Abstract
Preterm birth affects over 12% of all infants born in the US yet the biology of early delivery remains unclear, including whether epigenetic mechanisms are involved. We examined associations of maternal and umbilical cord blood long interspersed nuclear element-1 (LINE-1) DNA methylation with length of gestation and odds of preterm birth in singleton pregnancies in Project Viva. In white blood cells from maternal blood during 1st trimester (n=914) and 2nd trimester (n=922), and from venous cord blood at delivery (n=557), we measured LINE-1 by pyrosequencing (expressed as %5 methyl cytosines within the LINE-1 region analyzed [%5mC]). We ran linear regression models to analyze differences in gestation length, and logistic models for odds of preterm birth (<37 v. ≥37 weeks gestation), across quartiles of LINE-1. Mean(SD) LINE-1 levels were 84.3(0.6), 84.5(0.4), and 84.6(0.7) %5mC for 1st trimester, 2nd trimester and cord blood, respectively. Mean(SD) gestational age was 39.5(1.8) weeks, and 6.5% of infants were born preterm. After adjustment for maternal age, race/ethnicity, BMI, education, smoking status, and fetal sex, women with the highest vs. lowest quartile of 1st trimester LINE-1 had longer gestations (0.45 weeks [95% CI 0.12, 0.78]) and lower odds of preterm birth (OR 0.40 [0.17, 0.94]), whereas associations with cord blood LINE-1 were in the opposite direction (−0.45 weeks, −0.83, −0.08) and (OR 4.55 [1.18, 17.5]). In conclusion, higher early pregnancy LINE-1 predicts lower risk of preterm birth. In contrast, preterm birth is associated with lower LINE-1 in cord blood.
Keywords: Preterm, epigenetics, LINE-1, DNA methylation
INTRODUCTION
Despite much research and healthcare effort, preterm birth remains a major public health problem. In the United States over 12% of infants are born preterm (<37 weeks’ gestation).1 Preterm birth contributes to over a third of all US infant mortality,2 and is linked to major long-term morbidities.3, 4 While social determinants such as poverty,5 African-American race/ethnicity,6 and biologic risk factors such as genital infections7–9 and tobacco exposure10 affect a woman’s risk of delivering preterm, the mechanisms connecting risk factors to preterm birth remain unclear.11
Gene-environment interactions may account for much of the variation in risk of delivering preterm and may work through epigenetic phenomena.12 Epigenetics refers to heritable differences in gene expression in the absence of genetic sequence variation.13 DNA methylation of cytosine-guanine (CpG) dinucleotides represents one of several known epigenetic mechanisms. Typically, in eukaryotic cells, methylation of CpG sites within promoter regions of genes silences gene expression.14 One option of interrogating DNA methylation involves analyzing particular genes. Another approach is to analyze DNA repetitive sequences, 13, 15, 16 since they comprise more than half of the human genome including over 500,000 heavily methylated long interspersed nucleotide elements (LINE-1).17 Typically LINE-1 is heavily methylated, but in states of cellular stress, repetitive elements can be hypomethylated.18, 19
While no human cohort studies report associations between epigenetic marks and the length of gestation or preterm birth, recent human studies raise the possibility that epigenetics may underlie differences in risk of preterm birth. While DNA methylation of organ tissues specifically involved in preterm delivery, including the placenta and fetal tissues, may provide more information, the DNA methylation of circulating white blood cells has been associated with other non-hematologic disease processes.20, 21 Blood DNA hypomethylation of LINE-1 has been associated with cardiovascular disease,21 and with risk factors for both cardiovascular disease and preterm birth22, 23 including smoking24 and folate deficiency.23, 25 Cardiovascular disease and preterm delivery may share pathophysiologic mechanisms as women who have had a prior preterm delivery have been shown to have higher odds of developing cardiovascular disease.26 Both of these states have been associated with inflammation, raising the question as to whether LINE-1 hypomethylation represents an aggregate measure of inflammation over time that could be associated with an increased risk of preterm birth.
Other epidemiologic observations also suggest a role for epigenetic disruptions and an increased risk of preterm birth. Large meta-analyses have shown that pregnancies conceived via in vitro fertilization (IVF) have higher odds of preterm birth compared to non-IVF conceived pregnancies.27–29 During the process of IVF, manipulation of the cells occurs at a time when DNA is demethylated and remethylated30 which may be a window of particular epigenetic susceptibility, supported by studies linking higher risk of imprinting disorders and IVF.31 Whether epigenetic alterations caused by IVF lead to an increased risk of preterm birth or whether epigenetic factors contribute both to infertility and subsequent preterm birth remains unknown. However, the association between IVF and preterm birth suggests a potential role for epigenetic contributions to preterm birth.
The aim of this study was to examine the extent to which LINE-1 methylation in maternal peripheral blood during pregnancy, and in umbilical cord blood, is associated with length of gestation and risk of preterm birth. Because lower LINE-1 methylation has been associated with many states of poor health,20, 21, 32, 33 we hypothesized that higher LINE-1 methylation during pregnancy would be associated with longer gestations and lower odds of preterm delivery.
