Abstract
The role of glycogen synthase kinase 3 beta (GSK-3β) in modulating Notch control of vascular smooth muscle cell (vSMC) growth (proliferation and apoptosis) was examined in vitro under varying conditions of cyclic strain and validated in vivo following changes in medial tension and stress. Modulation of GSK-3β in vSMC following ectopic expression of constitutively active GSK-3β, siRNA knockdown and pharmacological inhibition with SB-216763 demonstrated that GSK-3β positively regulates Notch intracellular domain expression, CBF-1/RBP-Jκ transactivation and downstream target gene mRNA levels, while concomitantly promoting vSMC proliferation and inhibiting apoptosis. In contrast, inhibition of GSK-3β attenuated Notch signaling and decreased vSMC proliferation and survival. Exposure of vSMC to cyclic strain environments in vitro using both a Flexercell™ Tension system and a novel Sylgard™ phantom vessel following bare metal stent implantation revealed that cyclic strain inhibits GSK-3β activity independent of p42/p44 MAPK and p38 activation concomitant with reduced Notch signaling and decreased vSMC proliferation and survival. Exposure of vSMC to changes in medial strain microenvironments in vivo following carotid artery ligation revealed that enhanced GSK-3β activity was predominantly localized to medial and neointimal vSMC concomitant with increased Notch signaling, proliferating nuclear antigen and decreased Bax expression, respectively, as vascular remodeling progressed. GSK-3β is an important modulator of Notch signaling leading to altered vSMC cell growth where low strain/tension microenvironments prevail.
Keywords: Notch, GSK-3β, Vascular smooth muscle, Cyclic strain, Neointimal proliferation
Introduction
Glycogen synthase kinase 3β (GSK-3β) is a multifunctional kinase, ubiquitously expressed in eukaryotes, that regulates many diverse cellular processes including proliferation, differentiation and apoptosis [11]. Its activity is regulated by serine (inhibitory) and tyrosine (stimulatory) phosphorylation. GSK-3β is constitutively active in resting cells and subject to negative regulation in response to external stimuli by phosphorylation on serine 9 via activation of several kinases, including AKT and protein kinase c (PKC) [11]. GSK-3β is an important component of diverse signaling pathways (e.g., Wnt/β-catenin and Angiotensin type1 receptor) and aberrant regulation of GSK-3β has been implicated in several diseases including diabetes mellitus as well as cardiovascular [7] and neuro-degenerative diseases [11].
Control of vascular smooth muscle cell (vSMC) growth is critical to the structural integrity of blood vessels and the pathology of many vascular conditions including atherosclerosis, restenosis and neointimal hyperplasia [16, 41]. Pathological changes in vessel structure are induced, in part, by changes in the biomechanical environment and burden on vSMC and the subsequent activation of discrete signaling pathways that govern growth where reduced cyclic strain/tension can result in substantial changes in vSMC proliferation and apoptosis [16, 46]. The Notch signaling pathway is a highly conserved developmental pathway that controls cell differentiation during embryonic development of the vasculature and is recapitulated in adult cells following vascular injury [36]. Notch1 and 3 ICD control the modulation of SMC growth in response to growth factor stimulation and biomechanical activation [35, 38]. Notch signaling is significantly enhanced in low strain/tension environments in vitro [37] and in vivo [4, 35] concomitant with increased SMC proliferation and survival.
GSK-3β has been shown to modulate Notch signaling in mammalian cells with contradictory results reported [9, 10, 22]. The purpose of the current study was to evaluate the role of GSK-3β in regulating Notch function and mediating Notch control of vSMC growth (balance of proliferation and apoptosis) under static conditions and following exposure to varying strain environments both in vitro and in vivo.
Materials and methods
Materials
All items were of the highest purity commercially available and purchased from Sigma-Aldrich (Poole, Dorset, UK) unless otherwise stated. Antibodies against GSK-3α/β were purchased from Enzo Life Sciences (Exeter, UK), MAPK and p38 from Cell Signal (Beverly, MA), Hrt's from Santa Cruz Biotechnology, Inc. (Heidelberg, Germany) and Notch 1 and 3 ICD from Millipore Ltd (Watford, UK).
Cell culture
Rat vascular SMC (R354-05) were purchased from cell applications and grown in culture as previously described [44]. Bovine SMC were purchased from the Coriell Institute (New Jersey, USA; Cat No. AG08504) and grown as previously described [6].
Cyclic strain studies
Cells were seeded into 6-well pronectin™ coated Bioflex plates (Dunn Labortechnik, Asbach, Germany) at a density of 6 × 105 cells/well and exposed to physiological level of cyclic strain (0–10% strain, 60 cycles/min, 0–24 h with the Heartbeat simulation) as previously described [37].
Mock vascular phantom
Mock vascular phantoms (MVP) were manufactured from transparent Sylgard®184, a silicon elastomer as previously described [6]. A bare metal stent (BMS provided by Medtronic Ave) was deployed inside the MVP by means of a Basix 25 angioplasty inflation syringe (Merit Medical Systems, South Jordan, Utah) and expanded by a 9-mm angioplasty balloon catheter. Following coating with fibronectin, bovine aortic vSMC were seeded onto the MVP. The stented MVP was then placed into a culture chamber containing 100 ml of RPMI 1640 media supplemented with 10% FBS and primocin antibiotic (100 μg/ml) (Amaxa, MD, USA). The culture chamber consisted of a biocompatible Plexiglas® open box with an inlet and outlet for medium perfusion of the MVP. The culture chamber was then attached to a CellMax® bioreactor flow system. The cells were exposed to pulsatile flow for 7 days, following which the MVP was removed and cell growth analysed [6].
Mouse carotid artery ligation
The carotid artery ligation model of remodeling was performed after buprenorphine analgesia (2 mg/kg) and induction of anesthesia using inhalational isoflurane (2%) essentially as described previously [34] and conformed with the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH Publication No. 85–23, revised 1996)]. All procedures were approved by the University of Rochester Animal Care Committee.
Immunohistochemistry and histomorphometry
Mice were perfusion fixed with 10% paraformaldehyde in sodium phosphate buffer (pH 7.0), 14 days after ligation. A series of cross-sections (5 μm) were made from the bifurcation every 200 μm through a 2-mm length of carotid artery and stained with either hematoxylin and eosin and antibodies against PCNA, α-actin, Bax, Hrt-1 and GSK-3β, as described previously [35]. Media stress was calculated from media tension/h, where h is medial thickness (cm), determined histomorphometrically [20].
Immunocytochemistry
SMC were seeded onto 6-well plates 2 days before being stained at 2 × 105 cells per well. Cells were stained for phospho-GSK-3β, Notch3 or Notch1 at 80–90% confluency using the following protocol. Cells were washed three times in 1× PBS. The cells were then permeabilized and fixed in methanol (–20°C, 10 min), and subsequently rehydrated in 1× PBS/3% BSA (10 min). Cells were then incubated in the appropriate primary antibody (1:50 dilution in 1× PBS/3% BSA) at 4°C overnight with gentle agitation. Following three 10-min washes in 1× PBS, cells were incubated in the appropriate secondary antibody (1:200 dilution in 19 PBS/3% BSA using FITC or anti-goat AlexaFluor) for 2–3 h at 37°C. Cells were then washed once in 1× PBS before visualization with the use of an Olympus DP-50 fluorescent microscope, using appropriate excitation and emission spectra at 20× magnifications.
