Abstract
The capability of the liver to fully regenerate after injury is a unique phenomenon essential for the maintenance of its important functions in the control of metabolism and xenobiotic detoxification. The regeneration process is histologically well described, but the genes that orchestrate liver regeneration have been only partially characterized. Of particular interest are cytokines and growth factors, which control different phases of liver regeneration. Historically, their potential functions in this process were addressed by analyzing their expression in the regenerating liver of rodents. Some of the predicted roles were confirmed using functional studies, including systemic delivery of recombinant growth factors, neutralizing antibodies or siRNAs prior to liver injury or during liver regeneration. In particular, the availability of genetically modified mice and their use in liver regeneration studies has unraveled novel and often unexpected functions of growth factors, cytokines and their downstream signalling targets in liver regeneration. This review summarizes the results obtained by functional studies that have addressed the roles and mechanisms of action of growth factors and cytokines in liver regeneration after acute injury to this organ.
Keywords: apoptosis, growth factor, hepatectomy, liver regeneration, proliferation
INTRODUCTION
The liver plays a central role in the regulation of whole body metabolism as well as in compound detoxification. Due to these essential functions, injuries to this organ need to be rapidly and efficiently repaired. Various types of insults induce liver damage, such as cell loss caused by viruses, autoimmune diseases and toxins, including alcohol or commonly used anti-inflammatory, anti-convulsant or chemotherapeutic drugs as well as resection of liver tissue in patients with primary or metastatic liver tumours.
Interestingly, the liver has a unique ability to fully regenerate and thus differs significantly from other organs, which heal with a scar (Fausto et al, 2006; Michalopoulos, 2007). However, the regenerative capacity is insufficient after chronic injury as observed in chronic viral hepatitis or after long-term alcohol abuse, for example. These conditions often cause liver cirrhosis, which is characterized by the replacement of functional epithelial tissue by non-functional connective tissue. In the worst case, liver failure can occur. Although an enormous part of the global population is affected by these conditions, therapeutic options are still unsatisfactory and are mainly supportive. Therefore, the development of specific therapies to enhance the regenerative capacity of the liver is essential for the improvement of human health. This requires a thorough understanding of the mechanisms underlying liver regeneration and the identification of factors orchestrating this process.
A particularly useful model to study liver regeneration in mice and rats is partial hepatectomy (PH), in which the large and median lobes of the liver, which comprise approximately two-thirds of the organ, are surgically removed (Fig 1B). As a consequence, the normally quiescent and highly differentiated liver cells (Fig 1A) proliferate (Fig 1C and D) and the original liver mass is restored within a few days (approximately 10 days in rodents) by expansion of the remaining liver tissue. Most importantly, normal liver regeneration is not accompanied by massive inflammation or necrosis and thus does not induce a fibrotic response. Hepatocytes are the first to enter the cell cycle and undergo one to two rounds of cell division within 2–3 days. They initially receive signals to exit from G0 and to initiate the expression of a set of genes required for regeneration. Subsequently, hepatocytes progress through the cell cycle and undergo mitosis. This is followed by the proliferation of hepatic stellate cells, Kupffer cells and biliary epithelial cells. Furthermore, proliferation of endothelial cells and sprouting angiogenesis occur to re-establish the liver vasculature (Michalopoulos, 2007).
Figure 1. The different cell types of the liver and liver regeneration after partial hepatectomy (PH).
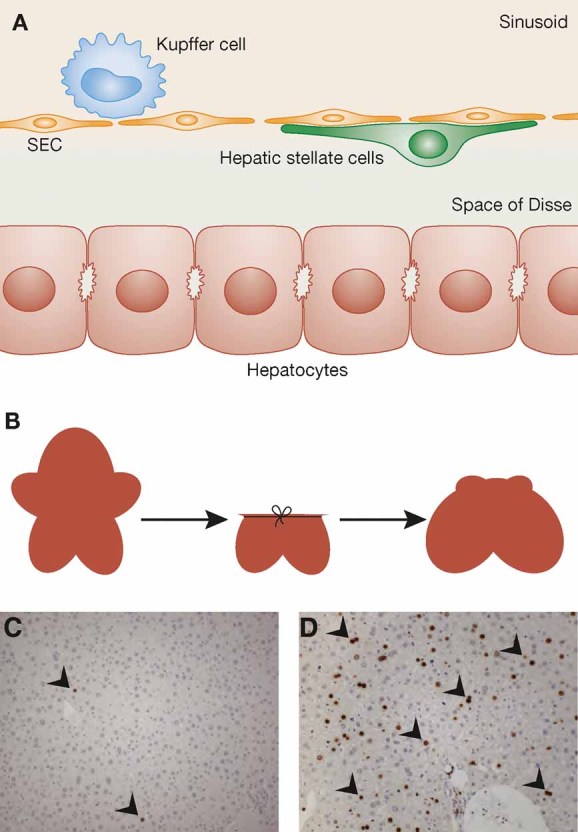
- A. The major cell types of the liver are shown schematically. They include hepatocytes (parenchymal cells of the liver), hepatic stellate cells, Kupffer cells and sinusoidal endothelial cells (SEC).
- B. The removal of liver tissue upon PH and the regeneration of the remaining liver are shown schematically.
- C, D. Liver cell proliferation as demonstrated by incorporation of 5-bromo-2′-deoxyuridine (BrdU) and subsequent staining of mouse liver sections with an antibody against BrdU is shown in non-injured liver (C) and 48 h after PH (D). BrdU-positive cells were detected using an alkaline phosphatase detection system and are indicated by arrowheads.
The activation, proliferation, migration, differentiation and survival of cells in the regenerating liver are controlled by a large number of growth factors and cytokines that are expressed at the site of injury or reach the liver via the circulatory system. However, the roles of endogenous growth factors in the regenerative process of the liver have been only partially elucidated and their proposed functions are frequently based on descriptive expression studies and/or results from cell culture assays.
The development of genetically modified mice that overexpress or have functional loss of growth factors, cytokines or their receptors and their use for liver regeneration studies has provided exciting and often unexpected results. In this review, we summarize the in vivo functions of cytokines and growth factors in liver regeneration reported so far. Because there are numerous reviews on liver fibrosis, we focus on the normal regeneration process, which is seen after PH.
Glossary
Complement component 5a
A protein fragment released from complement component 5, which can act as a pro-inflammatory cytokine. The function is mediated by the C5a receptor, a member of the G-protein coupled receptor family.
Hepatic stellate cells
Specialized pericytes that line the walls of liver sinusoids.
Hepatocytes
The liver parenchymal cells—specialized epithelial cells, which carry out most of the functions of the liver, including metabolism and detoxification.
Kupffer cells
Phagocytic cells of the liver that are considered as resident macrophages of this organ.
Lipopolysaccharide (LPS)
A major component of the cell wall of Gram-negative bacteria; LPSs are endotoxins and important antigens.
MyD88
Myeloid differentiation primary response gene (88), an adapter protein that is used by all Toll-like receptors to activate the transcription factor NF-κB.
Superior mesenteric vein
A blood vessel that drains blood from the small intestine; after fusion with the splenic vein it forms the hepatic portal vein.
TACE
Tumour necrosis factor-α converting enzyme—a membrane-bound disintegrin metalloproteinase that cleaves the membrane-associated cytokine proTNF-α, resulting in release of the soluble form.
Toll-like receptors
Proteins that recognize pathogen molecules and activate immune cell responses.