METHODS
Study Subjects
We studied participants enrolled in Project Viva, a pre-birth cohort study of mother-infant pairs recruited from obstetric offices of Harvard Vanguard Medical Associates, a multi-specialty group practice in eastern Massachusetts.34 Eligibility to participate in the study included English fluency, singleton pregnancy and gestational age less than 22 weeks at the time of enrollment. Details of recruitment and retention procedures are published elsewhere.34, 35 Of the 2128 mothers with live births enrolled in the study, we analyzed data from the subset of 1160 participants with maternal blood DNA from the first trimester (n=914), the second trimester (n=922), and/or umbilical cord blood DNA at delivery (n=577). Not all participants had samples available at each time point. However, there was substantial overlap; 729 women had both first and second trimester samples available and for the 557 infants with cord blood samples there were 428 maternal first trimester and 427 maternal second trimester samples. Comparison of the 1160 participants in this analysis 968 excluded participants showed a higher proportion of maternal white race (74% vs. 58%) and college or graduate education (70% vs. 59%) and a slightly lower proportion of infants born <37 weeks’ gestation (6% vs. 8%). Mothers provided written, informed consent to participate in the study and to have their DNA analyzed. The institutional review boards of the participating institutions, including Harvard Pilgrim Health Care, Brigham and Women’s Hospital and Beth Israel Deaconess Medical Center, approved the study.
Gestational Age
We calculated gestational age in weeks by subtracting the date of the last menstrual period from the date of delivery. Eighty-six percent of the mothers had ultrasound data available at 16–20 weeks. For 12% of subjects, ultrasound pregnancy dating estimates differed by more than 10 days from the LMP, and for them we used the dating obtained from the ultrasound to determine gestational age at birth. We categorized infants as preterm if the gestational age at birth was less than 37 completed weeks of gestation.
Covariate Ascertainment
Using a combination of interviews, study questionnaires and medical record reviews, we collected information on maternal age, self-designated race/ethnicity, parity, smoking habits, education, marital status, household income, and infant sex. We calculated prepregnancy body mass index (BMI, kg/m2) based on self-reported prepregnancy height and weight. We categorized deliveries as “spontaneous” if labor preceded either a vaginal birth or a cesarean section, or as “medically indicated” if cesarean or induced vaginal birth occurred in the absence of spontaneous labor.
LINE-1 DNA Methylation Analysis
We collected venous whole blood samples at the end of the first and second trimesters of pregnancy during in-person study visits and from the umbilical cord at delivery. We immediately refrigerated samples and transferred them to laboratory. There, they were spun and blood components were separated into aliquots for storage at −80°C. We extracted high molecular weight genomic DNA from the buffy coat with commercially available PureGene Kits (Gentra Systems, Minneapolis, MN) to prepare the samples for pyrosequencing.
As previously described,21, 36, 37 we quantified DNA methylation using bisulfite-PCR and pyrosequencing to analyze the methylation at 4 CpG sites repeated throughout the genome. We used primers designed toward a consensus LINE-1 sequence that repeats with no variation in the majority of the genomic LINE-1 repeated elements. To verify bisulfite conversion, we used non-CpG cytosines as built-in controls. We measured methylation as a percentage of 5-methyl cytosines (%5mC), within the regions as studied, in two replicates (runs) and combined measures from two runs and four sites as described below. As part of a pilot, we had previously analyzed a subset (n=48) of the cord blood samples at just 3 CpG sites.
Statistical Analysis
To estimate LINE-1 methylation we fit a mixed effects model38 to the direct LINE-1 measures at the 4 CpG sites allowing a different mean level at each site in each run, for 8 modeled means all together. For example, site 1 in run 1 had a different modeled mean from site 2 in run 1 and from site 1 in run 2. This approach was necessary because assuming a common constant mean across runs and sites was untenable as between-run, within-site Pearson correlation coefficients were as low as 0.23 (median 0.39, maximum 0.73). Between run correlations of mean LINE-1 were 0.6 for each of the three time points. We allowed a separate set of 6 additional means for the 48 subjects for whom we measured LINE-1 at only three sites. We used this approach because we observed differences between the samples from these 48 subjects and the other subjects. We fit a random intercept and a general covariance structure allowing different variances for each site and different correlations between sites within run, and different correlations between and within site across runs. We used the predicted random intercepts (the empirical Bayes’ estimates) as the underlying LINE-1 methylation level for the analysis.
