Abstract
The μ-opioid receptor is involved in the rewarding effects of not only opioids like morphine but also psychostimulants like amphetamine. This study aimed to investigate associations between subjective response to amphetamine and genetic polymorphisms and haplotypes in the μ-opioid receptor including the exonic variant rs1799971 (Asp40Asn). 162 Caucasian volunteers participated in three sessions receiving either placebo or d-amphetamine (10 and 20 mg). Associations between levels of self-reported Euphoria, Energy and Stimulation (ARCI-49) after d-amphetamine ingestion and polymorphisms in OPRM1 were investigated. The intronic SNPs rs510769 and rs2281617 were associated with significantly higher ratings of Euphoria, Energy and Stimulation after 10 mg amphetamine. Feelings of Euphoria, Energy and Stimulation were also found to be associated with a 2-SNP haplotype formed with rs1799971 and rs510769 and a 3-SNP haplotype formed with rs1918760, rs2281617 and rs1998220. These results support the hypothesis that genetic variability in the μ-opioid receptor gene influences the subjective effects of amphetamine and may suggest new strategies for prevention and treatment of psychostimulant abuse.
Keywords: amphetamine, euphoria, energy, stimulation, μ-opioid receptor, inter-individual differences
INTRODUCTION
Amphetamine, a prototypic drug of abuse, increases feelings of euphoria, energy and attention in most people (Brauer & de Wit, 1996). However, some individuals experience adverse effects such as increased anxiety or dysphoria (de Wit et al, 1986) There is evidence that some of the variations in subjective responses to amphetamine may be genetic in origin. For example, monozygotic twins have a higher concordance in subjective response to amphetamine compared to dizygotic twins (Crabbe et al., 1983; Nurnberger et al., 1982), and in healthy volunteers, polymorphisms in dopaminergic and other receptor mechanisms have been found to be associated with acute response to amphetamine (Dlugos et al., 2007; Mattay et al., 2003; Palmer et al., 2005).
Amphetamine acts mainly by inhibiting reuptake of presynaptic dopamine, norepinephrine and serotonin via interactions with membrane transporters involved in neurotransmitter vesicular storage and reuptake (Wallace & Connell, 2008; Schuldiner et al., 1993). However, its reinforcing effects are also modulated by μ-opioid receptors (OPRM1). OPRM1 are densely located in the mesolimbic dopamine system, which is composed of projections from the ventral tegmental area to the striatal nucleus accumbens (NAc) (Mathon et al., 2006). Amphetamine increases synaptic dopamine levels, which activate striatal neuropeptides in post synaptic neurons acting on opioid receptors, including OPRM1 (Dalia et al., 1998; Florin et al., 1998; Wang et al., 1998). The stimulation of OPRM1, in turn, inhibits GABA release and disinhibits dopamine neuronal firing. This results in further increases in extracellular dopamine levels in the NAc and striatum (Spanagel et al., 1992; Kieffer et al.,1992; Gerrits et al., 2003). The idea that endogenous opioids contribute to amphetamine responses is consistent with the observation that µ-receptor agonists and psychostimulants taken together produce strong euphorigenic effects (“speedball”) (Leri et al., 2003).
Controlled studies with rats and humans further support the hypothesis that responses to amphetamine are mediated by the opioid system. Oprm1-knockout mice show reduced reward from psychostimulants, (Hall et al., 2004) and opioid receptor antagonists attenuate amphetamine-induced dopamine release in the striatum and NAc (El Daly et al., 2004; Hooks et al., 2004). In healthy humans and in amphetamine-dependent patients the opioid receptor antagonist naltrexone decreases the subjective effects of amphetamine (Jayaram-Lindström et al., 2004a,b). Additionally, imaging studies show that human cocaine users have increased OPRM1 binding in several brain regions, and this increased binding is positively correlated with self-reported cocaine craving (Gorelick et al., 2005; Zubieta et al., 2005). OPRM1 binding also predicted time to relapse in the cocaine users (Gorelick et al., 2008). Thus, several lines of evidence suggest that the endogenous opioid system contributes to the rewarding effects of stimulant drugs.