CYTOKINES AND GROWTH FACTORS INVOLVED IN LIVER REGENERATION
Tumour necrosis factor (TNF)-α and lymphotoxins
An important regulator of the priming phase of liver regeneration is TNF-α. Expression of this cytokine is upregulated 30–120 min after PH, in particular in Kupffer cells, through activation of the nuclear factor κB (NF-κB) transcription factor (Yang et al, 2005). One of the major inducers is enteric-derived lipopolysaccharide (LPS) that reaches the liver via the blood stream (Cornell, 1985). This induction requires the adaptor protein MyD88, which is involved in most Toll-like receptor signalling pathways. In mice lacking MyD88, TNF-α mRNA levels in the liver as well as serum levels of interleukin-6 (IL-6) were much lower after PH compared to control mice (Campbell et al, 2006) and this was accompanied by impaired hepatocyte proliferation and delayed regeneration (Seki et al, 2005). In addition, activation of the receptor for the complement component C5a is important for TNF-α and IL-6 induction upon PH, as shown by treatment of mice with a C5a receptor inhibitory peptide (Strey et al, 2003). This is functionally relevant, as mice lacking C5a showed increased mortality and delayed regeneration after PH (Strey et al, 2003). Finally, levels of TNF-α and IL-6 were much lower in the injured liver of mice lacking intercellular adhesion molecule 1 (ICAM-1) compared to wild-type mice, and this was also associated with impaired regeneration. It seems most likely that activation of ICAM-1 by leukocytes at an early stage after liver injury is required for the efficient production of these cytokines by Kupffer cells (Selzner et al, 2003) (Fig 2).
Figure 2. Regulation and function of TNF-α and IL-6 in the regenerating liver.
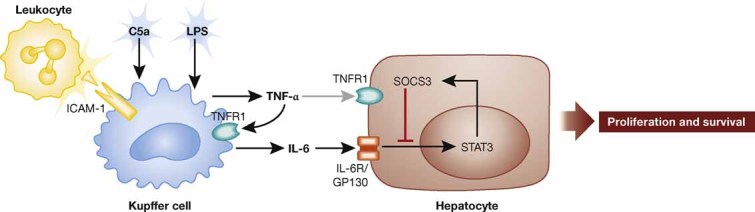
Upon PH, expression of both TNF-α and IL-6 is induced in Kupffer cells by LPS, by the complement component C5a and by leukocyte-mediated activation of ICAM-1. TNF-α further enhances the expression of IL-6 through activation of TNFR1. IL-6 signals through a complex of GP130 and the IL-6 receptor expressed by hepatocytes. This results in activation of STAT3 and subsequent production of SOCS3 (negative feedback). STAT3 activation induces hepatocyte proliferation and enhances survival after PH.
Blocking TNF-α signalling in rats by intraperitoneal injection of TNF-α neutralizing antibodies prior to PH prevented the expected increase in IL-6 serum levels and strongly reduced the proliferation of hepatocytes and non-parenchymal liver cells (Akerman et al, 1992). In contrast to these data, hepatocyte proliferation after PH was not affected in TNF-α knockout mice (Fujita et al, 2001). In this study, an unusually high mortality rate of wild-type mice was seen at day 1 after PH. The survival rate of the TNF-α deficient animals was higher than that of wild-type controls, most likely due to reduced neutrophil activation and liver necrosis. The difference between the TNF-α neutralization studies and the results obtained with knockout mice may result from use of different species (rats vs. mice) or from differences in the surgical procedure.
In agreement with the data obtained with TNF-α neutralizing antibodies, DNA synthesis after PH was severely impaired in mice lacking TNF receptor 1 (TNFR1), resulting in delayed regeneration (Yamada et al, 1997, 1998). An increased mortality rate was also observed after PH of these mice. These abnormalities were accompanied by a failure to activate NF-κB and signal transducer and activator of transcription 3 (STAT3) and to induce expression of IL-6 (Yamada et al, 1997, 1998). By contrast, liver regeneration was not affected in mice lacking TNFR2 (Yamada et al, 1998).
TNF receptor 1 can also be activated by lymphotoxin-α (LT-α) and mice lacking both TNF-α and LT-α showed a defect in liver regeneration similar to TNFR1-deficient mice (Knight & Yeoh, 2005). LT-α is strongly upregulated in intrahepatic lymphocytes after PH (Anders et al, 2005) and can also activate lymphotoxin β receptor (LTβR), another receptor on hepatocytes, when co-expressed with LT-β. In this case, membrane-anchored heterotrimers (LT-α1β2 and LT-α2β1) activate LTβR (Norris & Ware, 2007). Interestingly, mice lacking LTβR showed defects in liver regeneration similar to LT-α deficient mice, such as reduced survival, severe liver damage and impaired hepatocyte proliferation (Anders et al, 2005). This indicates that LT-α controls liver regeneration not only through activation of TNFR1 but also—or even predominantly—of LTβR. LT-α expressed by T cells is important for liver regeneration as shown by analysis of mice lacking LT-α in these immune cells. Vice versa, an agonistic antibody targeting LTβR improved liver regeneration by reduction of liver injury and stimulation of hepatocyte proliferation (Tumanov et al, 2009).
In spite of the important role of TNFR1 activation for liver regeneration, excessive production of TNF-α is deleterious. Thus, increased levels of TNF-α in the serum of mice lacking the epidermal growth factor receptor (EGFR) in hepatocytes (see below) are most likely responsible for the strongly increased lethality of these mice after PH (Natarajan et al, 2007). In addition, mice lacking tissue inhibitor of metalloproteinases 3 (TIMP-3) showed constitutive cleavage of the transmembrane precursor of TNF-α through enhanced activity of TNF-α converting enzmye (TACE), resulting in high serum levels of mature TNF-α. As a consequence, a large percentage of the TIMP-3 knockout mice died after completion of the proliferative phase of liver regeneration (5 days after PH or later) or at least suffered from severe hepatocyte necrosis and apoptosis. The phenotype was rescued by treatment of the knockout mice with neutralizing antibodies to TNF-α (Mohammed et al, 2004).
Interleukin-6 (IL-6)
Interleukin-6 is also strongly upregulated after PH and elevated serum levels are found early after surgery (Fausto et al, 2006). Similar to TNF-α, IL-6 is mainly produced by Kupffer cells and its expression is regulated by the LPS/MyD88 pathway, by complement components and by ICAM-1 activation (Campbell et al, 2006; Selzner et al, 2003; Strey et al, 2003). The PH-induced upregulation of TNF-α further contributes to the increase in IL-6 expression in an autocrine manner, as demonstrated by the reduced expression of IL-6 in the injured liver of TNFR1 knockout mice (Yamada et al, 1997) (Fig 2).
Cressman et al (1996) reported that the PH-induced proliferation of hepatocytes from IL-6-deficient mice is impaired and that the animals show liver necrosis and failure after surgery, resulting in increased mortality. A single injection of IL-6 before surgery could rescue this phenotype (Cressman et al, 1996). Interestingly, the liver regeneration defect of TNFR1 knockout mice was also rescued by a pre-operative injection of IL-6 (Yamada et al, 1997). This finding strongly suggests that the major role of TNF-α and TNFR1 in liver regeneration is the upregulation of IL-6, which in turn controls the regenerative process (Fig 2). Studies with bone marrow chimeric mice have further demonstrated that IL-6 derived from intrahepatic cells of bone marrow origin (most likely Kupffer cells) is required for efficient liver regeneration (Aldeguer et al, 2002).
The defect in hepatocyte proliferation following PH seen in IL-6 deficient mice was also rescued by treatment with stem cell factor (SCF). The latter is induced by IL-6 and mediates the mitogenic effects of this cytokine for hepatocytes (Ren et al, 2003). Consistent with this finding, hepatocyte proliferation after PH was strongly impaired in SCF-deficient mice or in wild-type mice treated with neutralizing antibodies against SCF during the regeneration period (Ren et al, 2003). Another downstream mediator of IL-6 in the injured liver is oncostatin M. This cytokine is also important for liver regeneration as demonstrated by the impaired restoration of liver mass following PH in mice lacking the oncostatin M receptor (Nakamura et al, 2004).
Interestingly, another group showed that some IL-6 deficient mice survive after PH, and hepatocyte proliferation and subsequent regeneration were not impaired in the surviving animals (Blindenbacher et al, 2003). This finding suggests that the mitogenic effect of IL-6 is of minor importance for liver regeneration. Rather, IL-6 seems to induce an adaptive response to PH that is required for survival.