We examined LINE-1 as a continuous variable as well as in quartiles. For cord blood LINE-1 we used sex-specific quartiles because males had higher levels. We ran unadjusted and multivariable adjusted linear regression models to analyze differences in gestation length, and logistic models for odds of preterm birth (<37 v. ≥37 weeks gestation). We adjusted multivariable models for maternal age, race/ethnicity, pre-pregnancy BMI, education, smoking during pregnancy, and fetal sex. To explore possible effect modification by sex on the relationship between cord blood LINE-1 and gestational age we introduced infant sex interaction terms to multivariable regression models. We also ran stratified logistic multivariable regression models to determine whether the relationship between preterm and LINE-1 differed by spontaneous vs. medically-indicated deliveries. We performed all analyses using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Mean (SD) LINE-1 %5mC were 84.3 (0.6), 84.5 (0.7) and 84.6 (0.7) in first trimester, second trimester and cord blood, respectively. Correlations among the 3 time points were very low; (Pearson r=0.02 for first and second trimester LINE-1, 0.01 for second trimester and cord LINE-1, and −0.04 for first trimester and cord). Women who were white, multiparous, or married/cohabitating had slightly higher 1st trimester LINE-1 than their counterparts (supplementary Table S1). Second trimester LINE-1 did not vary by covariates. Cord blood LINE-1 was higher among male vs. female infants (84.8 vs. 84.4 P <0.001). Mean (SD) gestational ages were 39.5 (1.7), 39.5 (1.7), and 39.7 (1.5) weeks among infants included in first trimester, second trimester and cord blood analyses respectively. The percent of preterm infants (born <37 weeks’ gestation) was 6.3, 6.6 and 4.9 in the first trimester, second trimester and cord LINE-1 analyses, respectively (Table 1).
Table 1.
Characteristics of Project Viva participants by blood sample in which LINE-1 was analyzed
| 1st Trimester Blood (n = 914) | 2nd Trimester Blood (n = 922) | Cord Blood (n = 557) | |
|---|---|---|---|
| n (%) | |||
| Maternal age (years) | |||
| 15 – < 25 | 72 (7.9) | 57 (6.2) | 52 (9.3) |
| 25 – < 30 | 180 (19.7) | 189 (20.5) | 114 (20.5) |
| 30 – < 35 | 370 (40.5) | 390 (42.3) | 231 (41.5) |
| 35 – < 45 | 292 (31.9) | 286 (31.0) | 160 (28.7) |
| Race/ethnicity | |||
| White | 683 (74.9) | 703 (76.5) | 407 (73.2) |
| Black | 97 (10.6) | 97 (10.6) | 58 (10.4) |
| Hispanic | 62 (6.8) | 50 (5.4) | 43 (7.7) |
| Other | 70 (7.7) | 69 (7.5) | 48 (8.6) |
| Prepregnancy BMI (kg/m2) | |||
| < 25 | 569 (62.5) | 590 (64.2) | 349 (62.9) |
| 25 – < 30 | 209 (22.9) | 204 (22.2) | 124 (22.3) |
| ≥ 30 | 133 (14.6) | 125 (13.6) | 82 (14.8) |
| Education | |||
| Less than college graduate | 273 (29.9) | 266 (28.9) | 175 (31.5) |
| College graduate | 639 (70.1) | 653 (71.1) | 381 (68.5) |
| Smoking during pregnancy | |||
| Never | 595 (67.0) | 620 (69.0) | 373 (69.5) |
| Former | 192 (21.6) | 190 (21.1) | 111 (20.7) |
| During pregnancy | 101 (11.4) | 89 (9.9) | 53 (9.9) |
| Infant sex | |||
| Male | 481 (52.6) | 470 (51.0) | 294 (52.8) |
| Female | 433 (47.4) | 452 (49.0) | 263 (47.2) |
| Gestational age at delivery (weeks) | |||
| < 34 | 15 (1.6) | 12 (1.3) | 6 (1.1) |
| 34 – <37 | 43 (4.7) | 49 (5.3) | 21 (3.8) |
| ≥ 37 | 856 (93.7) | 861 (93.4) | 530 (95.2) |
Values may not add up to total n because of missing data.
LINE-1, long interspersed nuclear element 1; %5mC, %5-methylated cytosines, BMI, body mass index
Maternal blood LINE-1
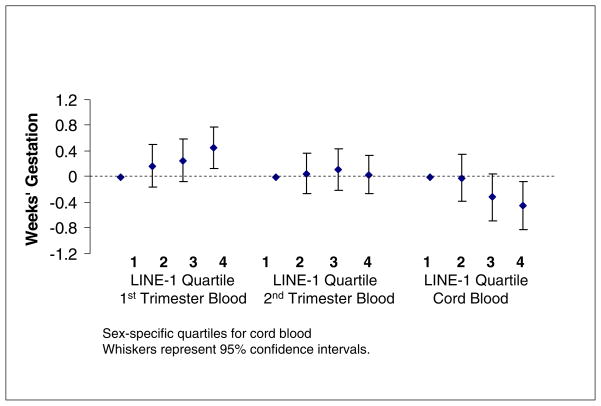
First trimester LINE-1 was positively associated with gestational age in unadjusted and adjusted models. Analysis of LINE-1 as a continuous variable revealed that each %5mC increase in LINE-1 predicted 0.20 (95% CI 0.01, 0.39) weeks longer gestation. Covariate adjustment did not change this estimate. Analyzing LINE-1 in quartiles, we found that mothers with the highest versus the lowest quartile of first trimester LINE-1 predicted longer gestations by an average of 0.45 weeks (95% CI 0.12, 0.78) (Fig.1). They also had substantially lower odds of preterm birth (OR 0.40, 95% CI 0.17, 0.93) (Table 3). Covariate adjustment did not materially alter this estimate. The odds ratios of preterm delivery associated with the lowest (vs. highest) quartile of LINE-1 did not differ by spontaneous (0.41, 95% CI 0.12, 1.34) and medically-indicated (0.35, 95% CI 0.10, 1.29) deliveries.