GSK-3β expressing vectors
The wild-type GSK-3β expression plasmid (HA-CTHUGSK-3β) and the constitutively active mutant GSK-3β-S9A, where the serine from position 9 has been replaced by an alanine, were kind gifts of Dr. Jim Woodgett of the Samuel Lenfeld Research Institute, Toronto, Canada. Plasmids were prepared for transfection according to the manufacturer's instructions using a Qiagen plasmid midi kit (Qiagen, Crawley, UK), as described previously [44].
Plasmid preparation, transient transfection, luciferase and β-galactosidase assays
Plasmids were prepared for transfection according to the manufacturers instructions using a Qiagen plasmid midi kit (Qiagen, Crawley, UK) as described previously [21, 37, 44]. The cells were transfected with a luciferase reporter construct, and various expression constructs. Transfection efficiency was confirmed and normalized to β-galactosidase activity following co-transfection with pCMV-LacZ (a plasmid-encoding β-galactosidase activity). Western blot analysis was also performed to confirm over expression of effector proteins. Cells were harvested 16–24 h post transfection, using 1× Reporter Lysis Buffer (Promega, Madison, WI). Transactivation of reporter genes was evaluated by the luciferase assay (Promega) and normalized to the β-galactosidase activity. The latter was performed according to the manufacturer's instructions (high-sensitivity β-galactosidase assay; Stratagene, La Jolla, CA, USA). In order to maximise the number of cells containing each plasmid encoded vector, transfected cells were puromycin selected and pooled as previously described and resulted in transfection efficiencies greater than 85% [21, 37, 44].
Western blot analysis
Proteins from cell lysates (10–30 μg) were resolved on SDS-PAGE (12% resolving, 5% stacking) before transfer onto nitrocellulose membrane (Amersham Biosciences, Little Chalfont, UK as described previously [44].
Proliferation and apoptosis assay by fluorescence-activated cell sorter analysis
Cell proliferation and apoptosis was determined by fluorescence-activated cell sorter analysis (FACS) analysis using the Vybrant™ CFDA-SE Cell Tracer Kit and the Vybrant™ Apoptosis Alexa Fluor 488™ Annexin V and propidium iodide Assay Kit #2, respectively, using a FACScan flow cytometer (Becton–Dickinson, Dublin, Ireland). Cells were designated as viable, apoptotic, or necrotic as previously described [13].
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR was carried out using the Rotor Gene (model RG-3000; Corbett Research, Sydney, Australia) and the SYBR green PCR kit (Qiagen), as described previously [44]. The gene-specific oligonucleotide sequences were the following: GSK-3β, forward 5′-GGA TCT GCC ATC GAG ACA TT-3′ and reverse 5′-GTG GCT CCA AAG ATC AGC TC-3′; Hrt-1, forward, 5′-CTG GAC GAG ACC ATC GAG G-3′, reverse, 5′-GCA GCA TTT TCA GGT GAT CCA C-3′; Hrt-2, forward, 5′-CTG CAC ACA GCT TCC CTC TGT C-3′, reverse, 5′-CTC CAA CTT CTG TCC CCC AGG G-3′; Hrt-3, forward, 5′-CGC AGA GGG ATC ATA GAG AAA C-3′, reverse, 5′-CAG GGC TCG GGC ATC AAA G-3′.
siRNA transfection/inhibition
For gene silencing studies, Lipofectamine 2000 Reagent (Invitrogen) was used to transiently transfect vSMCs with gene-specific siRNA duplexes for 24 h as previously described [35]. For inhibition studies, cells were treated with 25 μM SB216763 reagent (Sigma). Control cells were also treated with vehicle control (dimethyl sulfoxide).
Data analysis
Results are expressed as means ± SE. Experimental points were performed in triplicate with a minimum of three independent experiments. Kruskal–Wallis non-parametric ANOVA tests were used for comparison of the two groups. A value of p < 0.05 was considered significant.
Results
GSK-3β positively regulates notch signaling in vSMC
The presence of total GSK-3β protein, phospho GSK-3β (inactive pGSK-3β phosphorylated at ser9) and GSK-3β mRNA levels was confirmed in rat aortic vSMC by immunocytochemistry, immunoblotting and RT-PCR (Fig. 1a, b). Pharmacological inhibition of GSK-3β activity with SB-216763 resulted in a dose-dependent increase in the expression levels of inactive pGSK-3β in accordance with other inhibitors of GSK-3β [39] (Fig. 1c). A structurally distinct inhibitor, SB-415286 mimicked this effect (data not shown). Ectopic expression and puromycin selection of cells with constitutively active epitope tagged mut. GSK-3β (GSK-3β-S9A) (Fig. 1d, e) and selective silencing of GSK-3β but not GSK-3α using siRNA was also confirmed (Fig. 1f). Densitometric analysis further confirmed selective inhibition of GSK-3β (70 ± 4%) without any significant effect on GSK-3α (data not shown).
Fig. 1.
GSK-3β signaling components in vSMC. a Immunohistochemical staining and b western blot analysis of total GSK-3β and pGSK-3β in rat vSMC. c Pharmacological inhibition of GSK-3β activity with increasing concentrations of SB-216763 after 24 h treatment. d Ectopic expression of constitutively active mutant GSK-3β S9A (Mut.GSK-3β) in vSMC using anti-GFP after 72 h and e Western blot analysis of GSK-3β levels following ectopic expression of GSK-3β S9A (Mut.GSK-3β) with anti-HA and siRNA knockdown with anti-GSK-3β after 72 h. Data are representative of three independent experiments with similar results
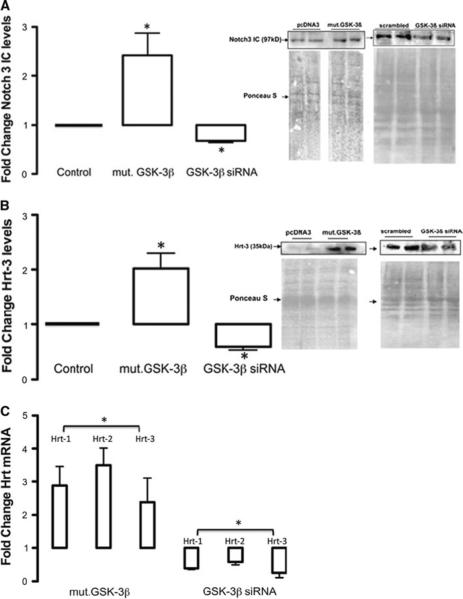
Ectopic expression of constitutively active GSK-3β-S9A resulted in a significant increase in Notch3 ICD protein levels (Fig. 2a) concomitant with a significant increase in Notch target gene expression (Hrt-3) (Fig. 2b) and mRNA levels (Fig. 2c). In contrast, selective GSK-3β knockdown with targeted siRNA significantly inhibited Notch3 ICD expression (Fig. 2a) concomitant with a significant decrease in Hrt-3 protein expression (Fig. 2b) and mRNA levels (Fig. 2c). In a similar manner, both interventions significantly modulated Notch target genes, Hrt-1 and Hrt-2 mRNA levels in these cells (Fig. 2c).
