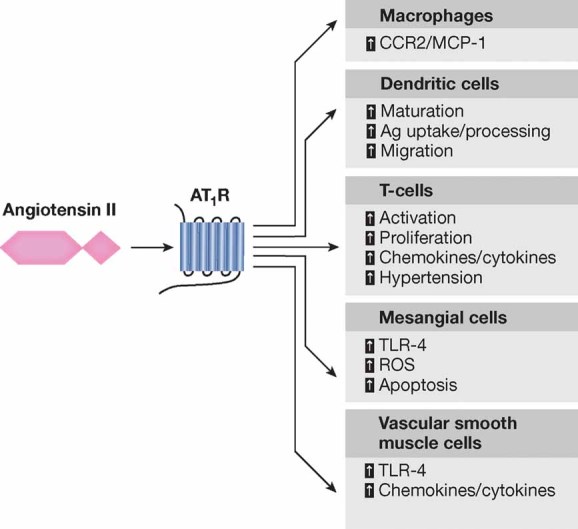
Angiotensin II via AT1 receptor signalling in immune cells as well as mesangial cells and vascular smooth muscle cells contributes to the localized activation of the immune system.
Angiotensin II and Autoimmunity
The recent observation that Ang II modulates T cell responses, suggests a possible role of the peptide in autoimmune diseases. RAS is critically involved in the development of Th1/Th17-mediated multiple sclerosis (MS) as shown in experimental autoimmune encephalomyelitis (EAE), a well-established mouse model for human MS (Platten et al, 2009). Peripheral CD4+T cells from EAE mice show increased levels of Ang II which acting through the AT1 receptor promote the synthesis of Th1 and Th17 cytokines, specifically IFN-γ and IL-17. Drugs that limit Ang II synthesis and its biological activity, such as the angiotensin converting enzyme inhibitor (ACEi) lisinopril or angiotensin receptor blocker (ARB) candesartan, result in the suppression of Th1 and Th17 cytokine release and the induction of powerful antigen-specific regulatory T cells (Treg) through the modulation of the NF-κB pathway. Of note, the adoptive transfer of Treg protects mice from severe signs of EAE (Platten et al, 2009). AT1 is also involved in promoting experimental autoimmune uveitis (EAU) and myocarditis (EAM) through its influence on T cell function. Administration of ARB suppresses EAU (Okunuki et al, 2009) and reduces the severity of myocardial lesions in EAM by inhibiting antigen-specific T cell activation (Liu et al, 2009) and contributing to the shift of Th1–Th2 immune response (Liu et al, 2009). A recent study also highlighted the role of AT1 receptors in glomerular inflammation associated with autoimmune disease in rodents by studying AT1A receptor-deficient (AT1A−/−) MLR-Faslpr/lpr (lpr) mice, which develop an autoimmune disease resembling human systemic lupus erythematosus (SLE) (Crowley et al, 2009). The Ang II type 1A receptor deficiency in lpr mice accelerates renal damage and mortality. Increased disease severity of AT1A−/− lpr mice is not a direct effect of immune cells since transplantation of bone marrow from AT1A−/− lpr donors does not affect survival of lpr wild-type recipient mice. Moreover, autoimmune injury in extrarenal tissues does not play a role in disease severity as the degree of injury in heart, joints and skin is comparable in wild-type and AT1A−/− lpr mice. Exacerbation of renal injury is instead attributed to the fact that in the absence of AT1A receptor exaggerated activation of the AT1B receptor, abundantly expressed in podocytes, occurs since blockade of all type 1 Ang II receptors by losartan reduces markers of kidney disease (Crowley et al, 2009). The role of the AT1B receptor as a pro-inflammatory mediator is also confirmed in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (MOG-EAE), another mouse model of MS (Stegbauer et al, 2009). In this model, the expression of renin, ACE, AT1A and in particular of the AT1B receptor is upregulated in macrophages, DCs and T cells and the course of the disease is ameliorated by treatment with RAS inhibitors (Stegbauer et al, 2009). Induction of EAE in AT1A−/− mice, which have unaltered levels of AT1B receptor, results in a severe disease which is cured by inhibiting the AT1B receptor with losartan highlighting the immunomodulatory and pro-inflammatory role of AT1B receptor (Stegbauer et al, 2009). In the late 1990s, Wallukat et al proposed that pre-eclampsia is a pregnancy-induced autoimmune disease in which the abnormalities in the formation of the placenta result from circulating autoantibodies that react with, and activate the AT1 receptor (Wallukat et al, 1999). Zhou et al have recently followed up on this suggestion and showed that pregnant mice injected with purified AT1-autoantibodies from women with pre-eclampsia develop typical signs of this condition such as hypertension, proteinuria, glomerular endotheliosis and placental abnormalities, all features that could be prevented by losartan administration (Zhou et al, 2008). This AT1-autoantibody-induced mouse model of pre-eclampsia is also accompanied by placental trophoblast cell apoptosis and significant foetal growth retardation (Irani et al, 2009).
Does Brain Ras Affect Cognitive Functions During Aging?