Figure 1.
Multivariable-adjusted Associations of LINE-1 DNA methylation with Gestational Age at Birth, Project Viva
Table 3.
Odds of Preterm Birth by Quartile of LINE-1 DNA Methylation among 1160a Participants Enrolled in Project Viva
| Preterm Cases | Unadjusted | Adjustedb | |
|---|---|---|---|
| n (%) | OR (95% CI) | ||
| First Trimester | |||
| 1st quartile | 19 (8.3) | 1.0 (reference) | 1.0 (reference) |
| 2nd quartile | 17 (7.4) | 0.88 (0.45, 1.74) | 0.91 (0.45, 1.81) |
| 3rd quartile | 14 (6.1) | 0.72 (0.35, 1.47) | 0.72 (0.35, 1.49) |
| 4th quartile | 8 (3.5) | 0.40 (0.17, 0.93) | 0.40 (0.17, 0.94) |
| Second Trimester | |||
| 1st quartile | 15 (6.5) | 1.0 (reference) | 1.0 (reference) |
| 2nd quartile | 12 (5.2) | 0.79 (0.36, 1.72) | 0.79 (0.36, 1.75) |
| 3rd quartile | 17 (7.4) | 1.14 (0.55, 2.34) | 1.14 (0.55, 2.37) |
| 4th quartile | 17 (7.4) | 1.14 (0.56, 2.35) | 1.16 (0.56, 2.40) |
| Cord Blood (sex-specific quartiles) | |||
| 1st quartile | 3 (2.2) | 1.0 (reference) | 1.0 (reference) |
| 2nd quartile | 6 (4.3) | 2.01 (0.49, 8.22) | 2.14 (0.50, 9.08) |
| 3rd quartile | 7 (5.0) | 2.37 (0.60, 9.35) | 2.29 (0.55, 9.48) |
| 4th quartile | 11 (7.9) | 3.87 (1.05, 14.2) | 4.55 (1.18, 17.5) |
First trimester n=914 (unadjusted) & n=884 (adjusted); second trimester n=922 (unadjusted) & n= 893 (adjusted); cord blood n=557 (unadjusted) & n= 534 (adjusted) because of missing data.
Adjusted for maternal age, BMI, race/ethnicity, education, smoking and infant sex in multivariable logistic regression models
LINE-1, long interspersed nuclear element-1, %5mC, %5-methylated cytosines
We did not detect any association between continuous second trimester LINE-1 and gestational age (−0.05 weeks, 95% CI −0.32, 0.22), or quartiles of second trimester LINE-1 and gestational age (Table 2). Nor did we detect associations between second trimester LINE-1 %5mC and preterm birth (OR for highest vs. lowest quartile 1.16, 95% CI 0.56, 2.40) (Table 3).
Table 2.
Gestation length differences according to quartile of LINE-1 methylation in leukocytes among participants enrolled in Project Vivaa
| LINE-1 %5mC | Unadjusted | Adjustedb | |||
|---|---|---|---|---|---|
| Mean (SD) | Min | Max | Mean Difference in Gestation Length Weeks (95% CI) | ||
| First Trimester Maternal Blood | |||||
| 1st Quartile | 83.5 (0.3) | 80.9 | 83.8 | 0.00 (reference) | 0.00 (reference) |
| 2nd Quartile | 84.1 (0.1) | 83.8 | 84.3 | 0.16 (−0.16, 0.48) | 0.17 (−0.17, 0.50) |
| 3rd Quartile | 84.5 (0.1) | 84.3 | 84.7 | 0.22 (−0.10, 0.54) | 0.25 (−0.08, 0.58) |
| 4th Quartile | 85.0 (0.2) | 84.7 | 85.7 | 0.45 (0.12, 0.77) | 0.45 (0.12, 0.78) |
| Second Trimester Maternal Blood | |||||
| 1st Quartile | 84.0 (0.2) | 83.0 | 84.3 | 0.00 (reference) | 0.00 (reference) |
| 2nd Quartile | 84.4 (0.1) | 84.3 | 84.5 | 0.06 (−0.25, 0.37) | 0.05 (−0.26, 0.37) |
| 3rd Quartile | 84.7 (0.1) | 84.5 | 84.8 | 0.09 (−0.22, 0.40) | 0.11 (−0.21, 0.43) |
| 4th Quartile | 85.1 (0.2) | 84.8 | 85.8 | 0.02 (−0.29, 0.33) | 0.02 (−0.30, 0.34) |
| Venous Umbilical Cord Bloodc | |||||
| 1st Quartile | 83.9 (0.6) | 79.5 | 84.5 | 0.00 (reference) | 0.00 (reference) |
| 2nd Quartile | 84.5 (0.2) | 84.1 | 84.8 | −0.02 (−0.38, 0.34) | −0.02 (−0.39, 0.35) |
| 3rd Quartile | 84.8 (0.2) | 84.4 | 85.2 | −0.33 (−0.69, 0.03) | −0.32 (−0.69, 0.05) |
| 4th Quartile | 85.4 (0.4) | 84.8 | 86.5 | −0.45 (−0.82,−0.09) | −0.45 (−0.83,−0.08) |
First trimester n=914 (unadjusted) & n=884 (adjusted); second trimester n=922 (unadjusted) & n= 893 (adjusted); cord blood n=557 (unadjusted) & n= 534 (adjusted) because of missing covariate data.