We hypothesized that genetic variations at the OPRM1 locus may influence subjective response to amphetamine. Two single nucleotide polymorphisms (SNPs) were of particular interest. The non-synonymous OPRM1 SNP rs1799971 (Asp40Asn) is reportedly a risk allele for addiction. Studies have shown that it is associated with methamphetamine-induced psychosis (Ide et al., 2006), alcoholism (Schinka et al., 2002), heroin addiction (Drankenberg et al., 2006), and incidence of completed suicide (Hishimoto et al., 2008). The intronic SNP rs510769 appears to be associated with vulnerability to heroin addiction (Levran et al., 2008). In addition, we performed association analyses between OPRM1 polymorphisms and physiological measures, such as blood pressure and heart rate, to determine whether OPRM1 polymorphisms might also influence physiological measures after amphetamine. We hypothesized that SNPs that modulate the subjective responses to drugs would also modulate physiological responses. In total we investigated the impact of seven SNPs in the OPRM1, including rs1799971 and rs510769, on physiological as well as prototypic behavioral and subjective responses to amphetamine: euphoria, energy and stimulation.
MATERIALS AND METHODS
Subjects
72 female and 90 male healthy volunteers, aged 18–35, were recruited by advertisements and posters. All subjects were of self-reported Caucasian origin, which was confirmed by analysis of ancestry informative markers in the sample (see Genotyping paragraph below). All participants took part in a screening that included a structured clinical psychiatric interview, screening questionnaires, a psychiatric symptom checklist (SCL-90) (Derogatis, 1983), the Michigan Alcoholism Screening Test (Selzer, 1971) and a health questionnaire with a detailed section on current and lifetime drug use. Volunteers received a physical examination by a physician and obtained an electrocardiogram. Subjects were excluded from participation if their BMI was less than 18 or greater than 26, if they had any current medical condition requiring medication or current or past medical condition that was considered to be a contraindication for amphetamine (e.g. hypertension, abnormal EKG), or any current Axis I psychiatric disorder (American Psychiatric Association, 1994). To reduce variability due to withdrawal or tolerance from caffeine and nicotine, volunteers were excluded if they consumed more than three cups of coffee per day, or smoked more than 10 cigarettes per week. Subjects were not included if they had been treated for a substance use disorder or had a history of legal, personal or employment problems related to drug use, if they were not fluent in English, if they had less than a high school education, or if they worked a night shift. Women who were pregnant or lactating, or planning to become pregnant during the study were also excluded. Women were only tested in their follicular phase as response to amphetamine is dampened during the luteal phase of the menstruation cycle because of altered pharmacodynamic effects (White et al., 2002).
Design
The study used a three-session crossover design. Each subject received placebo and d-amphetamine (10 and 20 mg), under double-blind conditions and in randomized order. After obtaining baseline measures, behavioral and physiological measures were recorded at regular intervals over 4 hours after capsule administration. Genotyping was performed blind to all behavioral data. A subset of subjects (N=101) also took part in a fourth session in which a 5 mg dose was used; data from this session are not included in this analysis because of the smaller sample size.
Procedure
Subjects first attended an orientation session to provide informed consent. They practiced behavioral tests and questionnaires, completed a personality questionnaire (data not presented) and gave a blood sample for genotyping. Participants were instructed to abstain from taking drugs including alcohol, nicotine or caffeine, for 24 h before each session and to fast from midnight the night before the sessions. Subjects were tested individually in a comfortably furnished room with television and reading materials for the 4-h session. Subjective and behavioral tasks were administered via computer. Volunteers were allowed to read during the sessions and watch emotionally neutral movies when no measurements were being taken. This study was approved by the Institutional Review Board of The University of Chicago and was carried out in accordance with the Helsinki Declaration of 1975.