The discovery of the existence of a paracrine, locally acting RAS operating independently from the circulatory RAS has fostered studies addressing the role of brain RAS, in particular in what concerns its involvement in the pathophysiology of Alzheimer's disease, the most common form of dementia. Main risk factors for Alzheimer's disease are age, accumulation of misfolded proteins in the aged brain and deterioration of the cardiovascular system. Accumulation of amyloid-(A-beta) peptide results in oxidative and inflammatory damage, which in turn leads to energy failure and synaptic dysfunction (Luchsinger & Mayeux, 2004; Querfurth & LaFerla, 2010). Increased ACE activity has been observed in homogenates of post mortem brain tissue from Alzheimer's disease patients and correlated with A-beta plaque load and the severity of amyloid angiopathy (Arregui et al, 1982). More recently, elevated neuronal and perivascular immunoreactivity of ACE and Ang II surrounding the parietal and frontal cortex vessels in the brain of Alzheimer's disease patients has also been reported (Miners et al, 2008; Savaskan et al, 2001). Increased ACE activity would be expected to reduce brain perfusion, characteristic of Alzheimer's disease possibly through elevated production of Ang II (Kehoe et al, 2009). Several studies indicate that treatment with ARB and ACEi help to preserve cognitive functions through a mechanism that is independent from their antihypertensive effect (reviewed by Poon (2008) and Shah et al (2009)). In the Tg2576 Alzheimer's disease mouse model, preventive treatment with an AT1 receptor blocker reduces Alzheimer's disease-type neuropathology and attenuates the aggregation of A-beta peptide into extracellular amyloid plaque deposits in the brain (despite no detectable blood pressure-lowering activity) (Wang et al, 2007). In Alzheimer's disease patients, treatment with ACEi that cross the blood–brain barrier (BBB), such as perindopril and captopril, has a beneficial effect on the rate of cognitive decline, as compared to enalapril and imidapril that cannot cross the BBB or an antihypertensive calcium channel blocker (Ohrui et al, 2004). Results from a cohort analysis of 819,491 (mostly male) patients with Alzheimer's disease or dementia show that ARB treatment reduces the incidence and progression of the disease—measured as admission to a nursing home—as compared to ACEi or other medications that control vascular disease or hypertension. Combined treatment with ARB and ACEi shows additive benefits as indicated by reduced risk of Alzheimer's or dementia progression (Li et al, 2010). This protection is consistent with the mutual activity of the drugs on the AT1 receptor. The strength and the consistency of the protective effect of ARB clearly support the involvement of AT1 and Ang II in cognitive impairment of the aged brain. Blockade of the AT1 receptor might also allow the conversion of endogenous Ang II to Ang III and Ang IV. In these circumstances activation of the AT4 receptor by Ang IV could result in memory-potentiating effects (Wright & Harding, 2008).
Activation of brain RAS is also involved in the pathogenesis and progression of Parkinson's disease (Mertens et al, 2010). Treatment with ACEi or ARB have neuroprotective effects in mice with Parkinson's disease mainly due to the reduction of oxidative stress, a key player of the disease (Lopez-Real et al, 2005; Munoz et al, 2006). Neuroprotective action of ARB against ischemic injury has also been shown to prevent stroke in experimental and clinical studies (Schmieder et al, 2007; Turnbull, 2003).
Angiotensin II: A Key Player In Oxidative Stress Toxicity and Aging
We have previously referred to the impact of Ang II in ROS production. In fact, Ang II robustly stimulates the production of molecular oxygen species that trigger mitochondrial dysfunction and cellular injury (de Cavanagh et al, 2007; Wilson, 1990). Ang II via AT1 receptor stimulation can activate NAD(P)H oxidase to produce ROS, resulting in oxidative stress damage (Griendling et al, 1994). Harman has proposed that ROS are the most prominent molecular species involved in the aging process (Harman, 1956). According to his theory, ROS contribute significantly to various age-associated organ failures, including hypertension, cardiovascular diseases and renal damage (de Cavanagh et al, 2007). Hence, Ang II could be involved in organ senescence given its ability to mediate the release of oxidant species. Supporting this hypothesis, Ang II-induced ROS production via AT1 receptor promotes the onset of vascular senescence associated with functional and structural changes of blood vessels that contribute to age-related vascular diseases (Min et al, 2009). Interestingly, homozygous mice deficient for AT1A grow-up normally and outlive their wild-type littermates by 26% (Benigni et al, 2009). These AT1A−/− mice also develop fewer aortic atherosclerotic lesions and less cardiac injury during aging. Oxidative stress is reduced in cardiomyocytes, aortas and kidneys from mice lacking AT1A receptor with respect to aged wild-type mice and ultrastructural analysis of mitochondria in proximal renal tubular cells show that AT1A−/− mice have an increased number of mitochondria (Benigni et al, 2009). Extension of lifespan is associated with upregulation of pro-survival genes including nicotinamide phosphoribosyltransferase (Nampt) and Sirtuin 3 (Sirt3) (but not Sirtuin 1) in the kidney from these mice (Benigni et al, 2009). Importantly, candesartan prevents Ang II-induced Nampt and Sirt3 mRNA down-regulation in cultured tubular epithelial cells indicating a possible molecular link between Ang II, AT1A and these survival genes. The effects in longevity observed in AT1A-deficient mice are likely the consequence of reduced mitochondrial damage due to attenuation of oxidative stress and the increased expression of Nampt and Sirt3 survival genes (Fig 3). Our results shed light on early reports that showed favourable effects of chronic long-term Ang II inhibition by either ACEi or ARBs in protecting rats from the deleterious effects of aging on cardiovascular system and prolonging life span (Basso et al, 2007). Other studies implicate the AT1 receptors in ROS-induced damage and aging; old mice lacking AT1 receptors also do not develop age-related cerebral circulation damage caused by the accumulation of oxygen radicals (Modrick et al, 2009). The inhibition of RAS reverses age-related advanced myocardiac hypertrophy and fibrosis in aged spontaneously hypertensive rats, and the protective effect presumably involves the attenuation of Ang II-mediated oxidative stress, as documented by reduced expression of NAD(P)H oxidative components in the hearts of aged animals (Ito et al, 2007). Chronic treatment with ACEi or ARB reduces kidney damage associated with age, and the beneficial effect of RAS inhibition involves the preservation of renal mitochondria (Ferder et al, 2002). Enalapril and losartan treatments prevent the age-associated decline in the renal mitochondrial capacity for energy production and attenuate the age-associated increase in mitochondrial oxidant production (de Cavanagh et al, 2003). RAS inhibition exerts a similar protective effect in the liver from old rats through the maintenance of an adequate mitochondrial function due to enhanced expression of genes responsible for mitochondrial respiration and biogenesis (de Cavanagh et al, 2008).