Adjusted for maternal age, prepregnancy BMI, smoking status, education and infant sex in multivariable linear regression models
Sex-specific quartiles are responsible for overlapping ranges.
LINE-1, Long interspersed nuclear element-1; %5mC, %5-methylated cytosines; BMI, body mass index
Cord blood LINE-1
Cord blood LINE-1 was inversely associated with gestational age (Table 4). For each %5mC increase in cord blood LINE-1, gestational age was 0.19 weeks shorter (95% CI 0.01, 0.38) in unadjusted models and the results did not change appreciably with adjustment for covariates (0.18 weeks shorter, 95% CI −0.03, 0.38). Infants with the highest quartile of cord blood LINE-1 were born 0.45 weeks (95% CI 0.08, 0.83) earlier than infants with the lowest quartile of LINE-1 (Table 2). Cord blood LINE-1 was inversely associated with preterm delivery. Infants in the highest sex-specific quartile of LINE-1 vs. the lowest quartile had higher odds of having been born preterm (OR 3.87, 95% CI 1.05, 14.2). Covariate adjustment only strengthened this association (OR 4.55, 95% CI 1.18, 17.5) (Table 3). We found no differences in associations with mean gestational age (interaction p = 0.97) or preterm birth (p = 0.84) according to infant sex.
Table 4.
Venous umbilical cord blood LINE-1 DNA methylation in leukocytes (%5mC) by gestational age category among 557 mother-infant pairs enrolled in Project Viva
| Overall | Female | Male | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Min | Max | n | Mean | n | Mean | |
| Gestational age (weeks) | ||||||||
| Preterm | ||||||||
| < 34 | 6 | 85.1 (0.6) | 84.3 | 85.7 | 1 | 84.3 | 5 | 85.3 |
| 34 to < 37 | 21 | 84.8 (0.7) | 82.8 | 86.0 | 9 | 84.5 | 12 | 85.1 |
| Full term | ||||||||
| 37 to < 39 | 109 | 84.6 (0.6) | 82.6 | 86.3 | 49 | 84.5 | 60 | 84.8 |
| 39 to < 41 | 307 | 84.7 (0.7) | 79.5 | 86.5 | 148 | 84.4 | 159 | 84.9 |
| >= 41 | 114 | 84.5 (0.6) | 82.8 | 86.1 | 56 | 84.3 | 58 | 84.7 |
%5mC, %5-methylated cytosines
DISCUSSION
In this cohort study, higher LINE-1 DNA methylation of maternal peripheral blood leukocytes during the first trimester predicted longer gestation and decreased odds of delivering preterm, as hypothesized. Conversely, we found cord blood LINE-1 DNA methylation to be inversely correlated with the length of gestation. These associations persisted after adjustment for many known risk factors for preterm birth. We observed similar associations of 1st trimester LINE-1 with preterm birth irrespective of whether the birth was spontaneous or medically induced. Medically indicated preterm birth may occur when a fetus has been showing signs of distress or poor growth, or if the mother has developed preeclampsia or other illness prompting delivery. Preeclampsia, characterized by hypertension and proteinuria, accounts for approximately 20% of extremely preterm (<28 weeks’ gestation) deliveries.39 A broader category of ischemic placental disease is responsible for over half of medically indicated preterm deliveries.40 Higher LINE-1 could be a marker of less ischemic placental disease or preeclampsia. We previously showed that higher LINE-1 expression is associated with lower risk of ischemic heart disease in non-pregnant adults.41 Since preeclampsia may have roots early in placental development secondary to implantation abnormalities, poor perfusion and subsequent endothelial damage,42 first trimester LINE-1 may be hypomethylated in preeclampsia as it is in ischemic heart disease. Mean LINE-1 was lower in the first trimester among women who developed preeclampsia (84.1 %5mC) compared to women who did not (84.3 %5mC) (P=0.15). Preliminary analysis suggests a relationship between increasing first trimester LINE-1 and decreased multivariable-adjusted odds of subsequent preeclampsia (OR 0.65, 95% CI 0.35, 1.19), but wide confidence intervals prevent conclusions about this association. Further work should be done to evaluate potential associations between maternal DNA methylation early in pregnancy and the development of preeclampsia.