Sessions were conducted from 9:00 AM to 1:00 PM, at least 48 hours apart. At the beginning of each session, subjects provided breath samples to confirm their abstinence from nicotine and alcohol and a urine sample that was tested for recent use of marijuana, cocaine, benzoylecgonine, phencyclidine, opiates, methamphetamine, Ecstasy, methadone, amphetamine, barbiturates and benzodiazepines. Subjects testing positive for these substances were excluded from the study. Volunteers completed baseline mood questionnaires of pre-drug subjective effects. At 9:30 AM, they ingested a capsule containing either placebo or d-amphetamine (10 or 20 mg) administered with 100 ml of water. Compared with clinically recommended daily doses of up to 40 mg of amphetamine for school-aged children with ADHD (Spencer et al., 2006; Greenhill et al., 2002) we used relatively low doses of amphetamine, in part to minimize risk to subjects and in part to enhance our ability to detect genotype-dependant differences. The doses were sufficient to produce measurable subjective and behavioral effects in the participating healthy volunteers. Subjective, behavioral and physiological measures including heart rate and blood pressure were obtained 30, 60, 90, 150 and 180 minutes after capsule intake.
Dependent Measures
To assess subjective drug effects, subjects completed the Addiction Research Center Inventory 49-item questionnaire (ARCI-49) (Martin et al., 1971). We examined the association between polymorphisms in OPRM1 and subjective response to amphetamine using the ARCI-49 in the present study. The ARCI-49 consists of 49 yes-no questions that relate to 5 factors corresponding to typical effects of psychoactive drugs. It is a sensitive instrument for determining subjective drug effects, and consists of five scales assessing (1) Euphoria (Morphine-Benzedrine Group scale), (2) intellectual Efficacy and Energy (Benzedrine Groups scale; hereafter referred to as Energy), (3) Stimulation (Amphetamine Group scale), (4) Sedation (Pentobarbital-Chlorpromazine-Alcohol Group scale) and (5) Dysphoria (Lysergic Diethylamide scale). The ARCI scales Euphoria, Energy and Stimulation were selected as primary outcome measures to capture the prototypic effects of amphetamine. Sedation was included as secondary outcome measure to examine whether ratings of Sedation decreased when ratings of Euphoria, Energy and Stimulation increased after amphetamine. In order to reduce variability between and within subjects peak change scores were calculated by subtracting the predrug baseline scores from the measured scores after drug ingestion. The value with the greatest distance from baseline was chosen as peak change score. In case of equal positive and negative maximum distances, 0 was used as peak change score.
Genotyping
Genotyping was performed using the Addictions Array (Hodgkinson et al., 2008). The Addictions Array aimed to develop a panel of markers able to extract full haplotype information for candidate genes in alcoholism, other addictions and disorders of mood and anxiety (Hodgkinson et al, 2008). Criteria for sample exclusion and classification as genotyping failure were previously described (Hodgkinson et al., 2008). Of 162 original subjects, only a single sample was uncallable at rs1799971; all other genotypes were successfully obtained. Genotyping accuracy was determined based on genotype concordance between DNA replicates (genotype error rate: 0%, 27 replicates). Subjects were genotyped at seven selected polymorphisms in the OPRM1 gene (Table 1) and were assigned to one of three genotype groups at five of the seven loci: homozygotes for the first or second allele and heterozygotes. There were only four subjects with genotype G/G at rs1799971 and three subjects with genotype T/T at rs2281617. Thus, participants with genotype G/G and A/G at rs1799971 and subjects with genotype T/T and C/T at rs2281617 were pooled into one group.
Table 1.