Figure 3. Ang II signalling affects aging.
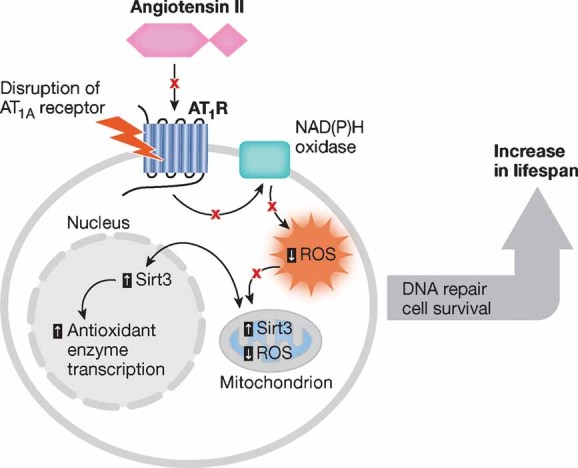
Ang II increases ROS generation and reduces the expression of the pro-survival gene Sirt3, affecting transcription of antioxidant enzymes. As depicted in red, the disruption of AT1A receptor preserves mitochondrial and cellular wellness and promotes longevity by modulating ROS production and sirtuin expression.
Clinical Implications
The synthesis of the first orally active ACEi, captopril (Ondetti et al, 1977) fostered the development of new therapeutic paradigms and opened a new era of research in the understanding of the clinical significance of RAS system. Despite the first warning of possible side effects of ACEi on the kidney, the efficacy of RAS blockers in treating hypertension, heart failure and renal diseases has gradually unfolded (Perico et al, 2008). Treatment of hypertension also benefited from the subsequent discovery of Ang II antagonists, selectively blocking AT1 receptor activation without influencing the vasodilatory kinins, which reduce blood pressure even in patients who do not have increased Ang II formation. The beneficial effect of inhibition of RAS activity by ACEi or ARB in patients with left ventricular dysfunction or systolic heart failure is now well established. Treatment with ACEi or ARB decreases mortality rate and reduces the risk for adverse cardiovascular outcome in high-risk patients with coronary artery disease, independently of the blood pressure control (Yusuf et al, 2000). Intensive treatment with ACEi of diabetic patients with microalbuminuria results in renoprotection and cardioprotection. In patients with non-diabetic chronic nephropathies, the integrated intervention including the use of ACEi, at dosages higher than those recommended for blood pressure control, together with ARB and a diuretic stabilizes the glomerular filtration rate and induces remission of the disease (Ruggenenti et al, 2008b). This approach has suggested that RAS blockers, by preventing progressive kidney function loss, possibly contribute to kidney repair (Benigni et al, 2010). While studying the beneficial effects of RAS blockade on heart and kidney, emerging evidence showed additional activities of RAS antagonists as anti-inflammatory and immunomodulatory agents and paved the way to new potential applications of these drugs in autoimmune diseases. The challenge for the next years is to establish whether these may translate in new medical applications of these drugs. Protection of brain blood vessels against damage by selective blockade of AT1 receptor alone, or even better in combination with ACEi, opens new perspectives to provide health benefits to patients with cognitive decline. Based on the recent studies on genetically modified animals and on aged rats chronically treated with ACEi or ARB, drugs that interfere with Ang II synthesis and/or biological activity may be promising candidates to regulate signalling cascades of senescence and extend lifespan. The excellent safety record of ACEi and ARB that have been administered chronically to millions of subjects with hypertension, cardiac and renal failure would indicate that long-term use of these medications might prove effective in preventing the progressive deterioration of organ function associated with aging without significant side-effects. Whether this would contribute to healthier aging and a longer live is a matter of intriguing speculation so far.
Pending issues
How does Ang II modulate TLR expression and activity in inflammation?