In contrast to the results obtained in the above-mentioned studies, a third group reported a much less severe phenotype of IL-6 knockout mice following PH (Sakamoto et al, 1999). These animals only showed delayed liver weight recovery, but no increase in mortality. The contradictory results regarding the role of IL-6 in liver regeneration may result from use of mice with different genetic background, from differences in the surgical procedure, or from the use of different anaesthesia/analgesia.
A protective role of IL-6 in the injured liver was demonstrated in a mouse model of severe liver injury (87% hepatectomy); the massive oxidative injury and mitochondrial dysfunction that occur under these conditions were strongly improved by IL-6 pre-treatment (Jin et al, 2007).
Mice lacking the IL-6 receptor component GP130 in the liver only displayed a minor defect in hepatocyte proliferation after PH, whereas increased hepatocyte apoptosis occurred when these knockout mice were injected with LPS 3 h after PH (Wuestefeld et al, 2003). These results further support a protective rather than a mitogenic role for IL-6 in liver regeneration. Finally, deletion of STAT3, a major component of the IL-6 signalling pathway, in hepatocytes using mice expressing Cre under the control of the transthyretin promoter resulted in higher mortality after PH, whereas hepatocyte proliferation and restoration of liver mass were only marginally affected in the surviving mice (Moh et al, 2007). A more severe defect in hepatocyte proliferation following PH was observed in another liver-specific Stat3 knockout strain in which Cre expression was driven by the albumin promoter (Li et al, 2002). However, this effect may be secondary to the obesity and metabolic abnormalities seen in these mice. Consistent with an important role of STAT3 in liver regeneration, mice lacking the suppressor of cytokine signalling 3 (SOCS3), an inhibitor of IL-6 signalling, showed prolonged activation of STAT3 after PH, which correlated with enhanced hepatocyte proliferation and accelerated liver weight restoration (Riehle et al, 2008) (Fig 2).
Hepatocyte growth factor (HGF)
A major hepatocyte mitogen is HGF, which performs its activity through activation of the receptor tyrosine kinase c-Met (Benvenuti & Comoglio, 2007). HGF expression is upregulated in non-parenchymal cells of the liver as well as in several extrahepatic organs after PH, and serum levels of HGF strongly increase within 1–3 h after PH (Michalopoulos, 2007). In addition, a biphasic increase in c-Met phosphorylation (activation) was observed 1–5 and 60 min after PH in rats (Stolz et al, 1999). Overexpression of full-length HGF or a truncated variant in the liver of transgenic mice led to increased hepatocyte proliferation and accelerated liver regeneration after PH (Bell et al, 1999; Sakata et al, 1996; Shiota & Kawasaki, 1998). The positive effect of HGF on liver regeneration also underlies the phenotype of mice overexpressing or lacking TIMP-1. Hepatocytes of TIMP-1-overexpressing transgenic mice exhibited a delay in cell cycle progression upon PH, whereas the opposite effect was seen in TIMP-1 knockout mice (Mohammed et al, 2005). TIMP-1 inhibits the metalloproteinase activity responsible for cleavage of the HGF precursor from the extracellular matrix and subsequent proteolytic maturation of HGF (Mohammed et al, 2005).
Mice lacking c-Met in the liver were generated to study the role of endogenous HGF and its receptor in liver regeneration. Crossing of mice with floxed c-Met alleles with transgenic mice expressing Cre recombinase under the control of the Mx-Cre promoter allowed inducible deletion of the c-Met gene in the liver of adult animals. Interestingly, regeneration after PH was delayed due to a defective exit of hepatocytes from quiescence and diminished S phase entry (Borowiak et al, 2004). In a second study, albumin-Cre transgenic mice were used to delete the c-Met gene in hepatocytes in a non-inducible manner (Huh et al, 2004). Surprisingly, most of the knockout mice died within 48 h after PH and showed severe liver necrosis and jaundice. Thus, c-Met activation obviously provides a survival signal for hepatocytes in the injured liver. In addition, a defect in tissue remodelling was detected in the c-Met mutant mice (Huh et al, 2004). To exclude the possibility that secondary changes resulting from long-term depletion of c-Met are responsible for the abnormalities seen upon PH, rats were injected with short hairpin RNAs (shRNAs) against HGF, c-Met or scrambled shRNA (Paranjpe et al, 2007). This resulted in moderate or even severe suppression of hepatocyte proliferation upon knock-down of HGF or c-Met, respectively, most likely due to abnormal expression of various cell cycle-related genes. In particular, entry of hepatocytes from the G1 into S-phase was affected (6–12 h after PH). A slight increase in apoptosis was also observed in the rats treated with c-Met shRNA. These results confirmed the important function of c-Met in liver regeneration that cannot be compensated for by other growth factors.
Epidermal growth factor (EGF) family
The seven members of the EGF family perform their functions by activation of four different high-affinity receptors, EGFR/ErbB1, HER2/ErbB2, HER3/ErbB3 and HER4/ErbB4 (Burgess, 2008). Several EGFR ligands are expressed in the normal and injured liver, and upregulation of transforming growth factor (TGF)-α, heparin-binding EGF (HB-EGF) and amphiregulin upon PH has been described (Michalopoulos, 2007). Consistent with this finding, phosphorylation (activation) of the EGFR was observed 60 min after PH in rats (Stolz et al, 1999). Surprisingly, mice lacking TGF-α showed normal liver regeneration after PH (Russell et al, 1996). Hepatocyte proliferation was delayed in HB-EGF knockout mice, but this deficit was only transient, possibly due to compensatory upregulation of TGF-α (Mitchell et al, 2005). Vice versa, when HB-EGF was overexpressed in the liver of transgenic mice, proliferation of hepatocytes was strongly enhanced and the regeneration process was accelerated (Kiso et al, 2003). A mitogenic effect in the injured liver was also demonstrated for amphiregulin, as mice lacking this growth factor showed impaired proliferative responses after PH (Berasain et al, 2005). Finally, a delay in hepatocyte proliferation after PH was observed in mice after removal of the submandibular glands, which are a potent source of EGF. The phenotype was rescued by treatment of the mice with recombinant EGF (Noguchi et al, 1991) (Fig 3).
Figure 3. Regulation of liver regeneration by EGFR signalling.
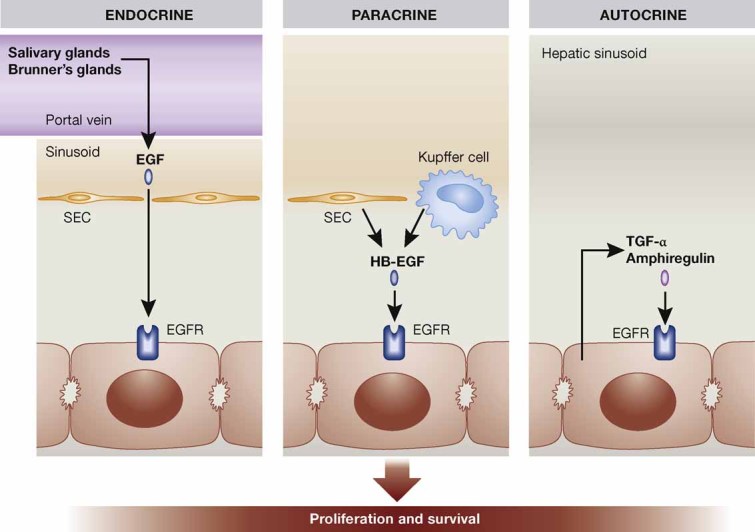
During liver regeneration the EGFR on hepatocytes is activated in an autocrine manner by amphiregulin and TGF-α, in a paracrine manner by HB-EGF derived from Kupffer cells and sinusoidal endothelial cells (SEC) and in an endocrine manner by EGF secreted from salivary glands and from Brunner's glands in the duodenum, from where it reaches the liver through the portal vein. EGFR activation stimulates hepatocyte proliferation and is important for survival after PH.