Allele and Genotype frequencies of the OPRM1 gene polymorphisms.
| Chr. Position |
Gene Position |
Allele | Genotype | HWE1 | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1/1 | 1/2 | 2/2 | p-Value | |||
| rs1799971A/G2 | 151924380 | 355 | 282 | 40 | 125 | 32 | 4 | 0.41 |
| rs510769 A/G3 | 151925602 | 1577 | 86 | 238 | 13 | 60 | 89 | 0.27 |
| rs660756A/C | 151979108 | 54992 | 206 | 118 | 63 | 80 | 19 | 0.53 |
| rs1918760 A/G | 152045410 | 121724 | 195 | 129 | 58 | 79 | 25 | 0.99 |
| rs2281617C/T | 152050664 | 126978 | 280 | 44 | 121 | 38 | 3 | 1.0 |
| rs1998220A/G | 152069596 | 145985 | 141 | 183 | 31 | 79 | 52 | 1.0 |
| rs9371781A/C | 152115349 | 191764 | 226 | 98 | 78 | 70 | 14 | 0.94 |
Hardy Weinberg Equilibrium: p-Values assessed by Haploview software version 4.0
Significant associations with methamphetamine-induced pychosis,26 alcoholism,27 heroin addiction,28 completed suicide,29 and HPA axis activation36,37 (p<0.05).
Significant associations with vulnerability to heroin addiction,30 (p<0.05)
A panel of 186 ancestry informative SNPs was selected as previously described (Hodgkinson et al., 2008). It was analyzed with Structure 2.1 (Pritchard et al., 2000) considering 1051 CEPH reference subjects to confirm the participants’ self-reported and experimenter-observed Caucasian designations. Proportions of membership for each subject in clusters corresponding to 7 geographic regions (Africa, Europe, Middle East, Central Asia, Far East Asia, America, Oceania) were estimated. Score means were compared between genotype groups at rs2281617, which was associated with amphetamine response.
Haplotype reconstruction
Haplotypes were derived using the program Phase 2.1.1 (stephenslab.uchicago.edu/software.html). Criteria to assess neighboring SNPs together as haplotypes were as follows: pairwise standardized linkage disequilibrium coefficient D`>.95 between polymorphisms; probability of reconstructed pairs of haplotypes for each individual D`≥.95, and haplotype frequency in the sample N>10. On the basis of these criteria, three haplotypes of rs1799971and rs510769 and four haplotypes of rs1918760, rs2281617 and rs1998220 were identified. The pairwise standardized linkage disequilibrium coefficients were D`<.95 between the markers rs660756 and rs9371781 and the ones included in the haplotype analysis. Thus, both SNPs were not included in the haplotype analysis. The haplotypes were coded as having two copies (2), as having one copy (1), or as not present (0) in an individual. Hardy-Weinberg equilibrium for each marker and linkage disequilibrium between the markers were analyzed using the Haploview software version 4.0 (http://www.broad.mit.edu/mpg/haploview/). Haploview was also used to generate a linkage disequilibrium map of the OPRM1 gene with the available HapMap data (The International HapMap Genome Browser B36).
Statistical Analyses
To assess genotype-independent main effects of Placebo and amphetamine (10 and 20 mg) a two-way ANOVA was performed using dose (0, 10 and 20 mg of drug) and time (five time points after capsule ingestion minus pre-drug (baseline) scores) as within subject factors for each dependent measure. Possible confounding variables (age, body mass index [BMI], gender and baseline responses) were assessed by performing separate two-way ANCOVAs with peak change scores as within-subject factors. A threshold of p<0.05 was used; confounding variables exceeding this threshold were included as covariates in further statistical analyses. Demographic characteristics for the different genotype groups, such as gender, BMI, education in years, age, current substance abuse and lifetime substance use were compared using ANOVA or χ2 tests.