Plausible mechanisms also support associations with spontaneous preterm birth, which can result from preterm premature rupture of membranes secondary to cervical insufficiency, or from preterm labor resulting from infection, uterine stretch from uterine fibroids, or trauma. Inflammation is the hallmark of spontaneous preterm labor.43 In a nested case-control study within Project Viva, we have shown that women with higher early pregnancy serum C-reactive protein had higher odds of preterm delivery.44 We have also demonstrated LINE-1 hypomethylation to be associated with elevation vascular cell adhesion molecule-1 (VCAM-1).45 Furthermore, interleukin-6 can induce LINE-1 hypomethylation in oral cancer cells studied in vitro.46 Others have proposed that LINE-1 hypomethylation itself could contribute to inflammation and the development of autoimmune disease through triggering the innate immunity pathway.47 LINE-1 hypomethylation may affect cellular function through encouraging transcription of sequences activated during conditions of cellular stress or inflammation.18, 19, 21
The lack of an association between second trimester LINE-1 methylation and preterm birth remains somewhat puzzling, given the inverse association of first trimester LINE-1 with odds of preterm birth. We speculate that LINE-1 status during the first trimester may reflect the inflammatory state of the mother closer to the timing of implantation, the success of which appears to be a key step for avoiding for pre-eclampsia and placental insufficiency more broadly.48 Furthermore, the low correlation between 1st and 2nd trimester LINE-1 suggests that LINE-1 at these time points reflect different processes (Pearson r= 0.02).
In contrast to our findings with 1st trimester LINE-1, we found that infants with higher cord blood LINE-1 had shorter gestations and higher odds of preterm birth. The cross-sectional nature of these observations prevents conclusions about causality. While Tabano et al. found that adult peripheral blood leukocytes had higher LINE-1 methylation (67.3%) than umbilical cord blood (60.1%), most studies of adults32 and across the lifespan49 suggest that global methylation decreases with age. We hypothesize that LINE-1 may become less methylated as cells divide and thus decreases with age in fetal life as it does in adult life. Furthermore, there are both maternal and fetal contributions to preterm birth. For example, infants with multiple congenital anomalies are more likely to be born preterm regardless of maternal health state.50 Thus, if indeed there is a causal relationship between DNA methylation and preterm birth, it is possible that the impacts on the length of gestation of maternal and fetal DNA methylation differ from one another.
A recent study comparing the global DNA methylation of placentas from preeclamptic pregnancies and normotensive pregnancies revealed that methylation levels were higher in preeclamptic placentas.51 While initially this may seem counter to our findings, another study suggests discordance between placental and fetal tissue methylation with placental tissue being relatively hypomethylated compared to the fetus.49 Tabano et al also found that the placenta (n=46) displayed relative hypomethylation of LINE-1 methylation (41.8%, range 34.0–52.3) compared to umbilical cord blood samples (n=10) (60.1%, range 57.5–77.7).52 Despite using the same technique,53 we found our mean cord blood methylation value of 84.6%5mC (range 71.5–86.5) is much higher. It is possible that simply by chance, their small sample size obtained estimates on the lower end of what is expected. However, it also highlights possible lack of comparability of DNA methylation values across studies.
Our findings should be considered in the context of inherent limitations. First, DNA methylation of peripheral blood leukocytes may or may not reflect the methylation of the tissues responsible for the conditions leading to preterm birth. Tissues such as the myometrium may be more appropriate for DNA methylation studies of preterm labor risk. Second, methylation of different leukocyte subtypes varies and we did not have the ability to adjust for the leukocyte differential, although adjustment has not affected the results of other cohort studies using LINE-1 methylation as an exposure.21, 45 Third, DNA methylation is one of several epigenetic mechanisms that work in conjunction with one another to regulate gene expression, and further work should be done to understand how histone modifications or other mechanisms may affect the risk of preterm birth. Fourth, gene-specific or genome-wide DNA methylation analyses may provide more insight into mechanisms underlying the processes prompting preterm birth, and while such technologies exist, they continue to be costly for cohort studies with large sample sizes. Fifth, the relatively high socio-economic status of our cohort may limit the generalizability of our findings, but our findings should be internally valid. Lastly, our preterm birth rate (6.5%) seems low compared to national estimates of 12% overall, and 10–11% among singleton births in the last 2 decades.54 However, a more appropriate comparison for our singleton-only, Massachusetts-based cohort may be the Massachusetts preterm birth rate of ~8%55 which includes multiples, who are at higher risk of preterm birth, suggesting that our rate of 6.5% among singletons may be representative of the region’s overall singleton rate.
In summary, we found that higher LINE-1 DNA methylation in maternal peripheral blood DNA during the first trimester was associated with longer gestations and decreased odds of preterm birth and that higher cord blood LINE-1 was associated with shorter gestations. Whether LINE-1 methylation simply serves as a potential biomarker for preterm birth or affects a woman’s risk of delivering preterm by altering gene expression and subsequent cellular function awaits further study.
Supplementary Material
Acknowledgments
We thank the Project Viva participants and staff. This work was supported by NIH grants R01 HD 034568, RC1 HD 063590, K24 HL 064081. Dr. Burris receives funding from the Klarman Scholars Program for Junior Faculty Development at Beth Israel Deaconess Medical Center. Dr. Baccarelli receives New Investigator Funding from the ES000002 NIH grant.