Does AT1 receptor inhibition modulate Th1/Th17 response and regulatory T cells activity in human multiple sclerosis? Do ACEi or ARBs represent a suitable therapy for this autoimmune disease?
Does RAS regulate other autoimmune diseases where antigen specific regulatory T cells may be a useful treatment strategy?
Do human AT1 receptors have the same effects observed in mice during aging? Do ACEi or ARBs represent a suitable therapy for human healthy aging?
How does Ang II via AT1 receptor influence the expression of Nampt and Sirt3 pro-survival genes?
Which are the mechanisms responsible for the protective effect of ARB treatment in Alzheimer's disease patients?
Acknowledgments
The authors declare that they have no conflict of interest.
Abbreviations
- RAS
renin–angiotensin system
- Ang II
angiotensin II
- ACE
angiotensin converting enzyme
- ARB
Angiotensin receptor blocker
- AT1
Ang II type 1 receptor
- AT2
Ang II type 2 receptor
- ROS
reactive oxygen species
References
- Aguilera G. Role of angiotensin II receptor subtypes on the regulation of aldosterone secretion in the adrenal glomerulosa zone in the rat. Mol Cell Endocrinol. 1992;90:53–60. doi: 10.1016/0303-7207(92)90101-b. [DOI] [PubMed] [Google Scholar]
- Alvarez A, Cerdá-Nicolás M, Naim Abu, Nabah Y, Mata M, Issekutz AC, Panés J, Loob RR, Sanz MJ. Direct evidence of leukocyte adhesion in arterioles by angiotensin II. Blood. 2004;104:402–408. doi: 10.1182/blood-2003-08-2974. [DOI] [PubMed] [Google Scholar]
- Arregui A, Perry EK, Rossor M, Tomlinson BE. Angiotensin converting enzyme in Alzheimer's disease increased activity in caudate nucleus and cortical areas. J Neurochem. 1982;38:1490–1492. doi: 10.1111/j.1471-4159.1982.tb07930.x. [DOI] [PubMed] [Google Scholar]
- Bacani C, Frishman WH. Chymase: a new pharmacologic target in cardiovascular disease. Cardiol Rev. 2006;14:187–193. doi: 10.1097/01.crd.0000195220.62533.c5. [DOI] [PubMed] [Google Scholar]
- Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A, Morigi M, Remuzzi G. Kidney regeneration. Lancet. 2010;375:1310–1317. doi: 10.1016/S0140-6736(10)60237-1. [DOI] [PubMed] [Google Scholar]
- Benndorf RA, Krebs C, Hirsch-Hoffmann B, Schwedhelm E, Cieslar G, Schmidt-Haupt R, Steinmetz OM, Meyer-Schwesinger C, Thaiss F, Haddad M, et al. Angiotensin II type 2 receptor deficiency aggravates renal injury and reduces survival in chronic kidney disease in mice. Kidney Int. 2009;75:1039–1049. doi: 10.1038/ki.2009.2. [DOI] [PubMed] [Google Scholar]
- Braun-Menéndez E, Page IH. Suggested revision of nomenclature—angiotensin. Science. 1958;127:242. doi: 10.1126/science.127.3292.242-a. [DOI] [PubMed] [Google Scholar]
- Braun-Menéndez E, Fascicolo C, Leloir LF, Munoz M. The substance causing renal hypertension. J Physiol. 1940;98:283–298. doi: 10.1113/jphysiol.1940.sp003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster UC, Perazella MA. The renin-angiotensin-aldosterone system and the kidney: effects on kidney disease. Am J Med. 2004;116:263–272. doi: 10.1016/j.amjmed.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Burson JM, Aguilera G, Gross KW, Sigmund CD. Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Physiol. 1994;267:E260–E267. doi: 10.1152/ajpendo.1994.267.2.E260. [DOI] [PubMed] [Google Scholar]
- Bush E, Maeda N, Kuziel WA, Dawson TC, Wilcox JN, DeLeon H, Taylor WR. CC chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II-induced hypertension. Hypertension. 2000;36:360–363. doi: 10.1161/01.hyp.36.3.360. [DOI] [PubMed] [Google Scholar]
- Cesari M, Rossi GP, Pessina AC. Biological properties of the angiotensin peptides other than angiotensin II: implications for hypertension and cardiovascular diseases. J Hypertens. 2002;20:793–799. doi: 10.1097/00004872-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Chua CC, Hamdy RC, Chua BH. Upregulation of vascular endothelial growth factor by angiotensin II in rat heart endothelial cells. Biochim Biophys Acta. 1998;1401:187–194. doi: 10.1016/s0167-4889(97)00129-8. [DOI] [PubMed] [Google Scholar]
- Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, et al. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol. 2008;295:F515–F524. doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SD, Vasievich MP, Ruiz P, Gould SK, Parsons KK, Pazmino AK, Facemire C, Chen BJ, Kim HS, Tran TT, et al. Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J Clin Invest. 2009;119:943–953. doi: 10.1172/JCI34862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Xu M, Yao M, Sun B. Angiotensin AT1 receptor antagonists exert anti-inflammatory effects in spontaneously hypertensive rats. Br J Pharmacol. 2007;152:1042–1048. doi: 10.1038/sj.bjp.0707454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res. 2006;98:463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest. 2000;106:103–106. doi: 10.1172/JCI10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cavanagh EM, Piotrkowski B, Basso N, Stella I, Inserra F, Ferder L, Fraga CG. Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB J. 2003;17:1096–1098. doi: 10.1096/fj.02-0063fje. [DOI] [PubMed] [Google Scholar]