Fig. 2.
GSK-3β enhances Notch signaling. a The effect of ectopic expression of constitutively active GSK-3β (Mut.GSK-3β) and siRNA knockdown of GSK-3β on Notch1 and 3 ICD levels in rat vSMC after 72 h. b The effect of ectopic expression of constitutively active GSK-3β (Mut.GSK-3β) and siRNA knockdown of GSK-3β on Hrt-3 protein levels after 72 h. c The effect of ectopic expression of constitutively active GSK-3β (Mut.GSK-3β) and siRNA knockdown of GSK-3β on Notch target gene mRNA levels. Data are representative of three independent experiments with similar results. Data were corrected for total protein content loaded and transferred using a ponceau S stain for each blot. RNA data were normalized to GAPDH mRNA levels. The cumulative data represents the mean values from three independent experiments ±SEM, *p < 0.05 versus control
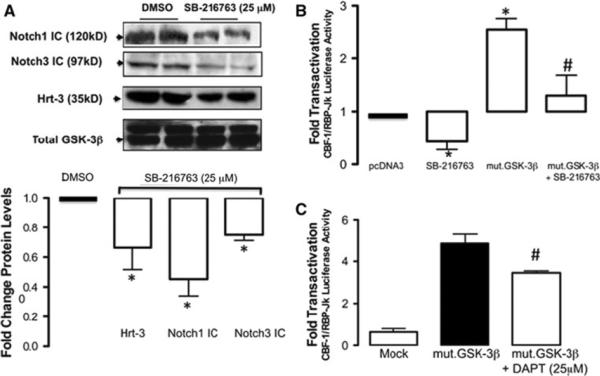
Pharmacological inhibition of GSK-3β activity with SB-216763 reduced Notch3 and Notch1 ICD levels with a concurrent decrease in Hrt-3 protein expression (Fig. 3a). In parallel studies, treatment of vSMC with SB-216763 decreased baseline CBF-1/RBP-Jκ promoter activity and significantly attenuated GSK-3β induced CBF-1/RBP-Jκ transactivation following ectopic expression of constitutively active mut. GSK-3β (GSK-3β-S9A) (Fig. 3b). In addition, treatment of cells with a γ-secretase inhibitor, DAPT, significantly attenuated GSK-3β induced CBF-1/RBP-Jκ promoter activity following ectopic expression of constitutively active mut. GSK-3β (GSK-3β-S9A) (Fig. 3c). The levels of Notch 1 receptor mRNA levels were also determined by realtime PCR following SB216763 treatment and exhibited a modest change in expression (22 ± 5% decrease, n = 3).
Fig. 3.
Inhibition of GSK-3β inhibits Notch signaling. a The effect of pharmacological inhibition of GSK-3β with SB-216763 (25 μM for 24 h) on Notch1, Notch3 ICD and Hrt-3 protein levels in rat vSMC after 24 h. b The effect of pharmacological inhibition of GSK-3β with SB-216763 (25 μM for 24 h) on CBF-1/RBP-Jκ promoter transactivation; reversal following ectopic expression with Mut.GSK-3β and c the effect of ectopic expression of constitutively active GSK-3β (Mut.GSK-3β) on CBF-1/RBP-Jκ promoter transactivation; inhibition with 25 μM DAPT. The cumulative data represents the mean values from three independent experiments ±SEM, *p < 0.05 versus control, #p < 0.05 versus Mut.GSK-3β
GSK-3β promotes vSMC proliferation and survival
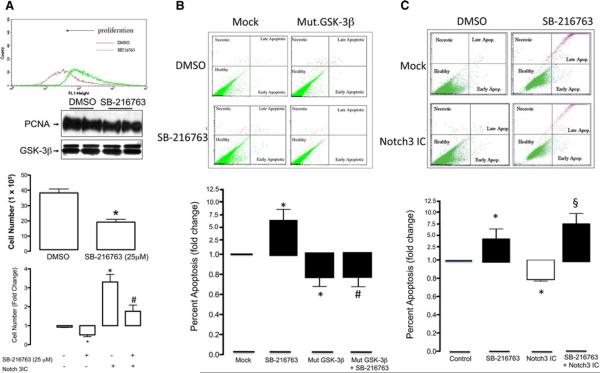
Pharmacological inhibition of GSK-3β activity with SB-216763 attenuated serum stimulated vSMC proliferation when assessed by FACS CFDA-SE analysis and cell counting while concurrently reducing serum stimulated proliferating cell nuclear antigen (PCNA) expression, a delta accessory protein of DNA polymerase synthesised in late G1 and S phases of the cell cycle (Fig. 4a). In parallel studies, pharmacological inhibition of GSK-3β activity with SB-216763 significantly increased the number of apoptotic nuclei when assessed by FACS analysis under low serum condition (0.5% FCS), an effect that was reversed following ectopic expression of constitutively active mut. GSK-3β (GSK-3β-S9A) (Fig. 4b). In addition, the significant pro-proliferative effect of forced expression of Notch3 ICD in quiesced vSMC exposed to 10% FCS was reversed following GSK-3β inhibition with SB-216763 (Fig. 4a). Moreover, the significant anti-apoptotic effect of forced expression of Notch3 ICD was reversed following inhibition of GSK-3β activity with SB-216763 (Fig. 4c) under high serum conditions (10% FCS) confirming a role for Notch in GSK-3β mediated vSMC proliferation and survival.
Fig. 4.