Footnotes
Statement of Interest
None
References
- 1.Hamilton BE, Martin JA, Ventura MA. Births: Preliminary Data for 2009. National Vital Statistics Reports. 2010:59. [PubMed] [Google Scholar]
- 2.MacDorman MF, Callaghan WM, Mathews TJ, Hoyert DL, Kochanek KD. Trends in preterm-related infant mortality by race and ethnicity, United States, 1999–2004. Int J Health Serv. 2007;37:635–641. doi: 10.2190/HS.37.4.c. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woythaler MA, McCormick MC, Smith VC. Late preterm infants have worse 24-month neurodevelopmental outcomes than term infants. Pediatrics. 2010;127:e622–629. doi: 10.1542/peds.2009-3598. [DOI] [PubMed] [Google Scholar]
- 5.Kramer MS, Seguin L, Lydon J, Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol. 2000;14:194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- 6.David RJ, Collins JW., Jr Differing birth weight among infants of U.S.-born blacks, African-born blacks, and U.S.-born whites. N Engl J Med. 1997;337:1209–1214. doi: 10.1056/NEJM199710233371706. [DOI] [PubMed] [Google Scholar]
- 7.Hitti J, Nugent R, Boutain D, et al. Racial disparity in risk of preterm birth associated with lower genital tract infection. Paediatr Perinat Epidemiol. 2007;21:330–337. doi: 10.1111/j.1365-3016.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- 8.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg RL, Andrews WW, Guerrant RL, et al. The preterm prediction study: cervical lactoferrin concentration, other markers of lower genital tract infection, and preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;182:631–635. doi: 10.1067/mob.2000.104211. [DOI] [PubMed] [Google Scholar]
- 10.Savitz DA, Dole N, Terry JW, Jr, Zhou H, Thorp JM., Jr Smoking and pregnancy outcome among African-American and white women in central North Carolina. Epidemiology. 2001;12:636–642. doi: 10.1097/00001648-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Behrman RE, Butler AS Institute of Medicine (U.S.) Preterm birth : causes, consequences, and prevention. Washington, D.C: National Academies Press; 2007. Committee on Understanding Premature Birth and Assuring Healthy Outcomes. [PubMed] [Google Scholar]
- 12.Burris HH, Collins JW., Jr Race and preterm birth--the case for epigenetic inquiry. Ethn Dis. 2010;20:296–299. [PubMed] [Google Scholar]
- 13.Bollati V, Baccarelli A. Environmental epigenetics. Heredity. 2010;105:105–112. doi: 10.1038/hdy.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- 15.Hedges DJ, Deininger PL. Inviting instability: Transposable elements, double-strand breaks, and the maintenance of genome integrity. Mutat Res. 2007;616:46–59. doi: 10.1016/j.mrfmmm.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bestor TH. The host defence function of genomic methylation patterns. Novartis Found Symp. 1998;214:187–195. doi: 10.1002/9780470515501.ch11. discussion 195–189, 228–132. [DOI] [PubMed] [Google Scholar]
- 17.Yang AS, Estecio MR, Doshi K, et al. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li TH, Schmid CW. Differential stress induction of individual Alu loci: implications for transcription and retrotransposition. Gene. 2001;276:135–141. doi: 10.1016/s0378-1119(01)00637-0. [DOI] [PubMed] [Google Scholar]
- 19.Schulz WA. L1 retrotransposons in human cancers. J Biomed Biotechnol. 2006;2006:83672. doi: 10.1155/JBB/2006/83672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bollati V, Galimberti D, Pergoli L, et al. DNA methylation in repetitive elements and Alzheimer disease. Brain Behav Immun. 2011;25:1078–1083. doi: 10.1016/j.bbi.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baccarelli A, Wright R, Bollati V, et al. Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology. 2010;21:819–828. doi: 10.1097/EDE.0b013e3181f20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timmermans S, Jaddoe VW, Silva LM, et al. Folic acid is positively associated with uteroplacental vascular resistance: The Generation R Study. Nutr Metab Cardiovasc Dis. 2009 doi: 10.1016/j.numecd.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Timmermans S, Jaddoe VW, Hofman A, Steegers-Theunissen RP, Steegers EA. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: the Generation R Study. Br J Nutr. 2009:1–9. doi: 10.1017/S0007114509288994. [DOI] [PubMed] [Google Scholar]
- 24.Hsiung DT, Marsit CJ, Houseman EA, et al. Global DNA methylation level in whole blood as a biomarker in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2007;16:108–114. doi: 10.1158/1055-9965.EPI-06-0636. [DOI] [PubMed] [Google Scholar]
- 25.Ingrosso D, Cimmino A, Perna AF, et al. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet. 2003;361:1693–1699. doi: 10.1016/S0140-6736(03)13372-7. [DOI] [PubMed] [Google Scholar]
- 26.Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth Characteristics and Subsequent Risks of Maternal Cardiovascular Disease: Effects of Gestational Age and Fetal Growth. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.111.034884. [DOI] [PubMed] [Google Scholar]
- 27.McGovern PG, Llorens AJ, Skurnick JH, Weiss G, Goldsmith LT. Increased risk of preterm birth in singleton pregnancies resulting from in vitro fertilization-embryo transfer or gamete intrafallopian transfer: a meta-analysis. Fertil Steril. 2004;82:1514–1520. doi: 10.1016/j.fertnstert.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 28.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–563. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 29.McDonald SD, Murphy K, Beyene J, Ohlsson A. Perinatel outcomes of singleton pregnancies achieved by in vitro fertilization: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2005;27:449–459. doi: 10.1016/s1701-2163(16)30527-8. [DOI] [PubMed] [Google Scholar]