To overcome the problem of redundancy among EGFR ligands, mice lacking the EGFR in hepatocytes were generated (Natarajan et al, 2007). This did not affect normal liver function, but approximately one-third of the animals died after PH and this became apparent 36–48 h after removal of liver tissue. The high levels of mature TNF-α present in the serum of the knockout mice likely contribute to the increased mortality. However, the number of apoptotic cells was not increased, suggesting that activation of the EGFR does not provide a direct survival signal for hepatocytes. In the surviving mice, delayed hepatocyte proliferation was observed, most likely due to delayed induction of cyclin D1 expression. Nevertheless, complete regeneration finally occurred, demonstrating that EGFR signalling is important but not essential for liver regeneration (Fig 3). In another study, rats were injected with shRNAs against EGFR (Paranjpe et al, 2010). This resulted in decreased hepatocyte proliferation 24 h after surgery and concomitant compensatory activation of c-Met, ErbB2 and ErbB3. However, liver weight restoration finally did occur, again demonstrating that EGFR signalling is important but not essential for liver regeneration. In contrast to these studies, the treatment of mice with a neutralizing antibody to the EGFR did not affect liver regeneration. This may be due to incomplete inhibition of EGFR signalling in the treated mice (Van Buren et al, 2008).
Fibroblast growth factors (FGFs)
The FGF family comprises 22 members that exert their functions through activation of four transmembrane tyrosine kinase receptors, designated FGF receptors (FGFR) 1–4 (Ornitz & Itoh, 2001). Several FGFs are expressed in the regenerating liver as well as in other organs from where they can reach the liver via the blood stream. This is likely to be important for liver regeneration, as a mitogenic effect of several FGFs for hepatocytes has been demonstrated in vitro and in vivo ((Steiling et al, 2003) and references therein). To determine the role of FGFR signalling in liver regeneration, mice expressing a dominant-negative mutant of FGFR2 in hepatocytes were subjected to PH. Proliferation of hepatocytes was delayed in these mice due to delayed G1/S transition (Steiling et al, 2003). Impaired liver regeneration was also observed in zebrafish expressing a dominant-negative FGFR mutant in an inducible manner (Kan et al, 2009). Because the dominant-negative receptor mutant blocks signalling through all FGFRs in response to common ligands, the type of receptor involved in liver regeneration remained to be determined. Loss of FGFR4 in mice did not affect the regeneration process after PH (Yu et al, 2000), suggesting that signalling by other FGFRs is also required. Indeed, mice lacking FGFR1 and FGFR2 in hepatocytes showed a strongly increased mortality rate after PH that resulted from severe necrosis of the remaining liver tissue. Mechanistic studies revealed that FGFR signalling controls the hepatectomy-induced upregulation of transcription factors, which induce the expression of detoxifying enzymes of the cytochrome P450 family. Therefore, detoxification of endogenous compounds and in particular of the drugs used for anaesthesia/analgesia was impaired in the FGFR1/FGFR2-deficient mice, resulting in liver damage and frequent failure. These data revealed an important cytoprotective role of FGFR1 and FGFR2 in the regenerating liver (Böhm et al, 2010).
Fibroblast growth factor receptors are also expressed on non-parenchymal cells, including endothelial cells, and FGF2 is a potent angiogenesis factor (Presta et al, 2009). When FGF2 knockout mice were subjected to PH, the overall regeneration process occurred normally. However, cell proliferation at day 4 after PH was reduced (Sturm et al, 2004). This may reflect delayed proliferation of endothelial cells, although this was not directly addressed. The observed upregulation of vascular endothelial growth factor (VEGF), another major endothelial cell mitogen, suggests that increased VEGF levels can compensate for the loss of FGF2. This hypothesis was supported by the delayed liver regeneration that occurred upon treatment of FGF2 knockout mice but not of control littermates with a VEGF receptor kinase inhibitor (Sturm et al, 2004).
Vascular endothelial growth factor (VEGF)
The VEGF family comprises five members in mammals, which control vasculogenesis, angiogenesis and lymphangiogenesis through activation of three receptor tyrosine kinases (VEGFR1-3) (Lohela et al, 2009). Of particular importance for blood vessel formation is VEGF-A, which is strongly upregulated in the regenerating liver, in particular in hepatocytes (Taniguchi et al, 2001). Injection of VEGF-A into rats promoted proliferation of sinusoidal endothelial cells and also of hepatocytes 48 h after PH (Taniguchi et al, 2001). The effect on hepatocytes most likely results from release of HGF from endothelial cells upon activation of VEGFR1 (LeCouter et al, 2003). Vessel density and diameter as well as liver to body weight ratio were also increased (Bockhorn et al, 2007). Furthermore, administration of recombinant adenovirus expressing the VEGFR splice variant VEGF-A165 prior to PH accelerated functional liver recovery in lean and also in obese mice (Redaelli et al, 2004). Vice versa, blockade of VEGF-A by treatment of rats with neutralizing antibodies suppressed proliferation of sinusoidal endothelial cells and also of hepatocytes after PH, and this impaired the regeneration process (Bockhorn et al, 2007; Taniguchi et al, 2001; Van Buren et al, 2008). By contrast, treatment with a VEGFR kinase inhibitor only affected liver regeneration in FGF2 knockout mice but not in wild-type mice (Sturm et al, 2004). The different results obtained with the neutralizing VEGF antibodies and the VEGFR kinase inhibitor may result from the use of different species (rats vs. mice), from different bioavailability of the agents or from differences in their efficiencies in blocking VEGF action.
Insulin-like growth factors (IGFs)
IGF-I and IGF-II are potent stimulators of mitogenesis and survival of different cell types. Their actions are predominantly mediated through the type I IGF receptor, a tyrosine kinase that resembles the insulin receptor, but also through the insulin receptor. The biological activity of IGFs is modulated by six insulin-like growth factor binding proteins (IGFBPs) (Pollak, 2008). Expression of IGFBP-1 is rapidly induced after PH, and the loss of this protein in mice caused abnormal liver regeneration characterized by severe necrosis, reduced and delayed DNA synthesis by hepatocytes, and blunted activation of Erk1/2. The last defect was rescued by treatment of the mice with a pre-operative dose of IGFBP-1, whereas this treatment was not sufficient to correct the defect in DNA synthesis (Leu et al, 2003). Although IGFBP-1 may also exert IGF-independent functions in the regenerating liver, a role for IGF in liver regeneration has been further demonstrated. Mice with a liver-specific IGF-1R knockout displayed a strong impairment in hepatocyte proliferation after PH. Surprisingly, this defect was only observed in male mice, indicating hormonal influences on the phenotype (Desbois-Mouthon et al, 2006). Finally, mice lacking the cytoprotective Nrf2 transcription factor showed delayed liver regeneration due to insulin/IGF-1 resistance that was caused by enhanced levels of reactive oxygen species (Beyer et al, 2008). These studies provide evidence for an important role of the IGF/insulin system in liver regeneration.
Wnt proteins
Wnt proteins constitute a large family of growth and differentiation factors. Upon binding to the frizzled transmembrane receptor, different signalling pathways are initiated. Of particular importance is the canonical wnt pathway that results in stabilization and nuclear translocation of β-catenin, which then acts as a transcriptional regulator (van Amerongen & Nusse, 2009). Wnt-1 and β-catenin are expressed by hepatocytes, and stabilization and nuclear translocation of β-catenin occur immediately after PH (Monga et al, 2001). To determine the function of wnt/β-catenin signalling in liver regeneration, morpholino-mediated knockdown of β-catenin was performed following PH via injection of anti-sense phospho-morpholino oligonucleotides into the superior mesenteric vein. This caused impaired hepatocyte proliferation and reduction of the liver/body weight ratio in comparison to mice treated with control morpholinos (Sodhi et al, 2005). The important role of β-catenin in liver regeneration was confirmed by analysis of mice lacking this protein in hepatocytes (Tan et al, 2006). These hepatocytes showed a delayed onset of DNA synthesis after PH and the mice appeared sick and lethargic. In addition, enhanced apoptosis was seen in the liver at all time points during regeneration, though the liver eventually regenerated. The regeneration defect may be a direct consequence of the loss of β-catenin. Alternatively, the reduced liver size of these mice and/or the reduction in EGFR expression observed in these livers may contribute to the regeneration phenotype (Tan et al, 2006). Indeed, while impaired liver regeneration in mice with hepatocyte-specific loss of β-catenin was also observed in another study (Sekine et al, 2007), these investigators did not find a correlation between activation of β-catenin signalling and hepatocyte proliferation. This observation argues for an indirect mechanism that remains thus far uncharacterized.