GSK-3β promotes vSMC proliferation and apoptosis. a The effect of GSK-3β inhibition with SB-216763 (25 μM) on vSMC cell proliferation (24 h), PCNA expression (24 h) and cell number (48 h) following exposure to 10% FCS. Proliferation was assessed following FACS analysis using the Vybrant™ CFDA-SE Cell Tracer Kit Cell, PCNA levels by western blot and cell counting using a hemocytometer. The effect of pharmacological inhibition of GSK-3β activity with SB-216763 (25 μM) in the absence or presence of forced expression of Notch3 ICD on serum-stimulated vSMC proliferation was also assessed after 48 h. b The effect of pharmacological inhibition of GSK-3β activity with SB-216763 (25 μM) on the number of apoptotic nuclei following exposure to serum (10% FCS) by FACS analysis using a Vybrant™ Apoptosis Alexa Fluor 488™ Annexin V and propidium iodide assay; an effect that was reversed following ectopic expression of constitutively active mut.GSK-3β (GSK-3β-S9A) and c the effect of pharmacological inhibition of GSK-3β activity with SB-216763 (25 μM) following exposure to low serum (0.5% FCS) by FACS analysis using a Vybrant™ Apoptosis Alexa Fluor 488™ Annexin V and propidium iodide assay in the absence or presence of forced expression of Notch3 ICD. The cumulative data represents the mean values from three independent experiments ±SEM, *p < 0.05 versus control, #p < 0.05 versus SB-216763, §p < 0.05 versus Notch3 ICD
Biomechanical regulation of GSK-3β activity
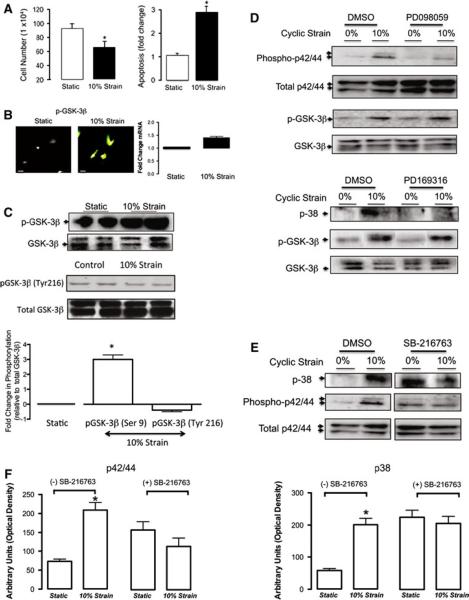
The functional involvement of GSK-3β in modulating vSMC growth in response to changes in cyclic strain was examined in vitro. Exposure of vSMC to static or cyclic strain conditions (24 h, 10%, 1 Hz) resulted in a strain-induced decrease in cell number (Fig. 5a), an increase in apoptosis (Fig. 5a) concomitant with a robust increase in immunocytochemical staining of inactive pGSK-3β independent of any significant change in GSK-3β mRNA levels (Fig. 5b) or pGSK-3β Try216 expression (Fig. 5c). These data suggest that zero strain environments promote GSK-3β activity and growth in these cells. Since the regulatory phosphorylation of GSK-3β and its activity in vascular cells is under the control of MAPK-dependent signaling [29] and since we have previously shown that MAPK inhibition significantly attenuated strain-induced decreases in NICD expression [38], confluent serum-deprived vSMC were exposed to cyclic strain in the absence or presence of p42/44 MAPK and p38 inhibitors before GSK-3β activity was evaluated. Inhibition of p42/p44 MAPK or p38 with PD098059 and PD169316, respectively, failed to reverse the strain-induced increase in pGSK-3β expression in these cells (Fig. 5d). In contrast, inhibition of GSK-3β activity with SB-216763 significantly attenuated the strain-induced changes in p42/p44 MAPK and p38, respectively (Fig. 5e, f).
Fig. 5.
Cyclic strain enhances GSK-3β phosphorylation. a The effect of 10% cyclic strain for 24 h on rat vSMC (a) cell number and apoptosis. The baseline `static' level of apoptosis was 4 ± 1%, n = 6. b Representative immunocytochemical staining and QRT-PCR of GSK-3β mRNA levels following exposure to 10% cyclic strain for 24 h. c Representative western blot analysis of pGSK-3β Ser9 and Tyr216 levels following exposure to 10% cyclic strain for 24 h. d Representative western blot analysis of the effect of 10% cyclic strain on pGSK-3β activity after 30 min in the absence or presence of inhibitors of phospho-p44/42 and phospho-p38, respectively. e , f Representative western blot analysis and cumulative data of the effect of cyclic strain on phospho-p44/42 and phosphop38 activity after 30 min following inhibition of GSK-3β with SB-216763 (25 μM). The cumulative data represents the mean values from three independent experiments ± SEM, *p < 0.05 versus static control
To further substantiate a functional involvement of GSK-3β in modulating vSMC growth in response to changes in cyclic strain, the expression of GSK-3β and Notch in vSMC was examined within a stented microenvironment in vitro. The MVP reproduces the mechanical conditions of lower cyclic strain amplitude within a stent in vivo [46]. The expression of inactive pGSK-3β and Notch1 was examined 7 days following implantation of a BMS. In parallel experiments, the level of proliferation and apoptosis was determined in situ. The level of strain amplitude was measured `upstream' and within the `stented' region of the BMS in each MVP by videoextensometry within each region and was calculated at 6 and 1.5%, respectively. There was a significant decrease in the level of immunocytochemical staining for inactive pGSK-3β within the stented region when compared with the upstream regions concomitant with a dramatic increase in Notch1 staining (Fig. 6a). In parallel, the number of cells was significantly higher within the stented region of the MVP when compared with upstream regions (Fig. 6b). In contrast, the number of apoptotic cells was significantly lower within the stented region of the MVP when compared with upstream regions (Fig. 6b). Taken together, these data clearly demonstrate that low strain amplitude microenvironments increase both GSK-3β activity and Notch1 expression while concomitantly promoting vSMC growth in vitro.
Fig. 6.
GSK-3β phosphorylation within a stented microenvironment in vitro and following vascular remodeling in vivo. a Representative immunocytochemical staining of pGSK-3β and Notch1 in bovine vSMC upstream and within the stented regions of a MVP following stent implantation after 4 days. Cells were visualised using a DAPI solution (4', 6-diamidino-2-phenylindole) and cell number determined by fluorescent microscopy in multiple random fields of view. Magnification 40× lm. b Cumulative data on the number of cells upstream and within the stented region of a MVP after 4 days in culture. Cells were counted blindly by determining the number of DAPI stained nuclei in situ from digital images of different constant regions of 300 × 300 pixels in random visual fields. Cells counts were confirmed using a hemocytometer following trypsinisation of individual cells. Data are the mean of four independent experiments performed in triplicate, *p < 0.05 vs upstream. c Representative immunohistochemical staining for pGSK-3β, total GSK-3β, α-actin, PCNA, Bax and Hrt-1 from ligated carotid arteries from C57B16/J mice after 14 days. d Quantitative realtime PCR analysis of GSK-3β mRNA levels from sham and ligated left carotid arteries from C57B16/J mice 3 and 14 days after ligation (4 vessels per preparation). RNA data were normalized to GAPDH mRNA levels. The cumulative data represents the mean values from three independent experiments ±SEM, *p < 0.05 versus sham control
To examine the functional involvement of GSK-3β in modulating vSMC growth in response to changes in cyclic strain/tension in vivo, we utilized the murine carotid ligated artery model in which reduced blood flow results in decreased vessel wall tension and stress, triggering vessel remodeling and neointimal formation [24, 35]. We confirmed that medial tension and stress was reduced by 40% in the ligated left carotid artery after 14 days ligation when compared with sham [20]. The expression and localization of both GSK-3β and Notch components within the media and developing neointima of these vessels was then evaluated. Vascular SMC were stained for total and inactive pGSK-3β (ser 9) and compared to cells stained for smooth muscle α-actin, proliferating cell nuclear antigen (PCNA, a proliferative marker), Bax (a pro-apoptotic marker) and Hrt-1 (a Notch target gene). Immunohistochemical analysis 14 days post-ligation revealed that GSK-3β expression was predominantly localized to vSMC within the medial and neointimal layers of these vessels (Fig. 6c) concomitant with increased PCNA, decreased Bax levels and enhanced Notch target gene expression (Fig. 6c). In addition, the expression of pGSK-3β (ser 9) within the remodeled vessel was minimal relative to the total GSK-3β levels present after 14 days of injury suggesting that the majority of GSK-3β was active in vSMC following ligation (Fig. 6c). Quantification of GSK-3β mRNA levels using QRT-PCR demonstrated that GSK-3β mRNA levels initially decreased after 3 days following carotid artery ligation but increased thereafter as vascular remodeling progressed (Fig. 6d).