- de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol. 2007;27:545–553. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]
- de Cavanagh EM, Flores I, Ferder M, Inserra F, Ferder L. Renin-angiotensin system inhibitors protect against age-related changes in rat liver mitochondrial DNA content and gene expression. Exp Gerontol. 2008;43:919–928. doi: 10.1016/j.exger.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- de Mello W. Effect of extracellular and intracellular angiotensin on heart cell function; on the cardiac renin-angiotensin system. Regul Pept. 2003;114:87–90. doi: 10.1016/s0167-0115(03)00121-6. [DOI] [PubMed] [Google Scholar]
- Donadelli R, Abbate M, Zanchi C, Corna D, Tomasoni S, Benigni A, Remuzzi G, Zoja C. Protein traffic activates NF-kB gene signaling and promotes MCP-1-dependent interstitial inflammation. Am J Kidney Dis. 2000;36:1226–1241. doi: 10.1053/ajkd.2000.19838. [DOI] [PubMed] [Google Scholar]
- Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- Esteban V, Lorenzo O, Ruperez M, Suzuki Y, Mezzano S, Blanco J, Kretzler M, Sugaya T, Egidio J, Ruiz-Ortega M. Angiotensin II, via AT1 and AT2 receptors and NF-kB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J Am Soc Nephrol. 2004;15:1514–1529. doi: 10.1097/01.asn.0000130564.75008.f5. [DOI] [PubMed] [Google Scholar]
- Ferder LF, Inserra F, Basso N. Advances in our understanding of aging: role of the renin-angiotensin system. Curr Opin Pharmacol. 2002;2:189–194. doi: 10.1016/s1471-4892(02)00139-x. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Chappell MC. Novel angiotensin peptides. Cell Mol Life Sci. 2004;61:2720–2727. doi: 10.1007/s00018-004-4243-4. [DOI] [PubMed] [Google Scholar]
- Fleming I. Signaling by the angiotensin-converting enzyme. Cir Res. 2006;98:887–896. doi: 10.1161/01.RES.0000217340.40936.53. [DOI] [PubMed] [Google Scholar]
- Ghiani BU, Masini MA. Angiotensin II bindings sites in the rat pancreas and their modulation after sodium loading and depletion. Comp Biochem Physiol A Physiol. 1995;111:439–444. doi: 10.1016/0300-9629(95)00030-b. [DOI] [PubMed] [Google Scholar]
- Gleibermann L. Blood pressure and dietary salt in human populations. Ecol Food Nutrition. 1973;2:143–156. [Google Scholar]
- Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulaes NADH and NADPH oxidase activity in cultures vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton TA, Handa RK, Harding JW, Wright JW. A role for angiotensin IV/AT4 system in mediating natiuresis in the rat. Peptides. 2001;22:935–944. doi: 10.1016/s0196-9781(01)00405-3. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Helwig J, Jr, Rhoads JE, Roberts B. The metabolic response to trauma. Annu Rev Med. 1956;7:141–156. doi: 10.1146/annurev.me.07.020156.001041. [DOI] [PubMed] [Google Scholar]
- Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XR, Chen WY, Truong LD, Lan HY. Chymase is upregulated in diabetic nephropathy: implications for an alternative pathway of angiotensin II-mediated diabetic renal and vascular disease. J Am Soc Nephrol. 2003;14:1738–1747. doi: 10.1097/01.asn.0000071512.93927.4e. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- Irani RA, Zhang Y, Blackwell SC, Zhou CC, Ramin SM, Kellems RE, Xia Y. The detrimental role of angiotensin receptor agonistic autoantibodies in intrauterine growth restriction seen in preeclampsia. J Exp Med. 2009;206:2809–2822. doi: 10.1084/jem.20090872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Ohishi M, Yamamoto K, Tatara Y, Shiota A, Hayashi N, Komai N, Yanagitani Y, Rakugi H, Ogihara T. Renin-angiotensin inhibition reverses advanced cardiac remodeling in aging spontaneously hypertensive rats. Am J Hypertens. 2007;20:792–799. doi: 10.1016/j.amjhyper.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Iwai N, Inagami T, Ohmichi N, Kinoshita M. Renin is expressed in rat macrophage/monocyte cells. Hypertension. 1996;27:399–403. doi: 10.1161/01.hyp.27.3.399. [DOI] [PubMed] [Google Scholar]
- Ji Y, Liu J, Wang Z, Liu N. Angiotensin II induces inflammatory response partly via toll-like receptor 4-dependent signaling pathway in vascular smooth muscle cells. Cell Physiol Biochem. 2009;23:265–276. doi: 10.1159/000218173. [DOI] [PubMed] [Google Scholar]
- Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, Milford E, Abdi R. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18:1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- Karlsson C, Lindell K, Ottoson M, Sjöström L, Carlsson B, Carlsson LM. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab. 1998;83:3925–3929. doi: 10.1210/jcem.83.11.5276. [DOI] [PubMed] [Google Scholar]
- Kehoe PG, Miners S, Love S. Angiotensins in Alzheimer's disease—friend or foe. Trends Neurosci. 2009;32:619–628. doi: 10.1016/j.tins.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Kim S, Hiroshi I. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2003;52:11–33. [PubMed] [Google Scholar]