Jagged, Delta and Notch
There are four different Notch receptors (Notch-1–4) in mammals, that are activated by their membrane-bound ligands Jagged-1 and -2, and Delta-like 1, 3 and 4. Upon ligand binding, Notch proteins are cleaved and the carboxyterminal peptide translocates to the nucleus to control gene expression (Rampal et al, 2007). Several components of this pathway are expressed in the normal and regenerating liver and both Notch-1 and Jagged-1 are upregulated upon PH. Most importantly, nuclear translocation of the intracellular part of Notch-1 was observed within 15 min after PH, followed by upregulation of the Notch target genes Hes-1 and Hes-5 (Kohler et al, 2004). In vitro studies revealed that Jagged-1 is a mitogen for hepatocytes, and injection of plasmids encoding silencing RNAs for Notch-1 or Jagged-1 into the superior mesenteric vein 2 days prior PH suppressed hepatocyte proliferation in the regenerating liver (Kohler et al, 2004). The important role of Notch-1 in liver regeneration was confirmed in a genetic model where Notch-1 was deleted in the liver in an inducible manner. This already caused nodular regenerative hyperplasia of the liver prior to PH. Upon surgery, restoration of the liver mass was delayed in the knockout animals due to a reduced proliferative response of the Notch-1 deficient hepatocytes. However, complete regeneration finally occurred and the increased liver weight that was seen in the knockout mice prior to PH was restored (Croquelois et al, 2005).
Notch signalling is also important for the regeneration of the liver vasculature (Wang et al, 2009). This was shown in mice with an inducible deletion of a major Notch signalling regulator—the recombination signal binding protein-Jκ (RBP-J). This protein associates with the cleaved part of the Notch receptors and mediates Notch-induced gene regulation. RBP-J was deleted in hepatocytes and endothelial cells of mice and an abnormal arrangement and narrowing of liver sinusoids was observed after PH. Most importantly, proliferation of liver sinusoidal endothelial cells was impaired, and obstruction of the sinusoid microcirculation occurred. As a consequence, proliferation of hepatocytes was reduced and their rate of apoptosis was increased. Nevertheless, the liver finally regenerated, albeit with a strong delay (Wang et al, 2009).
Transforming growth factor β (TGF-β)
The three types of TGF-β that exist in mammals (TGF-β1–3) exert their functions through activation of heteromeric receptor complexes consisting of type I and type II transmembrane receptors (Moustakas & Heldin, 2009). TGF-β is a potent growth inhibitor for different types of epithelial cells, including hepatocytes. TGF-β1 expression is strongly upregulated upon PH, but the responsiveness to this factor declines transiently in the regenerating liver, which may be important for hepatocyte proliferation (Houck & Michalopoulos, 1989). The transient resistance may result from down-regulation of the receptors or upregulation of signalling inhibitors, including SnoN and Ski (Macias-Silva et al, 2002). When TGF-β1 was administered intravenously at different time points during the liver regeneration process, hepatocyte proliferation was efficiently reduced. However, the effect was transient and the TGF-β treatment did not prevent regeneration (Russell et al, 1988). A delay in hepatocyte proliferation after PH was also seen in transgenic mice overexpressing mature TGF-β in hepatocytes (Bouzahzah et al, 2000).
To determine the role of endogenous TGF-β in liver regeneration, mice lacking the type II TGF-β receptor in hepatocytes were generated. Upon PH, hepatocyte proliferation was strongly accelerated in these mice up to 7 days after surgery and the liver to body weight ratio was increased. However, the hyperproliferation was only transient and normal regeneration was achieved (Romero-Gallo et al, 2005). A similar approach to study TGF-β function in liver regeneration was used by another group (Oe et al, 2004). These investigators also found accelerated hepatocyte proliferation, although the effect was no longer observed 120 h after PH. Interestingly, enhanced expression of activin A and its type II receptor as well as persistent SMAD activation were seen at this time point, indicating a compensatory activity of activin under these conditions. This hypothesis was confirmed by treatment of mice with the activin antagonist follistatin, which resulted in enhanced hepatocyte proliferation at 120 h, particularly in the mice lacking the type II TGF-β receptor (Oe et al, 2004). These findings suggest that activin and TGF-β collaborate to terminate hepatocyte proliferation in the regenerating liver (Fig 4), although a contribution of additional factors to this process cannot be excluded.
Figure 4. Possible collaborative functions of TGF-β and activin in the termination of liver regeneration.
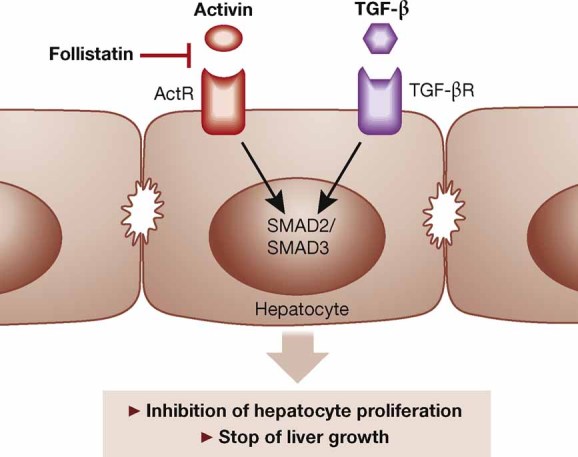
Activin A and TGF-β activate their individual receptor complexes on hepatocytes, resulting in activation of SMAD2 and SMAD3 and inhibition of cell proliferation. This is likely to be important for the termination of liver regeneration. Activin A activity is inhibited by the secreted glycoprotein follistatin, which acts as a positive regulator of liver regeneration.
Activins
Activins are members of the TGF-β superfamily, which exert their functions through heterodimeric receptor complexes consisting of type I and type II receptors that differ from the TGF-β receptors. The most abundant activin variant is activin A, but activins AB, B, C and E also exist in mammals (Werner & Alzheimer, 2006). Although activin C and E are strongly expressed in the liver, mice lacking these proteins showed normal liver development and regeneration after PH (Lau et al, 2000). On the other hand, adenovirus-mediated overexpression of the activin βC subunit (which gives rise to activin C) accelerated hepatocyte proliferation and reestablishment of the liver mass after PH (Wada et al, 2005). Consistent with the pro-mitogenic role of activin C for hepatocytes, mitotic hepatocytes frequently express the activin βC subunit in the regenerating rat liver (Gold et al, 2005). By contrast, apoptotic cells often express the activin βA subunit (Gold et al, 2005) and activin A was identified as a pro-apoptotic factor for hepatocytes in vivo (Hully et al, 1994). Activin A also inhibits proliferation of these cells, as administration of the activin A antagonist follistatin immediately after PH accelerated the initial round of DNA synthesis and stimulated liver regeneration in rats (Kogure et al, 1995). Furthermore, hepatocyte proliferation was prolonged when a booster of follistatin was injected 48 h after PH into mice (Oe et al, 2004). These results identify activin A as a negative regulator of hepatocyte proliferation in the regenerating liver with a potential role in the termination of the regeneration process (Fig 4).