To analyze the impact of genotypes on drug response, either separate two-way ANOVAS or two-way ANCOVAS (SPSS 16.0) were performed for each outcome measure. Genotype or number of haplotype copies were used as grouping factors and peak change scores for placebo, 10 and 20 mg amphetamine were chosen as within-subjects factors, comprising two-way ANOVAs and ANCOVAs. Assessing drug by genotype and haplotype interactions Lavene´s test for equality of error variances was always included in the analyses. Greenhouse-Geisser correction was used when Lavene´s test for equality of error variances was significant. To investigate main effects of genotype groups further, and to determine whether differences in drug response were due to placebo or amphetamine, post hoc analyses were conducted by performing one-way ANOVAs or ANCOVAs with peak change scores as dependent measures. Alpha was set at p<0.05 (two-tailed) for all analyses. However, because we examined seven SNPs and primary outcome measures (Euphoria, Energy and Stimulation), a conservative Bonferroni-Holm correction would require a p-value of 0.002 for statistical significance.
RESULTS
Demographic and Genetic Characteristics of the Subjects
All subjects were of Caucasian origin. They were aged 18–35 years and reported 12–20 years of formal education. Table 2 shows demographic characteristics including current and lifetime substance abuse of the overall sample.
Table 2.
Demographic characteristics for all subjects.
| Overall | |
|---|---|
| Demographic Characteristics | |
| Overall, n | 162 |
| Age (mean (years) ±SEM1) | 22.8±0.3 |
| Gender (%female) | 44.1 |
| BMI (mean (kg/m2) ±SEM1) | 22.7±0.2 |
| Current substance use | |
| Alcohol (mean drinks/week) | 4.5±0.3 |
| Cigarettes (mean cig./week) | 0.8±0.1 |
| Caffeine (mean cups/week) | 7.3±0.5 |
| Marijuana (mean times/month) | 0.9±0.2 |
| Lifetime substance use | |
| Stimulants (% ever used) | 52.2 |
| Sedatives (% ever used) | 6.2 |
| Opiates (% ever used) | 21.7 |
| Marijuana (% ever used) | 44.1 |
| Hallucinogens (% ever used) | 28.6 |
| Inhalants (% ever used) | 9.3 |
SEM: Standard Error of Mean
Comparisons across genoptype groups for all OPRM1 SNPs were made using one way ANOVA for continuous data and χ2 for frequency data (*p<.05).
162 subjects completed ARCI questionnaires for all three sessions. Genotype and allele frequencies for the investigated SNPs are shown in Table 1. All SNPs were in Hardy-Weinberg-equilibrium. Allele frequency proportions did not significantly differ from those given in the Hapmap project (The International HapMap Genome Browser B36). Polymorphisms within each gene were in high intermarker linkage disequilibrium forming two haplotype blocks. Haplotype blocks and D’ values between the genetic variants are shown in Figure 1. Rs1799971 and rs510769 were in high intermarker linkage disequilibrium as well as the SNPs rs1918760, rs2281617 and rs1998220 (r2<1.0). These observations were consistent with the HapMap data (The International HapMap Genome Browser B36). Analysis of ancestry informative markers (Structure 2.1.) confirmed self-reported Caucasian origin in all study participants. Caucasian origin was confirmed by score means of the analyzed 7 clusters corresponding to 7 geographic regions (Africa, Europe, Middle East, Central Asia, Far East Asia, America, Oceania). The genotype groups at locus rs2281617 did not differ in ethnicity, and because all subjects were confirmed to be Caucasian we did not repeat the ethnicity check with other polymorphisms.
Figure 1.