- Kitayama H, Maeshima Y, Takazawa Y, Yamamoto Y, Wu Y, Ichinose K, Hirokoshi K, Sugiyama H, Yamasaki Y, Makino H. Regulation of angiogenic factors in angiotensin II infusion model in association with tubulointerstitial injuries. Am J Hypertens. 2006;19:718–727. doi: 10.1016/j.amjhyper.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Pieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Harding JW, Wright JW. Angiotensin II- and IV-induced changes in cerebral blood flow. Roles of AT1 and AT2, and AT4 receptor subtypes. Regul Pept. 1997;68:131–138. doi: 10.1016/s0167-0115(96)02116-7. [DOI] [PubMed] [Google Scholar]
- Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- Lapteva N, Nieda M, Ando Y, Ide K, Hatta-Ohashi Y, Dymshits G, Ishikawa Y, Juji T, Tokunaga K. Expression of renin-angiotensin system genes in immature and mature dendritic cells identified using human cDNA microarray. Biochem Biophys Res Commun. 2001;285:1059–1065. doi: 10.1006/bbrc.2001.5215. [DOI] [PubMed] [Google Scholar]
- Lapteva N, Ide K, Nieda M, Ando Y, Hatta-Ohashi Y, Minami M, Dymshits G, Egawa K, Juji T, Tokunaga K. Activation and suppression of renin-angiotensin system in human dendritic cells. Biochem Biophys Res Commun. 2002;296:194–200. doi: 10.1016/s0006-291x(02)00855-0. [DOI] [PubMed] [Google Scholar]
- Lev-Ran A, Porta M. Salt and hypertension: a phylogenetic perspective. Diabetes Metab Res Rev. 2005;21:118–131. doi: 10.1002/dmrr.539. [DOI] [PubMed] [Google Scholar]
- Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin BM. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu X, Wang A, Fan H, Yuan H. Effects of angiotensin-II receptor blockers on experimental autoimmune myocarditis. Int J Cardiol. 2009;137:282–288. doi: 10.1016/j.ijcard.2009.09.540. [DOI] [PubMed] [Google Scholar]
- Lopez-Real A, Rey P, Soto-Otero R, MEndez-Alvarez E, Labandeira-Garcia JL. Angiotensin-converting enzyme inhibition reduces oxidative stress and protects dopaminergic neurons in a 6-hydroxydopamine rat model of Parkinsonism. J Neurosci Res. 2005;81:865–873. doi: 10.1002/jnr.20598. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Mayeux R. Cardiovascular risk factors and Alzheimer's disease. Curr Atheroscler Rep. 2004;6:261–266. doi: 10.1007/s11883-004-0056-z. [DOI] [PubMed] [Google Scholar]
- Lv J, Jia R, Yang D, Zhu J, Ding G. Candesartan attenuates Angiotensin II-induced mesangial cell apoptosis via TLR4/MyD88 pathway. Biochem Biophys Res Commun. 2009;380:81–86. doi: 10.1016/j.bbrc.2009.01.035. [DOI] [PubMed] [Google Scholar]
- Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29:367–374. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- Mertens B, Vanderheyden P, Michotte Y, Sarre S. The role of central renin-angiotensin system in Parkinson's disease. J Renin Angiotensin Aldosterone Syst. 2010;11:49–56. doi: 10.1177/1470320309347789. [DOI] [PubMed] [Google Scholar]
- Min LJ, Mogi M, Iwai M, Horiuchi M. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev. 2009;8:113–121. doi: 10.1016/j.arr.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Miners JS, Ashby E, Van Helmond Z, Chalmers KA, Palmer LE, Love S, Kehoe PG. Angiotensin-converting enzyme (ACE) levels and activity in Alzheimer's disease, and relationship of perivascular ACE-1 to cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2008;34:181–193. doi: 10.1111/j.1365-2990.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Takai S. Tissue angiotensin II generating system by angiotensin-converting enzyme and chymase. J Pharmacol Sci. 2006;100:391–397. doi: 10.1254/jphs.cpj06008x. [DOI] [PubMed] [Google Scholar]
- Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2009;296:H1914–H1919. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulik S, Speth RC, Turner BB, Rowe BP. Angiotensin II receptor subtype distribution in the rabbit brain. Exp Brain Res. 2002;142:275–283. doi: 10.1007/s00221-001-0940-5. [DOI] [PubMed] [Google Scholar]
- Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, et al. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz A, Rey P, Guerra MJ, Mendez-Alvarez E, Soto-Otero R, Labandeira-Garcia JL. Reduction of dopaminergic degeneration and oxidative stress by inhibion of angiotensin converting enzyme in a MPTP model of parkinsonism. Neuropharmacology. 2006;51:112–120. doi: 10.1016/j.neuropharm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Nahmod KA, Vermeulen ME, Raiden S, Salamone G, Gamberale R, Fernandez-Calotti P, Alvarez A, Nahmod V, Giordano M, Geffner JR. Control of dendritic cell differentiation by angiotensin II. FASEB J. 2003;17:491–493. doi: 10.1096/fj.02-0755fje. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Wooding S, Sakagami T, Emi M, Tokunaga K, Tamiya G, Ishigami T, Umemura S, Munkhbat B, Jin F, et al. Natural selection and population history in the human angiotensinogen gene (AGT): 736 complete AGT sequences in chromosomes from around the world. Am J Hum Genet. 2004;74:898–916. doi: 10.1086/420793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrui T, Tomita N, Sato-Nakagawa T, Matsui T, Maruyama M, Niwa K, Arai H, Sasaki H. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology. 2004;63:1324–1325. doi: 10.1212/01.wnl.0000140705.23869.e9. [DOI] [PubMed] [Google Scholar]