Bone morphogenetic proteins (BMPs)
In contrast to TGF-β and activin A, BMPs have been described as positive regulators of liver regeneration. Thus, inducible expression of a dominant-negative BMP receptor in zebrafish strongly impaired regeneration after 1/3 hepatectomy (Kan et al, 2009). In mice, systemic administration of BMP-7 stimulated hepatocyte proliferation after PH and enhanced the restoration of liver mass, whereas application of a soluble BMP type I receptor (Alk3) or a neutralizing antibody to BMP-7 had the opposite effect, which was rescued by application of BMP-7 (Sugimoto et al, 2007). The roles of other BMPs in the liver regeneration process remain to be determined.
Open questions
The past few years have seen a remarkable increase in research on liver regeneration and the functional roles of various growth factors and cytokines in this process have been explored. Nevertheless, numerous open questions remain that need to be addressed in the future:
Although a large number of growth factors and cytokines are expressed in the regenerating liver, only a few of them have been functionally characterized. Therefore, the analysis of additional genetically modified mice will most likely identify further key players in this process.
Functional redundancy and/or compensation have most likely masked the function of several growth factors and cytokines in liver regeneration as demonstrated for members of the EGF family. Therefore, the generation and analysis of mice lacking two or more growth factors of the same family will provide further insight into the role of these factors in liver regeneration.
It is as yet unclear whether different growth factor families have unique or overlapping functions in liver regeneration. Therefore, liver regeneration studies using mice deficient in two or more growth factors or their receptors that do not belong to the same family (e.g. the EGFR and the c-Met receptor) will be of interest.
In some cases, controversial results have been obtained with regard to the function of individual genes in liver regeneration. This may be due to differences in the surgical procedure and its timing, to the use of different anesthesia/analgesia, to different nutrition, to the use of rats vs. mice or to differences in the genetic background of mice. These issues need to be considered when comparing results from different laboratories, and the international standardization of experimental procedures is an obvious goal.
Only few studies have addressed the mechanisms of action of growth factors and cytokines in liver regeneration at the molecular level. Future studies using microarray analyses to identify the mRNAs and micro-RNAs expressed in the normal and regenerating liver of mice with overexpression or loss of specific growth factors or their receptors will identify that the target genes of these factors are important for liver regeneration. This should be complemented by proteomics and phospho-proteomics studies to identify the signalling pathways and downstream effectors involved in liver regeneration and the role of certain growth factors in this regulation.
In most studies the classical procedure of a two-third hepatectomy has been used for the analysis of growth factor function in liver regeneration. It will be interesting to determine the roles of growth factors in more severe injury models, such as 87% hepatectomy or PH followed by treatment with LPS (see, e.g. (Jin et al, 2007; Wuestefeld et al, 2003)).
Liver surgery is frequently performed in patients with a pre-damaged liver, e.g. in patients with liver fibrosis/cirrhosis. Therefore, it will be important to characterize the role of different growth factors and cytokines in the repair of diseased liver and to determine if treatment with exogenous growth factors or neutralization/down-regulation of proteins that inhibit growth factor action can be used to stimulate liver regeneration under such conditions.
Inhibition of several growth factors or their receptors in rodents has been shown to impair the liver regeneration process. Since various antagonists for growth factors or their receptors have been approved or are in clinical trials for the treatment of cancer, it will be interesting to determine whether these antagonists impair liver regeneration in humans. This is particularly important in cancer patients undergoing liver surgery for removal of metastases. Initial results did not reveal a major impairment of liver regeneration in patients treated with antibodies against VEGF-A or EGFR (Cleary et al, 2009; Gruenberger & Gruenberger, 2008), but data from additional clinical trials are required to address this question and to optimize the timing of surgery for these patients upon cessation of their treatment with inhibitors. Furthermore, the effects of antagonists against other growth factors—such as HGF and FGFs—on liver regeneration remain to be determined.
Finally, the challenge we face is to use our knowledge of the role of growth factors in liver regeneration to improve this process in humans, e.g. by systemic application of growth factors or neutralizing antibodies against proteins that inhibit growth factor signalling. This is of major importance, as augmenting liver regeneration is essential to improving the survival rate of patients after surgical resection of large amounts of liver tissue; for example, in patients with liver metastases. In addition, the improvement of the liver regeneration process would reduce the amount of liver tissue required for liver transplantation. This will reduce the risk for the donors and would also enhance the growth of the transplant in the recipient. Finally, the factors that facilitate hepatocyte proliferation and normal regeneration—and thus limit or even prevent the development of fibrosis—need to be identified. These factors and their downstream effectors are likely to be important targets for therapeutic intervention in patients with different types of liver disease.
Pending issues
Role of other growth factors and cytokines in liver regeneration, as well as thorough analysis of redundancy and compensation among well characterized players.
Molecular dissection of the signalling cascades downstream of growth factor and cytokine receptors.
International standardization of PH experimental procedures.
Liver regeneration studies in rodents with pre-damaged liver, e.g. with liver fibrosis.
Translation of mouse studies to human studies.
Acknowledgments
Work on liver regeneration in the laboratory of S. W. is supported by grants from the ETH Zurich (TH 41/04-2) and the Swiss National Science Foundation (3100A0-109340). U.K. is supported by a Boehringer Ingelheim predoctoral fellowship.
References
- Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579–G585. doi: 10.1152/ajpgi.1992.263.4.G579. [DOI] [PubMed] [Google Scholar]
- Aldeguer X, Debonera F, Shaked A, Krasinkas AM, Gelman AE, Que X, Zamir GA, Hiroyasu S, Kovalovich KK, Taub R, et al. Interleukin-6 from intrahepatic cells of bone marrow origin is required for normal murine liver regeneration. Hepatology. 2002;35:40–48. doi: 10.1053/jhep.2002.30081. [DOI] [PubMed] [Google Scholar]
- Anders RA, Subudhi SK, Wang J, Pfeffer K, Fu YX. Contribution of the lymphotoxin beta receptor to liver regeneration. J Immunol. 2005;175:1295–1300. doi: 10.4049/jimmunol.175.2.1295. [DOI] [PubMed] [Google Scholar]
- Bell A, Chen Q, DeFrances MC, Michalopoulos GK, Zarnegar R. The five amino acid-deleted isoform of hepatocyte growth factor promotes carcinogenesis in transgenic mice. Oncogene. 1999;18:887–895. doi: 10.1038/sj.onc.1202379. [DOI] [PubMed] [Google Scholar]
- Benvenuti S, Comoglio PM. The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol. 2007;213:316–325. doi: 10.1002/jcp.21183. [DOI] [PubMed] [Google Scholar]