Discussion
GSK-3β, originally identified as a serine/threonine kinase that phosphorylates glycogen synthase, has since been shown to phosphorylate and regulate the activity of many diverse proteins involved in several signaling pathways such as β-catenin, p53 and Notch [9, 15, 22]. As GSK-3β is located at the focal point where multiple cell signals merge to control cell proliferation, apoptosis and migration, it represents a potential novel molecular target to treat vascular proliferative disease. Several studies have highlighted the importance of GSK-3β targets in controlling vSMC proliferation and apoptosis in vitro [5] and in vivo [2, 23]. One such target, Notch is known to play a putative role in dictating venous to arterial differentiation during embryogenesis [25] and the vascular response to injury [28].
GSK-3β may modulate Notch signaling through phosphorylation of NICD which protects it from proteosomal degradation [10], by directly binding to NICD [22], via a direct interaction with the Notch co-activator MAMl1 [40], and/or via modifying γ-secretase activity [45]. Initial studies reported that GSK-3β phosphorylated Notch1 ICD in vitro enhancing its activity while Notch signaling was reduced in GSK-3β deficient fibroblasts [10]. However, subsequent studies suggested that Notch1 and 2IC phosphorylation by GSK-3β negatively regulated Notch transcriptional activity [3, 9]. In the current study, we demonstrate that GSK-3β positively regulates the activity of Notch 1 and 3 ICD in vSMC in vitro. Ectopic expression of GSK-3β in vSMC increased NICD levels, promoted CBF-1/RBP-Jκ transactivation and enhanced downstream Notch target gene expression. Coincidentally, inhibition of GSK-3β activity using a pharmacological inhibitor or reduction in GSK-3β levels following selective siRNA knockdown (without any effect on GSK-3α levels) resulted in attenuation of Notch activity. The enhanced Notch activity was due, in part, to increased NICD levels since DAPT, a γ-secretase inhibitor that reduces NICD levels [14] partially attenuated the enhanced transactivation of CBF-1/RBP-Jκ promoters following ectopic expression of active GSK-3β in these cells. These data suggest that changes in NICD levels contribute in part to the enhanced CBF-1/RBP-Jκ transactivation following GSK-3β activation since the level of transactivation is reduced concomitant with a similar level of reduction in NICD expression at this concentration of DAPT (data not shown). In addition, the effect of SB216763 on the repercussions of forced expression of NICD on vSMC proliferation and apoptosis suggests that one of the target(s) of SB216763 is also likely to be NICD. However, because CBF-1/RBP-Jκ transactivation by constitutively active GSK-3β remains robust even when NICD levels are decreased, there is also the possibility that GSK-3β promotes CBF-1/RBP-Jκ activity downstream from NICD. Indeed, activation of Notch and β-catenin signaling through PI3K (and GSK-3β) in vascular progenitors (as well as differentiating ECs) has been reported [15]. Notch and β-catenin signaling subsequently converge into a single protein complex with CBF-1/RBP-Jκ, NICD, and β-catenin (arterial complex) on arterial genes. It is likely that Notch signaling from Notch ligand binding and β-catenin signaling from Wnt and VE-cadherin participate in forming the complex and can be modulated by GSK-3β [49].
The positive regulation of Notch signaling following GSK-3β activation resulted in enhanced vSMC proliferation and survival in vitro. In addition, the pro-proliferative effect of Notch3 ICD overexpression was reversed following GSK-3β inhibition suggesting that GSK-3β phosphorylation of one of its substrates significantly interferes with Notch promotion of vSMC proliferation. While the pro-apoptotic response of vSMC following GSK-3β inhibition was unaffected by Notch 3 ICD over expression, the anti-apoptotic effect of Notch 3 ICD over expression was reversed by GSK-3β inhibition further highlighting that GSK-3β phosphorylation also significantly interferes with Notch promotion of vSMC survival. These data are in agreement with previous studies confirming a disparate role for GSK-3β in cell survival where GSK-3β oppositely regulated two major apoptotic signaling pathways (mitochondrial intrinsic apoptotic pathway and the death receptor-mediated extrinsic apoptotic signaling pathway) [1, 19]. Consequently, inhibition of GSK-3β provides protection from intrinsic apoptosis but may potentiate extrinsic apoptotic signaling. Furthermore, inhibition of CBF-1/RBP-Jκ transactivation with SB-216367 blunted the effect of constitutively active GSK-3β. However, SB-216367 did not inhibit the anti-apoptotic effect of this active mutant further reinforcing the disparate effects of GSK inhibition on cell survival and highlighting the potential role of a potential Notch mediated CBF-1/RBP-Jκ independent pathway for vSMC apoptosis. Indeed, since inhibition of γ-secretase activity using DAPT failed to robustly affect CBF-1/RBP-Jκ transactivation induced by the active mutant of GSK-3β, a CBF-1/RBP-Jκ process that is independent of the Notch pathway is further implicated. This may also explain in part the inability of Notch 3 ICD overexpression to overcome the pro-apoptotic effects of GSK-3β inhibition in these cells. Moreover, while these data are consistent with GSK-3β phosphorylation of NICD, it is also likely that Notch receptors are phosphorylated and primed by other kinases. Recent studies suggest that GSK-3β directly interacts with MAML proteins that are transcriptional co-activators for Notch signaling by recruiting CycC:CDK8 to phosphorylate NICD and coordinate activation with turnover [12].