- Okunuki Y, Usui Y, Nagai N, Kezuka T, Ishida S, Takeuchi M, Goto H. Suppression of experimental autoimmune uveitis by angiotensin II type 1 receptor blocker telmisartan. Invest Ophthalmol Vis Sci. 2009;50:2255–2261. doi: 10.1167/iovs.08-2649. [DOI] [PubMed] [Google Scholar]
- Oliverio MI, Coffman TM. Angiotensin II receptor physiology using gene targeting. News Physiol Sci. 2000;15:171–175. doi: 10.1152/physiologyonline.2000.15.4.171. [DOI] [PubMed] [Google Scholar]
- Ondetti MA, Rubin B, Cushman DW. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science. 1977;196:441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- Page IH, Helmer OH. A crystalline pressor substance (angiotonin) resulting from the interaction between renin and renin-activator. J Exp Med. 1940;71:29–42. doi: 10.1084/jem.71.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perico N, Benigni A, Remuzzi G. Present and future drug treatments for chronic kidney diseases: evolving targets in renoprotection. Nat Rev Drug Discov. 2008;7:936–953. doi: 10.1038/nrd2685. [DOI] [PubMed] [Google Scholar]
- Piqueras L, Kubes P, Alvarez A, O'Connor E, Issekutz AC, Esplugues JV, Sanz MJ. Angiotensin II induces leukocyte-endothelial cell interactions in vivo via AT(1) and AT(2) receptor-mediated P-selectin upregulation. Circulation. 2000;102:2118–2123. doi: 10.1161/01.cir.102.17.2118. [DOI] [PubMed] [Google Scholar]
- Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, Phillips LK, Goldstein MJ, Bhat R, Raine CS, et al. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci USA. 2009;106:14948–14953. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- Poon IO. Effects of antihypertensive drug treatment on the risk of dementia and cognitive impairment. Pharmacotherapy. 2008;28:366–375. doi: 10.1592/phco.28.3.366. [DOI] [PubMed] [Google Scholar]
- Porrello ER, Delbridge LM, Thomas WG. The angiotensin II type 2 (AT2) receptor: an enigmatic seven transmembrane receptor. Front BioSci. 2009;14:958–972. doi: 10.2741/3289. [DOI] [PubMed] [Google Scholar]
- Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Artherocler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Re RN, Cook JL. The intracrine hypothesis: an update. Regul Pept. 2006;133:1–9. doi: 10.1016/j.regpep.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Reaux A, Fournie-Zaluski MC, Llorens-Cortes C. Angiotensin III: a central regulator of vasopressin release and blood pressure. Trends Endocrinol Metab. 2001;12:157–162. doi: 10.1016/s1043-2760(01)00381-2. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Zoja C, Gagliardini E, Corna D, Abbate M, Benigni A. Combining an antiproteinuric approach with mycophenolate mofetil fully suppresses progressive nephropathy of experimental animals. J Am Soc Nephrol. 1999;10:1542–1549. doi: 10.1681/ASN.V1071542. [DOI] [PubMed] [Google Scholar]
- Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggenenti P, Bettinaglio P, Pinares F, Remuzzi G. Angiotensin converting enzyme insertion/deletion polymorphism and renoprotection in diabetic and nondiabetic nephropathies. Clin J Am Soc Nephrol. 2008a;3:1511–1525. doi: 10.2215/CJN.04140907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggenenti P, Perticucci E, Cravedi P, Gambara V, Costantini M, Sharma SK, Perna A, Remuzzi G. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol. 2008b;19:1213–1224. doi: 10.1681/ASN.2007090970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Lorenzo O, Egido J. Angiotensin III increases MCP-1 and activates NF-kappaB and AP-1 in cultured mesangial and mononuclear cells. Kidney Int. 2000;57:2285–2298. doi: 10.1046/j.1523-1755.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Esteban V, Suzuki Y, Ruperez M, Mezzano S, Ardiles L, Justo P, Ortiz A, Egidio J. Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney Int. 2003;(Suppl 86):S21–S26. doi: 10.1046/j.1523-1755.64.s86.5.x. [DOI] [PubMed] [Google Scholar]
- Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17:2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Hock C, Olivieri G, Bruttel S, Rosenberg C, Hulette C, Muller-Spahn F. Cortical alterations of angiotensin converting enzyme, angiotensin II and AT1 receptor in Alzheimer's dementia. Neurobiol Aging. 2001;22:541–546. doi: 10.1016/s0197-4580(00)00259-1. [DOI] [PubMed] [Google Scholar]
- Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BMW. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- Schulman IH, Raij L. The angiotensin II type 2 receptor: what is its clinical significance. Curr Hypertens Rep. 2008;10:188–193. doi: 10.1007/s11906-008-0036-8. [DOI] [PubMed] [Google Scholar]
- Shah K, Qureshi SU, Johnson M, Parikh N, Schulz PE, Kunik ME. Does use of antihypertensive drugs affect the incidence or progression of dementia? A systematic review. Am J Geriatr Pharmacother. 2009;7:250–261. doi: 10.1016/j.amjopharm.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Smith HW. 1953. From fish to philosopher. The Natural History Library.