- Berasain C, Garcia-Trevijano ER, Castillo J, Erroba E, Lee DC, Prieto J, Avila MA. Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology. 2005;128:424–432. doi: 10.1053/j.gastro.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Beyer TA, Xu W, Teupser D, auf dem Keller U, Bugnon P, Hildt E, Thiery J, Kan YW, Werner S. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212–223. doi: 10.1038/sj.emboj.7601950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blindenbacher A, Wang X, Langer I, Savino R, Terracciano L, Heim MH. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology. 2003;38:674–682. doi: 10.1053/jhep.2003.50378. [DOI] [PubMed] [Google Scholar]
- Bockhorn M, Goralski M, Prokofiev D, Dammann P, Grunewald P, Trippler M, Biglarnia A, Kamler M, Niehues EM, Frilling A, et al. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res. 2007;138:291–299. doi: 10.1016/j.jss.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Böhm F, Speicher T, Hellerbrand C, Dickson C, Partanen JM, Ornitz DM, Werner S. FGF receptors 1 and 2 control chemically-induced injury and compound detoxification in the regenerating liver. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.06.069. (in press), July 2, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiak M, Garratt AN, Wustefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA. 2004;101:10608–10613. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzahzah B, Fu M, Iavarone A, Factor VM, Thorgeirsson SS, Pestell RG. Transforming growth factor-beta1 recruits histone deacetylase 1 to a p130 repressor complex in transgenic mice in vivo. Cancer Res. 2000;60:4531–4537. [PubMed] [Google Scholar]
- Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008;26:263–274. doi: 10.1080/08977190802312844. [DOI] [PubMed] [Google Scholar]
- Campbell JS, Riehle KJ, Brooling JT, Bauer RL, Mitchell C, Fausto N. Proinflammatory cytokine production in liver regeneration is MyD88-dependent, but independent of Cd14, Tlr2, and Tlr4. J Immunol. 2006;176:2522–2528. doi: 10.4049/jimmunol.176.4.2522. [DOI] [PubMed] [Google Scholar]
- Cleary JM, Tanabe KT, Lauwers GY, Zhu AX. Hepatic toxicities associated with the use of preoperative systemic therapy in patients with metastatic colorectal adenocarcinoma to the liver. Oncologist. 2009;14:1095–1105. doi: 10.1634/theoncologist.2009-0152. [DOI] [PubMed] [Google Scholar]
- Cornell RP. Gut-derived endotoxin elicits hepatotrophic factor secretion for liver regeneration. Am J Physiol. 1985;249:R551–R562. doi: 10.1152/ajpregu.1985.249.5.R551. [DOI] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- Croquelois A, Blindenbacher A, Terracciano L, Wang X, Langer I, Radtke F, Heim MH. Inducible inactivation of Notch1 causes nodular regenerative hyperplasia in mice. Hepatology. 2005;41:487–496. doi: 10.1002/hep.20571. [DOI] [PubMed] [Google Scholar]
- Desbois-Mouthon C, Wendum D, Cadoret A, Rey C, Leneuve P, Blaise A, Housset C, Tronche F, Le Bouc Y, Holzenberger M. Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. Faseb J. 2006;20:773–775. doi: 10.1096/fj.05-4704fje. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- Fujita J, Marino MW, Wada H, Jungbluth AA, Mackrell PJ, Rivadeneira DE, Stapleton PP, Daly JM. Effect of TNF gene depletion on liver regeneration after partial hepatectomy in mice. Surgery. 2001;129:48–54. doi: 10.1067/msy.2001.109120. [DOI] [PubMed] [Google Scholar]
- Gold EJ, Zhang X, Wheatley AM, Mellor SL, Cranfield M, Risbridger GP, Groome NP, Fleming JS. betaA- and betaC-activin, follistatin, activin receptor mRNA and betaC-activin peptide expression during rat liver regeneration. J Mol Endocrinol. 2005;34:505–515. doi: 10.1677/jme.1.01657. [DOI] [PubMed] [Google Scholar]
- Gruenberger B, Gruenberger T. Using bevacizumab and cetuximab before liver surgery. Curr Colorectal Cancer Rep. 2008;4:126–129. [Google Scholar]
- Houck KA, Michalopoulos GK. Altered responses of regenerating hepatocytes to norepinephrine and transforming growth factor type beta. J Cell Physiol. 1989;141:503–509. doi: 10.1002/jcp.1041410308. [DOI] [PubMed] [Google Scholar]
- Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hully JR, Chang L, Schwall RH, Widmer HR, Terrell TG, Gillett NA. Induction of apoptosis in the murine liver with recombinant human activin A. Hepatology. 1994;20:854–862. doi: 10.1002/hep.1840200413. [DOI] [PubMed] [Google Scholar]
- Jin X, Zhang Z, Beer-Stolz D, Zimmers TA, Koniaris LG. Interleukin-6 inhibits oxidative injury and necrosis after extreme liver resection. Hepatology. 2007;46:802–812. doi: 10.1002/hep.21728. [DOI] [PubMed] [Google Scholar]
- Kan NG, Junghans D, Izpisua Belmonte JC. Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. Faseb J. 2009;23:3516–3525. doi: 10.1096/fj.09-131730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiso S, Kawata S, Tamura S, Inui Y, Yoshida Y, Sawai Y, Umeki S, Ito N, Yamada A, Miyagawa J, et al. Liver regeneration in heparin-binding EGF-like growth factor transgenic mice after partial hepatectomy. Gastroenterology. 2003;124:701–707. doi: 10.1053/gast.2003.50097. [DOI] [PubMed] [Google Scholar]
- Knight B, Yeoh GC. TNF/LTalpha double knockout mice display abnormal inflammatory and regenerative responses to acute and chronic liver injury. Cell Tissue Res. 2005;319:61–70. doi: 10.1007/s00441-004-1003-6. [DOI] [PubMed] [Google Scholar]
- Kogure K, Omata W, Kanzaki M, Zhang YQ, Yasuda H, Mine T, Kojima I. A single intraportal administration of follistatin accelerates liver regeneration in partially hepatectomized rats. Gastroenterology. 1995;108:1136–1142. doi: 10.1016/0016-5085(95)90212-0. [DOI] [PubMed] [Google Scholar]
- Kohler C, Bell AW, Bowen WC, Monga SP, Fleig W, Michalopoulos GK. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39:1056–1065. doi: 10.1002/hep.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AL, Kumar TR, Nishimori K, Bonadio J, Matzuk MM. Activin betaC and betaE genes are not essential for mouse liver growth, differentiation, and regeneration. Mol Cell Biol. 2000;20:6127–6137. doi: 10.1128/mcb.20.16.6127-6137.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- Leu JI, Crissey MA, Craig LE, Taub R. Impaired hepatocyte DNA synthetic response posthepatectomy in insulin-like growth factor binding protein 1-deficient mice with defects in C/EBP beta and mitogen-activated protein kinase/extracellular signal-regulated kinase regulation. Mol Cell Biol. 2003;23:1251–1259. doi: 10.1128/MCB.23.4.1251-1259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277:28411–28417. doi: 10.1074/jbc.M202807200. [DOI] [PubMed] [Google Scholar]
- Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Macias-Silva M, Li W, Leu JI, Crissey MA, Taub R. Up-regulated transcriptional repressors SnoN and Ski bind Smad proteins to antagonize transforming growth factor-beta signals during liver regeneration. J Biol Chem. 2002;277:28483–28490. doi: 10.1074/jbc.M202403200. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Nivison M, Jackson LF, Fox R, Lee DC, Campbell JS, Fausto N. Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem. 2005;280:2562–2568. doi: 10.1074/jbc.M412372200. [DOI] [PubMed] [Google Scholar]
- Moh A, Iwamoto Y, Chai GX, Zhang SS, Kano A, Yang DD, Zhang W, Wang J, Jacoby JJ, Gao B, et al. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest. 2007;87:1018–1028. doi: 10.1038/labinvest.3700630. [DOI] [PubMed] [Google Scholar]
- Mohammed FF, Smookler DS, Taylor SE, Fingleton B, Kassiri Z, Sanchez OH, English JL, Matrisian LM, Au B, Yeh WC, et al. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004;36:969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- Mohammed FF, Pennington CJ, Kassiri Z, Rubin JS, Soloway PD, Ruther U, Edwards DR, Khokha R. Metalloproteinase inhibitor TIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration. Hepatology. 2005;41:857–867. doi: 10.1002/hep.20618. [DOI] [PubMed] [Google Scholar]
- Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Nonaka H, Saito H, Tanaka M, Miyajima A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology. 2004;39:635–644. doi: 10.1002/hep.20086. [DOI] [PubMed] [Google Scholar]
- Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci USA. 2007;104:17081–17086. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S, Ohba Y, Oka T. Influence of epidermal growth factor on liver regeneration after partial hepatectomy in mice. J Endocrinol. 1991;128:425–431. doi: 10.1677/joe.0.1280425. [DOI] [PubMed] [Google Scholar]
- Norris PS, Ware CF. The LT beta R signaling pathway. Adv Exp Med Biol. 2007;597:160–172. doi: 10.1007/978-0-387-70630-6_13. [DOI] [PubMed] [Google Scholar]
- Oe S, Lemmer ER, Conner EA, Factor VM, Leveen P, Larsson J, Karlsson S, Thorgeirsson SS. Intact signaling by transforming growth factor beta is not required for termination of liver regeneration in mice. Hepatology. 2004;40:1098–1105. doi: 10.1002/hep.20426. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe S, Bowen WC, Bell AW, Nejak-Bowen K, Luo JH, Michalopoulos GK. Cell cycle effects resulting from inhibition of hepatocyte growth factor and its receptor c-Met in regenerating rat livers by RNA interference. Hepatology. 2007;45:1471–1477. doi: 10.1002/hep.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe S, Bowen WC, Tseng GC, Luo JH, Orr A, Michalopoulos GK. RNA interference against hepatic epidermal growth factor receptor has suppressive effects on liver regeneration in rats. Am J Pathol. 2010;176:2669–2681. doi: 10.2353/ajpath.2010.090605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- Presta M, Andres G, Leali D, Dell'era P, Ronca R. Inflammatory cells and chemokines sustain FGF2-induced angiogenesis. Eur Cytokine Netw. 2009;20:39–50. doi: 10.1684/ecn.2009.0155. [DOI] [PubMed] [Google Scholar]
- Rampal R, Luther KB, Haltiwanger RS. Notch signaling in normal and disease states: possible therapies related to glycosylation. Curr Mol Med. 2007;7:427–445. doi: 10.2174/156652407780831593. [DOI] [PubMed] [Google Scholar]
- Redaelli CA, Semela D, Carrick FE, Ledermann M, Candinas D, Sauter B, Dufour JF. Effect of vascular endothelial growth factor on functional recovery after hepatectomy in lean and obese mice. J Hepatol. 2004;40:305–312. doi: 10.1016/j.jhep.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Ren X, Hogaboam C, Carpenter A, Colletti L. Stem cell factor restores hepatocyte proliferation in IL-6 knockout mice following 70% hepatectomy. J Clin Invest. 2003;112:1407–1418. doi: 10.1172/JCI17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle KJ, Campbell JS, McMahan RS, Johnson MM, Beyer RP, Bammler TK, Fausto N. Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. J Exp Med. 2008;205:91–103. doi: 10.1084/jem.20070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Gallo J, Sozmen EG, Chytil A, Russell WE, Whitehead R, Parks WT, Holdren MS, Her MF, Gautam S, Magnuson M, et al. Inactivation of TGF-beta signaling in hepatocytes results in an increased proliferative response after partial hepatectomy. Oncogene. 2005;24:3028–3041. doi: 10.1038/sj.onc.1208475. [DOI] [PubMed] [Google Scholar]
- Russell WE, Coffey RJ, Jr, Ouellette AJ, Moses HL. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci USA. 1988;85:5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell WE, Kaufmann WK, Sitaric S, Luetteke NC, Lee DC. Liver regeneration and hepatocarcinogenesis in transforming growth factor-alpha-targeted mice. Mol Carcinog. 1996;15:183–189. doi: 10.1002/(SICI)1098-2744(199603)15:3<183::AID-MC4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli V, Demetris AJ. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology. 1999;29:403–411. doi: 10.1002/hep.510290244. [DOI] [PubMed] [Google Scholar]
- Sakata H, Takayama H, Sharp R, Rubin JS, Merlino G, LaRochelle WJ. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Differ. 1996;7:1513–1523. [PubMed] [Google Scholar]
- Seki E, Tsutsui H, Iimuro Y, Naka T, Son G, Akira S, Kishimoto T, Nakanishi K, Fujimoto J. Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration. Hepatology. 2005;41:443–450. doi: 10.1002/hep.20603. [DOI] [PubMed] [Google Scholar]
- Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology. 2007;45:361–368. doi: 10.1002/hep.21523. [DOI] [PubMed] [Google Scholar]
- Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692–700. doi: 10.1053/gast.2003.50098. [DOI] [PubMed] [Google Scholar]
- Shiota G, Kawasaki H. Hepatocyte growth factor in transgenic mice. Int J Exp Pathol. 1998;79:267–277. doi: 10.1046/j.1365-2613.1998.730403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi D, Micsenyi A, Bowen WC, Monga DK, Talavera JC, Monga SP. Morpholino oligonucleotide-triggered beta-catenin knockdown compromises normal liver regeneration. J Hepatol. 2005;43:132–141. doi: 10.1016/j.jhep.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Steiling H, Wustefeld T, Bugnon P, Brauchle M, Fassler R, Teupser D, Thiery J, Gordon JI, Trautwein C, Werner S. Fibroblast growth factor receptor signalling is crucial for liver homeostasis and regeneration. Oncogene. 2003;22:4380–4388. doi: 10.1038/sj.onc.1206499. [DOI] [PubMed] [Google Scholar]
- Stolz DB, Mars WM, Petersen BE, Kim TH, Michalopoulos GK. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res. 1999;59:3954–3960. [PubMed] [Google Scholar]
- Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm J, Keese M, Zhang H, Bonninghoff R, Magdeburg R, Vajkoczy P, Dono R, Zeller R, Gretz N. Liver regeneration in FGF-2-deficient mice: VEGF acts as potential functional substitute for FGF-2. Liver Int. 2004;24:161–168. doi: 10.1111/j.1478-3231.2004.0896.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Yang C, LeBleu VS, Soubasakos MA, Giraldo M, Zeisberg M, Kalluri R. BMP-7 functions as a novel hormone to facilitate liver regeneration. Faseb J. 2007;21:256–264. doi: 10.1096/fj.06-6837com. [DOI] [PubMed] [Google Scholar]
- Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem. 2001;49:121–130. doi: 10.1177/002215540104900112. [DOI] [PubMed] [Google Scholar]
- Tumanov AV, Koroleva EP, Christiansen PA, Khan MA, Ruddy MJ, Burnette B, Papa S, Franzoso G, Nedospasov SA, Fu YX, et al. T cell-derived lymphotoxin regulates liver regeneration. Gastroenterology. 2009;136:694–704. doi: 10.1053/j.gastro.2008.09.015. e 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Van Buren G, II, Yang AD, Dallas NA, Gray MJ, Lim SJ, Xia L, Fan F, Somcio R, Wu Y, Hicklin DJ, et al. Effect of molecular therapeutics on liver regeneration in a murine model. J Clin Oncol. 2008;26:1836–1842. doi: 10.1200/JCO.2007.11.6566. [DOI] [PubMed] [Google Scholar]
- Wada W, Medina J, Hasegawa Y, Kuwano H, Kojima I. Adenovirus-mediated overexpression of the activin betaC subunit accelerates liver regeneration in partially hepatectomized rats. J Hepatol. 2005;43:823–828. doi: 10.1016/j.jhep.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang CM, Hou LH, Dou GR, Wang YC, Hu XB, He F, Feng F, Zhang HW, Liang YM, et al. Disruption of the transcription factor recombination signal-binding protein-Jkappa (RBP-J) leads to veno-occlusive disease and interfered liver regeneration in mice. Hepatology. 2009;49:268–277. doi: 10.1002/hep.22579. [DOI] [PubMed] [Google Scholar]
- Werner S, Alzheimer C. Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev. 2006;17:157–171. doi: 10.1016/j.cytogfr.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wuestefeld T, Klein C, Streetz KL, Betz U, Lauber J, Buer J, Manns MP, Muller W, Trautwein C. Interleukin-6/glycoprotein 130-dependent pathways are protective during liver regeneration. J Biol Chem. 2003;278:11281–11288. doi: 10.1074/jbc.M208470200. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Webber EM, Kirillova I, Peschon JJ, Fausto N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor. Hepatology. 1998;28:959–970. doi: 10.1002/hep.510280410. [DOI] [PubMed] [Google Scholar]
- Yang L, Magness ST, Bataller R, Rippe RA, Brenner DA. NF-kappaB activation in Kupffer cells after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2005;289:G530–G538. doi: 10.1152/ajpgi.00526.2004. [DOI] [PubMed] [Google Scholar]
- Yu C, Wang F, Kan M, Jin C, Jones RB, Weinstein M, Deng CX, McKeehan WL. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J Biol Chem. 2000;275:15482–15489. doi: 10.1074/jbc.275.20.15482. [DOI] [PubMed] [Google Scholar]