Several studies have previously addressed the regulatory phosphorylation of GSK-3β in response to biomechanical stimulation in vitro and confirmed an AKT-dependent downstream inhibition of GSK-3β activity in response to cyclic strain [42, 50]. MAPK are also known to act as a priming kinase for GSK-3β [32] where the regulatory phosphorylation of GSK-3β in vascular cells is also under the control of MAPK-dependent signaling [29]. We have previously demonstrated that MAPK inhibition significantly attenuated strain-induced decreases in NICD expression in vSMC [38]. In these studies, the strain-induced decrease in vSMC growth was associated with a cyclic strain-induced down regulation of Notch receptors that was Gi-protein and ERK1/2-dependent. The significant attenuation of Notch signaling and vSMC growth was reversed following ectopic expression of NICD's. In this context, the present study addressed whether strain-induced MAPK signaling contributed to changes in downstream GSK-3β activity in these cells. While the strain-induced increase in MAPK activities and inactivation of GSK-3β coincided with a significant decrease in vSMC proliferation and survival, inhibition of ERK and p38 activity failed to attenuate the strain-induced phosphorylation and inactivation of GSK-3β. These data suggest that unlike AKT [42, 50] neither ERK nor p38 act upstream of GSK-3β in vSMC to phospho-relay and transduce biomechanical stimuli and are therefore unlikely to act as the priming kinases for GSK-3β in response to cyclic strain. In contrast, inhibition of GSK-3β resulted in significant increases in both baseline levels of ERK and p38 activity and subsequent attenuation of strain-induced phospho-ERK and -p38 activity, respectively. Multiple signaling pathways besides those directed towards GSK-3β are activated by cyclic strain [43, 48]. Nevertheless, our data suggest that the strain-induced changes in vSMC proliferation and apoptosis that occur concomitant with an ERK1/2-dependent attenuation of Notch signaling are clearly due in part to increases in GSK-3β phosphorylation at Ser 9 since inhibition with SB216763 modulates Notch signaling and inhibits the strain-induced changes ERK1/2 activity and therefore Notch signaling downstream. These data further indicate that GSK-3β signaling may play a critical role in promoting downstream MAPK signaling in vSMC in response to strain.
The functional significance of GSK-3β in modulating vSMC growth in response to changes in cyclic strain was further confirmed in vSMC grown within, and upstream from, a stent in vitro. The stented MVP fully reproduces the mechanical microenvironment within a stent and mimics the significant decrease in arterial wall compliance and distensibility following stent implantation [46]. A decrease in cyclic strain amplitude within the stent resulted in a marked increase in vSMC cell growth concomitant with an increase in GSK-3β activation and enhanced Notch1 signaling. In similar studies, stent implantation in vivo, with the associated reduction in cyclic strain amplitude, stimulated both AKT and pGSK-3β phosphorylation while also increasing neointima formation in the stented rat aorta [50]. Thus, activation of GSK-3β following stent implantation represents an important phospho-relay and transduction mechanism for decreases in cyclic strain within arterial media during restenosis in vivo. Moreover, modulation of Notch signaling components as a direct result of increased GSK-3β activity in vSMC within the microenvironment of the stent has important implications for vSMC growth following stent deployment.
The functional involvement of GSK-3β in modulating vSMC growth in response to changes in cyclic strain/tension was further validated in vivo following carotid artery ligation where reduced blood flow results in decreased vessel wall tension and stress [20]. Moreover, the increase in active GSK-3β within the medial and neointimal layer was associated with increased vSMC proliferation (PCNA), decreased apoptosis (Bax) and enhanced Notch1 signaling (Hrt). Previous studies have revealed that GSK-3β is acutely inactivated (within 24 h) following balloon injury and carotid ligation in vivo [17, 26]. However, the levels of active GSK-3β significantly rise as neointimal formation progresses in a manner such that treatment with a ROS scavenger [50] or TNF-α inhibition [47], which both inhibit GSK-3β activity, attenuated the vascular remodeling response in vivo. Taken together, these data strongly support an important role for GSK-3β in modulating the phenotypic and growth response of vSMC to low strain microenvironments in vivo where vSMC growth can occur unabated. In this context, pharmacological inhibition of GSK-3β on drug-eluting stents results in a marked attenuation of neointimal formation in vivo [18, 30].
It is clear that maintenance of an appropriate physiological level of GSK-3β activity is crucial since either too little or too much GSK-3β activity can promote vascular cell fate changes [8]. Consistent with our data, recent studies now suggest that GSK-3β may present as a target gene of specific microRNA's in airway smooth muscle and moreover cyclic strain inhibits endogenous GSK-3β activity in these cells through miRNA-26a [33]. As miRNA-26a levels are significantly downregulated in vSMC during vascular remodeling [27], the enhanced GSK-3β activity within neointimal and medial cells following carotid ligation is consistent with a reduction in miRNA-26a regulation of GSK-3β activity in these cells. Our data clearly identify GSK-3β control of Notch function as a target for intervention and highlight GSK-3β inhibitors as a potential treatment option for vascular proliferative disease.
In conclusion, we have identified GSK-3β as a positive modulator of Notch signaling in vSMC. The enzyme offers a potential therapeutic target for vascular disease states that display impaired or exaggerated Notch signaling due to decreases in strain/tension within the vasculature, and subsequent exaggerated SMC proliferation. In this context, dose-dependent modulation of GSK-3β and control of the timing and extent of its inhibition has been proposed as a novel mechanism to treat cancer, diabetes and mood disorders [31]. A similar strategy may be useful in exploiting the therapeutic potential of Notch in vascular disease.
Acknowledgments
This work was supported in part by grants from Science Foundation Ireland (PAC and CL) and the Health Research Board of Ireland (PAC) and by funds from the National Institutes of Health, (AA-12610 to E.M.R and K99HL095650 to D.M.) and the American Heart Association (Grant-in-Aid 0855865D to J.P.C).
Footnotes
Conflict of interest None.