- Steckelings UM, Kaschina E, Unger T. The AT2 receptor—a matter of love and hate. Peptides. 2005;26:1401–1409. doi: 10.1016/j.peptides.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Stegbauer J, Lee DH, Seubert S, Ellrichmann G, Manzel A, Kvakan H, Muller DN, Gaupp S, Rump LC, Gold R, et al. Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc Natl Acad Sci USA. 2009;106:14942–14947. doi: 10.1073/pnas.0903602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- Thekkumkara TJ, Cookson R, Linas SL. Angiotensin (AT1A) receptor-mediated increases in transcellular sodium transport in proximal tubule cells. Am J Physiol. 1998;274:F897–F905. doi: 10.1152/ajprenal.1998.274.5.F897. [DOI] [PubMed] [Google Scholar]
- Thomas WG, Sernia C. The immunocytochemical localization of angiotensinogen in the rat ovary. Cell tissue Res. 1990;261:367–373. doi: 10.1007/BF00318679. [DOI] [PubMed] [Google Scholar]
- Tigerstedt R, Bergman P. Niere and Kreislauf. Scand Arch Physiol (Germany) 1898;8:223–271. [Google Scholar]
- Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1582. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- Urata H, Boehm KD, Philip A, Kinoshita A, Gabrovsek J, Bumpus FM, Husain A. Cellular localization and regional distribution of an angiotensin II-forming chymase in the heart. J Clin Invest. 1993;91:1269–1281. doi: 10.1172/JCI116325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kats JP, Danser AH, van Meegen JR, Sassen LM, Verdouw PD, Schalekamp MA. Angiotensin production by the heart: a quantitative study in pigs with the use of radiolabeled angiotensin infusion. Circulation. 1998;98:73–81. doi: 10.1161/01.cir.98.1.73. [DOI] [PubMed] [Google Scholar]
- Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, Humala N, Seror I, Bartholomew S, Rosendorff C, et al. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch WJ. Angiotensin II-dependent superoxide: effects on hypertension and vascular dysfunction. Hypertension. 2008;52:51–56. doi: 10.1161/HYPERTENSIONAHA.107.090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SK. Role of oxygen-derived free radicals in acute angiotensin II-induced hypertensive vascular disease in the rat. Circ Res. 1990;66:722–734. doi: 10.1161/01.res.66.3.722. [DOI] [PubMed] [Google Scholar]
- Wolf G, Wenzel U, Burns KD, Harris RC, Stahl RAK, Thaiss F. Angiotensin II activates nuclear transcription factor-B through AT1 and AT2 receptors. Kidney Int. 2002;61:1986–1995. doi: 10.1046/j.1523-1755.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- Wooding S. Natural selection: sign, sign, everywhere a sign. Curr Biol. 2004;14:R700–R701. doi: 10.1016/j.cub.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Wright JW, Harding JW. The angiotensin AT4 receptor subtype as a target for the treatment of memory dysfunction associated with Alzheimer's disease. J Renin Angiotensin Aldosterone Syst. 2008;9:226–237. doi: 10.1177/1470320308099084. [DOI] [PubMed] [Google Scholar]
- Wu R, Laplante MA, de Champlain J. Cyclooxygenase-2 inhibitors attenuate angiotensin II-induced oxidative stress, hypertension, and cardiac hypertrophy in rats. Hypertension. 2005;45:1139–1144. doi: 10.1161/01.HYP.0000164572.92049.29. [DOI] [PubMed] [Google Scholar]
- Wu K, Zhou T, Sun G, Wang W, Zhang Y, Hao L, Chen N. Valsartan inhibited the accumulation of dendritic cells in rat fibrotic renal tissue. Cell Mol Immunol. 2006;3:213–220. [PubMed] [Google Scholar]
- Yang J, Jiang H, Ding JW, Chen LH, Li S, Zhang XD. Valsartan preconditioning protects against myocardial ischemia-reperfusion injury through TLR4/NF-kappaB signaling pathway. Mol Cell Biochem. 2009;330:39–46. doi: 10.1007/s11010-009-0098-1. [DOI] [PubMed] [Google Scholar]
- Yun AJ, Doux JD, Lee PY. Contrast nephropathy may be partly mediated by autonomic dysfunction: renal failure considered as a modern maladaptation of the prehistoric trauma response. Med Hypotheses. 2006;66:776–783. doi: 10.1016/j.mehy.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]