- 30.Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14(Spec No 1):R47–58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- 31.Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009;91:305–315. doi: 10.1016/j.fertnstert.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bollati V, Schwartz J, Wright R, et al. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev. 2009;130:234–239. doi: 10.1016/j.mad.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu ZZ, Sparrow D, Hou L, et al. Repetitive element hypomethylation in blood leukocyte DNA and cancer incidence, prevalence, and mortality in elderly individuals: the Normative Aging Study. Cancer Causes Control. 2010;22:437–447. doi: 10.1007/s10552-010-9715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, et al. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 35.Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009;201:58, e51–58. doi: 10.1016/j.ajog.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baccarelli A, Wright RO, Bollati V, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179:572–578. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarantini L, Bonzini M, Apostoli P, et al. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation. Environ Health Perspect. 2009;117:217–222. doi: 10.1289/ehp.11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 39.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168:980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ananth CV, Vintzileos AM. Medically indicated preterm birth: recognizing the importance of the problem. Clin Perinatol. 2008;35:53–67. viii. doi: 10.1016/j.clp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Lucchinetti E, Feng J, Silva R, et al. Inhibition of LINE-1 expression in the heart decreases ischemic damage by activation of Akt/PKB signaling. Physiol Genomics. 2006;25:314–324. doi: 10.1152/physiolgenomics.00251.2005. [DOI] [PubMed] [Google Scholar]
- 42.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 43.Gravett MG, Novy MJ. Endocrine-immune interactions in pregnant non-human primates with intrauterine infection. Infect Dis Obstet Gynecol. 1997;5:142–153. doi: 10.1155/S1064744997000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pitiphat W, Gillman MW, Joshipura KJ, et al. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol. 2005;162:1108–1113. doi: 10.1093/aje/kwi323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baccarelli A, Tarantini L, Wright RO, et al. Repetitive element DNA methylation and circulating endothelial and inflammation markers in the VA normative aging study. Epigenetics. 2010:5. doi: 10.4161/epi.5.3.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gasche JA, Hoffmann J, Boland CR, Goel A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer. 2011;129:1053–1063. doi: 10.1002/ijc.25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crow MK. Long interspersed nuclear elements (LINE-1): potential triggers of systemic autoimmune disease. Autoimmunity. 2010;43:7–16. doi: 10.3109/08916930903374865. [DOI] [PubMed] [Google Scholar]
- 48.Cheng MH, Wang PH. Placentation abnormalities in the pathophysiology of preeclampsia. Expert Rev Mol Diagn. 2009;9:37–49. doi: 10.1586/14737159.9.1.37. [DOI] [PubMed] [Google Scholar]
- 49.Fuke C, Shimabukuro M, Petronis A, et al. Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: an HPLC-based study. Ann Hum Genet. 2004;68:196–204. doi: 10.1046/j.1529-8817.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- 50.Purisch SE, DeFranco EA, Muglia LJ, Odibo AO, Stamilio DM. Preterm birth in pregnancies complicated by major congenital malformations: a population-based study. Am J Obstet Gynecol. 2008;199:287, e281–288. doi: 10.1016/j.ajog.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 51.Kulkarni A, Chavan-Gautam P, Mehendale S, Yadav H, Joshi S. Global DNA methylation patterns in placenta and its association with maternal hypertension in pre-eclampsia. DNA Cell Biol. 2011;30:79–84. doi: 10.1089/dna.2010.1084. [DOI] [PubMed] [Google Scholar]
- 52.Tabano S, Colapietro P, Cetin I, et al. Epigenetic modulation of the IGF2/H19 imprinted domain in human embryonic and extra-embryonic compartments and its possible role in fetal growth restriction. Epigenetics. 2010;5:313–324. doi: 10.4161/epi.5.4.11637. [DOI] [PubMed] [Google Scholar]
- 53.Bollati V, Fabris S, Pegoraro V, et al. Differential repetitive DNA methylation in multiple myeloma molecular subgroups. Carcinogenesis. 2009;30:1330–1335. doi: 10.1093/carcin/bgp149. [DOI] [PubMed] [Google Scholar]
- 54.Martin JA, Hamilton BE, Sutton PD, et al. Births: Final data for 2008. Natl Vital Stat Rep. 2010:59. [PubMed] [Google Scholar]
- 55.Massachusetts Department of Public Health. [Accessed September 15, 2011]; http://www.mass.gov/?pageID=eohhs2terminal&&L=4&L0=Home&L1=Consumer&L2=Community+Health+and+Safety&L3=Population+Health+Statistics&sid=Eeohhs2&b=terminalcontent&f=dph_research_epi_c_births&csid=Eeohhs2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.