References
- 1.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. doi:1016/j.pneurobio. 2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, Nagendran J, Haromy A, Dyck JR, Michelakis ED. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-β NFAT axis. Circulation. 2009;120:1231–1240. doi: 10.1161/CIRCULATIONAHA.109.848911. doi:10.1161/CIRCULATIONAHA.109.848911. [DOI] [PubMed] [Google Scholar]
- 3.Camenzind E, Wijns W, Mauri L, Boersma E, Parikh K, Kurowski V, Gao RL, Bode C, Greenwood JP, Gershlick A, O'Neill W, Serruys PW, Jorissen B, Steg PG, Investigtors PSC Rationale and design of the Patient Related OuTcomes with Endeavor versus Cypher stenting Trial (PROTECT): randomized controlled trial comparing the incidence of stent thrombosis and clinical events after sirolimus or zotarolimus drug-eluting stent implantation. Am Heart J. 2009;158:U902–U947. doi: 10.1016/j.ahj.2009.10.002. doi:10.1016/J.Ahj.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Campos AH, Wang W, Pollman MJ, Gibbons GH. Determinants of Notch-3 receptor expression and signaling in vascular smooth muscle cells: implications in cell-cycle regulation. Circ Res. 2002;91:999–1006. doi: 10.1161/01.res.0000044944.99984.25. doi:10.1161/01.RES.0000044944.99984.25. [DOI] [PubMed] [Google Scholar]
- 5.Chow W, Hou G, Bendeck MP. Glycogen synthase kinase 3beta regulation of nuclear factor of activated T-cells isoform c1 in the vascular smooth muscle cell response to injury. Exp Cell Res. 2008;314:2919–2929. doi: 10.1016/j.yexcr.2008.07.010. doi:10.1016/j.yexcr.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Colombo A, Zahedmanesh H, Toner DM, Cahill PA, Lally C. A method to develop mock arteries suitable for cell seeding and in vitro cell culture experiments. J Mech Behav Biomed Mater. 2010;3:470–477. doi: 10.1016/j.jmbbm.2010.04.003. doi:10.1016/j.jmbbm.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Diniz GP, Carneiro-Ramos MS, Barreto-Chaves ML. Angiotensin type 1 receptor mediates thyroid hormone-induced cardiomyocyte hypertrophy through the Akt/GSK-3beta/mTOR signaling pathway. Basic Res Cardiol. 2009;104:653–667. doi: 10.1007/s00395-009-0043-1. doi:10.1007/s00395-009-0043-1. [DOI] [PubMed] [Google Scholar]
- 8.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. doi:10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinosa L, Ingles-Esteve J, Aguilera C, Bigas A. Phosphorylation by glycogen synthase kinase-3 beta down-regulates Notch activity, a link for Notch and Wnt pathways. J Biol Chem. 2003;278:32227–32235. doi: 10.1074/jbc.M304001200. doi:10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- 10.Foltz DR, Santiago MC, Berechid BE, Nye JS. Glycogen synthase kinase-3beta modulates notch signaling and stability. Curr Biol. 2002;12:1006–1011. doi: 10.1016/s0960-9822(02)00888-6. doi:10.1016/S0960-9822(02)00888-6. [DOI] [PubMed] [Google Scholar]
- 11.Force T, Woodgett JR. Unique and overlapping functions of GSK-3 isoforms in cell differentiation and proliferation and cardiovascular development. J Biol Chem. 2009;284:9643–9647. doi: 10.1074/jbc.R800077200. doi: 10.1074/jbc.R800077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fryer CJ, White JB, Jones KA. Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol Cell. 2004;16:509–520. doi: 10.1016/j.molcel.2004.10.014. doi:10.1016/j. molcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Gao W, Ferguson G, Connell P, Walshe T, Murphy R, Birney YA, O'Brien C, Cahill PA. High glucose concentrations alter hypoxia-induced control of vascular smooth muscle cell growth via a HIF-1alpha-dependent pathway. J Mol Cell Cardiol. 2007;42:609–619. doi: 10.1016/j.yjmcc.2006.12.006. doi:10.1016/j.yjmcc.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. doi:10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gosens R, Meurs H, Schmidt M. The GSK-3/beta-cateninsignalling axis in smooth muscle and its relationship with remodelling. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:185–191. doi: 10.1007/s00210-008-0269-8. doi:10.1007/s00210-008-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Bio. 2009;10:53–62. doi: 10.1038/nrm2596. doi:10.1038/Nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall JL, Chatham JC, Eldar-Finkelman H, Gibbons GH. Upregulation of glucose metabolism during intimal lesion formation is coupled to the inhibition of vascular smooth muscle cell apoptosis. Role of GSK3beta. Diabetes. 2001;50:1171–1179. doi: 10.2337/diabetes.50.5.1171. doi: 10.2337/diabetes.50.5.1171. [DOI] [PubMed] [Google Scholar]
- 18.Hibbert B, Ma X, Pourdjabbar A, Holm E, Rayner K, Chen YX, Sun J, Filion L, O'Brien ER. Inhibition of endothelial progenitor cell glycogen synthase kinase-3beta results in attenuated neointima formation and enhanced re-endothelialization after arterial injury. Cardiovasc Res. 2009;83:16–23. doi: 10.1093/cvr/cvp156. doi:10.1093/cvr/cvp156. [DOI] [PubMed] [Google Scholar]
- 19.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim J, Berk BC. Flow-mediated vascular remodeling in hypertension: relation to hemodyamics. Stroke. 2009;40:582–590. doi: 10.1161/STROKEAHA.108.529826. doi:10.1161/STROKEAHA.108.529826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin J, Wang Y, Wang F, Kerns JK, Vinader VM, Hancock AP, Lindon MJ, Stevenson GI, Morrow DM, Rao P, Nguyen C, Barrett VJ, Browning C, Hartmann G, Andrew DP, Sarau HM, Foley JJ, Jurewicz AJ, Fornwald JA, Harker AJ, Moore ML, Rivero RA, Belmonte KE, Connor HE. Oxazolidinones as novel human CCR8 antagonists. Bioorg Med Chem Lett. 2007;17:1722–1725. doi: 10.1016/j.bmcl.2006.12.076. doi:10.1016/j.bmcl.2006.12.076. [DOI] [PubMed] [Google Scholar]
- 22.Jin YH, Kim H, Oh M, Ki H, Kim K. Regulation of Notch1/NICD and Hes1 expressions by GSK-3alpha/beta. Mol Cells. 2009;27:15–19. doi: 10.1007/s10059-009-0001-7. doi:10.1007/s10059-009-0001-7. [DOI] [PubMed] [Google Scholar]
- 23.Jung JH, Min PK, Kim JY, Park S, Choi EY, Ko YG, Choi D, Jang Y, Shim WH, Cho SY. Systemic immunosuppressive therapy inhibits in-stent restenosis in patients with renal allograft. Catheter Cardiovasc Interv. 2006;68:567–573. doi: 10.1002/ccd.20799. doi:10.1002/ccd.20799. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 25.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 26.Lee CS, Kwon YW, Yang HM, Kim SH, Kim TY, Hur J, Park KW, Cho HJ, Kang HJ, Park YB, Kim HS. New mechanism of rosiglitazone to reduce neointimal hyperplasia: activation of glycogen synthase kinase-3beta followed by inhibition of MMP-9. Arterioscler Thromb Vasc Biol. 2009;29:472–479. doi: 10.1161/ATVBAHA.108.176230. doi: 10.1161/ATVBAHA.108.176230. [DOI] [PubMed] [Google Scholar]
- 27.Leeper NJ, Raiesdana A, Kojima Y, Chun HJ, Azuma J, Maegdefessel L, Kundu RK, Quertermous T, Tsao PS, Spin JM. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol. 2010 doi: 10.1002/jcp.22422. doi:10.1002/jcp.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Takeshita K, Liu PY, Satoh M, Oyama N, Mukai Y, Chin MT, Krebs L, Kotlikoff MI, Radtke F, Gridley T, Liao JK. Smooth muscle Notch1 mediates neointimal formation after vascular injury. Circulation. 2009;119:2686–2692. doi: 10.1161/CIRCULATIONAHA.108.790485. doi:10.1161/CIRCULATIONAHA.108.790485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J. 2002;16:950–962. doi: 10.1096/fj.01-0870com. doi:10.1096/fj.01-0870com. [DOI] [PubMed] [Google Scholar]
- 30.Ma X, Hibbert B, Dhaliwal B, Seibert T, Chen YX, Zhao X, O'Brien ER. Delayed re-endothelialization with rapamycin-coated stents is rescued by the addition of a glycogen synthase kinase-3beta inhibitor. Cardiovasc Res. 2010;86:338–345. doi: 10.1093/cvr/cvq047. doi:10.1093/cvr/cvq047. [DOI] [PubMed] [Google Scholar]
- 31.Martinez A, Perez DI. GSK-3 inhibitors: a ray of hope for the treatment of Alzheimer's disease? J Alzheimers Dis. 2008;15:181–191. doi: 10.3233/jad-2008-15204. [DOI] [PubMed] [Google Scholar]
- 32.Miura T, Miki T. GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ J. 2009;73:1184–1192. doi: 10.1253/circj.cj-09-0284. doi:10.1253/circj.CJ-09-0284. [DOI] [PubMed] [Google Scholar]
- 33.Mohamed JS, Lopez MA, Boriek AM. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3beta. J Biol Chem. 2010;285:29336–29347. doi: 10.1074/jbc.M110.101147. doi:10.1074/jbc.M110. 101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrow D, Cullen JP, Liu W, Cahill PA, Redmond EM. Alcohol inhibits smooth muscle cell proliferation via regulation of the notch signaling pathway. Arterioscler Thromb Vasc Biol. 2010;30:2597–2603. doi: 10.1161/ATVBAHA.110.215681. doi:10.1161/ATVBAHA.110.215681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrow D, Cullen JP, Liu W, Guha S, Sweeney C, Birney YA, Collins N, Walls D, Redmond EM, Cahill PA. Sonic Hedgehog induces Notch target gene expression in vascular smooth muscle cells via VEGF-A. Arterioscler Thromb Vasc Biol. 2009;29:1112–1118. doi: 10.1161/ATVBAHA.109.186890. doi:10.1161/ATVBAHA.109.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrow D, Guha S, Sweeney C, Birney Y, Walshe T, O'Brien C, Walls D, Redmond EM, Cahill PA. Notch and vascular smooth muscle cell phenotype. Circ Res. 2008;103:1370–1382. doi: 10.1161/CIRCRESAHA.108.187534. doi: 10.1161/CIRCRESAHA.108.187534. [DOI] [PubMed] [Google Scholar]
- 37.Morrow D, Scheller A, Birney YA, Sweeney C, Guha S, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA. Notch-mediated CBF-1/RBP-J{kappa}-dependent regulation of human vascular smooth muscle cell phenotype in vitro. Am J Physiol Cell Physiol. 2005;289:C1188–C1196. doi: 10.1152/ajpcell.00198.2005. doi:10.1152/ajpcell. 00198.2005. [DOI] [PubMed] [Google Scholar]
- 38.Morrow D, Sweeney C, Birney YA, Cummins PM, Walls D, Redmond EM, Cahill PA. Cyclic strain inhibits Notch receptor signaling in vascular smooth muscle cells in vitro. Circ Res. 2005;96:567–575. doi: 10.1161/01.RES.0000159182.98874.43. doi:10.1161/01.RES.0000159182.98874.43. [DOI] [PubMed] [Google Scholar]
- 39.Park HJ, Kim HJ, Bae GS, Seo SW, Kim DY, Jung WS, Kim MS, Song MY, Kim EK, Kwon KB, Hwang SY, Song HJ, Park CS, Park RK, Chong MS, Park SJ. Selective GSK-3beta inhibitors attenuate the cisplatin-induced cytotoxicity of auditory cells. Hearing Res. 2009;257:53–62. doi: 10.1016/j.heares.2009.08.001. doi:10.1016/j.heares.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Just Ribeiro M, Hansson ML, Lindberg MJ, Popko-Scibor AE, Wallberg AE. GSK3beta is a negative regulator of the transcriptional coactivator MAML1. Nucleic Acids Res. 2009;37:6691–6700. doi: 10.1093/nar/gkp724. doi:10.1093/nar/gkp724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz RS, Henry TD. Pathophysiology of coronary artery restenosis. Rev Cardiovasc Med. 2002;3(Suppl 5):S4–S9. [PubMed] [Google Scholar]
- 42.Sedding DG, Seay U, Fink L, Heil M, Kummer W, Tillmanns H, Braun-Dullaeus RC. Mechanosensitive p27Kip1 regulation and cell cycle entry in vascular smooth muscle cells. Circulation. 2003;108:616–622. doi: 10.1161/01.CIR.0000079102.08464.E2. doi:10.1161/01.CIR.0000079102.08464.E2. [DOI] [PubMed] [Google Scholar]
- 43.Shyu KG. Cellular and molecular effects of mechanical stretch on vascular cells and cardiac myocytes. Clin Sci. 2009;116:377–389. doi: 10.1042/CS20080163. doi:10.1042/Cs20080163. [DOI] [PubMed] [Google Scholar]
- 44.Sweeney C, Morrow D, Birney YA, Coyle S, Hennessy C, Scheller A, Cummins PM, Walls D, Redmond EM, Cahill PA. Notch 1 and 3 receptor signaling modulates vascular smooth muscle cell growth, apoptosis, and migration via a CBF-1/RBP-Jk dependent pathway. FASEB J. 2004;18:1421–1423. doi: 10.1096/fj.04-1700fje. doi: 10.1096/fj.04-1700fje. [DOI] [PubMed] [Google Scholar]
- 45.Uemura K, Kuzuya A, Shimozono Y, Aoyagi N, Ando K, Shimohama S, Kinoshita A. GSK3beta activity modifies the localization and function of presenilin 1. J Biol Chem. 2007;282:15823–15832. doi: 10.1074/jbc.M610708200. doi:10.1074/jbc.M610708200. [DOI] [PubMed] [Google Scholar]
- 46.Vernhet H, Demaria R, Juan JM, Oliva-Lauraire MC, Senac JP, Dauzat M. Arterial stenting and overdilation: does it change wall mechanics in small-caliber arteries? J Endovasc Ther. 2002;9:855–862. doi: 10.1177/152660280200900620. [DOI] [PubMed] [Google Scholar]
- 47.Wang AB, Li HL, Zhang R, She ZG, Chen HZ, Huang Y, Liu DP, Liang CC. A20 attenuates vascular smooth muscle cell proliferation and migration through blocking PI3k/Akt singling in vitro and in vivo. J Biomed Sci. 2007;14:357–371. doi: 10.1007/s11373-007-9150-x. doi:10.1007/ s11373-007-9150-x. [DOI] [PubMed] [Google Scholar]
- 48.Willert M, Augstein A, Poitz DM, Schmeisser A, Strasser RH, Braun-Dullaeus RC. Transcriptional regulation of Pim-1 kinase in vascular smooth muscle cells and its role for proliferation. Basic Res Cardiol. 2010;105:267–277. doi: 10.1007/s00395-009-0055-x. doi:10.1007/s00395-009-0055-x. [DOI] [PubMed] [Google Scholar]
- 49.Yamamizu K, Matsunaga T, Uosaki H, Fukushima H, Katayama S, Hiraoka-Kanie M, Mitani K, Yamashita JK. Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors. J Cell Biol. 2010;189:325–338. doi: 10.1083/jcb.200904114. doi:10.1083/jcb. 200904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou RH, Lee TS, Tsou TC, Rannou F, Li YS, Chien S, Shyy JY. Stent implantation activates Akt in the vessel wall: role of mechanical stretch in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:2015–2020. doi: 10.1161/01.ATV.0000095161.06906.ED. doi:10.1161/01.ATV.0000095 161.06906.ED. [DOI] [PubMed] [Google Scholar]