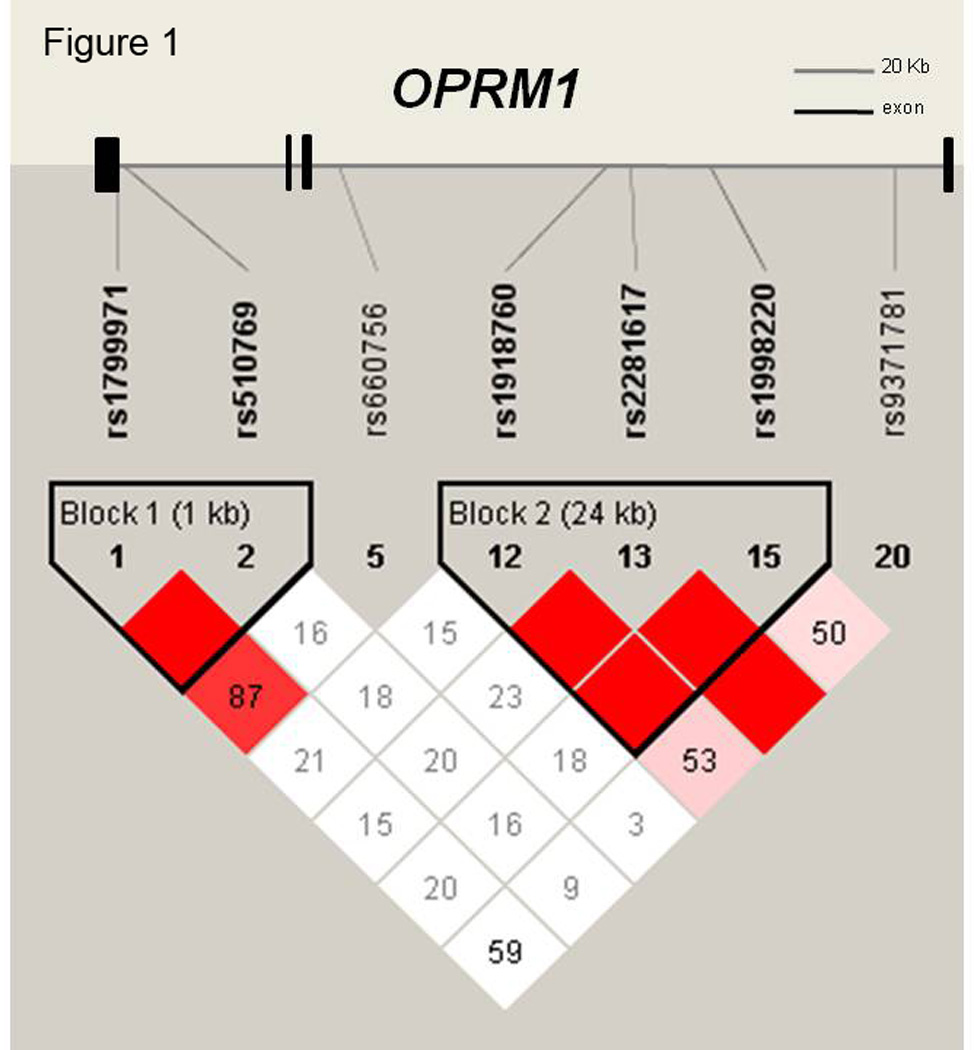
Genomic structure of OPRM1 gene, mapped to chromosome 6, is shown to scale including 4 exons spanning 207.6 kb as well as results of linkage disequilibrium analyses: D’ values of single nucleotide polymorphisms along the OPRM1 gene, illustrating two haplotype blocks. D’ values were calculated by Haploview version 4.0.
Correlation analyses for all demographic information with SNPs and haplotypes were performed to determine whether demographic measures should be included as covariates in statistical analyses. Genotype groups for each SNP and haplotype were similar on most demographic information. However, subjects with genotype C/C at rs660756 reported significantly higher lifetime opioid use (p<.001, χ2: 36,60, df=2) and higher lifetime sedative use (p<.001, χ2: 36,60, df=2). Participants with genotype A/G at rs1799971 reported higher marijuana consumption per month (one-way ANOVA: p<.05, F(2,152)=3.90) than people of the other genotype groups. In a separate analysis we found no relationship between lifetime opiate use or marijuana use per month and the outcome measures. Response to placebo did not differ across any of the genotype groups.
Genotype-Independent Effects of Amphetamine
The ARCI scales Euphoria, Energy and Stimulation were selected as primary outcome measures to capture the prototypic drug effects. Sedation was included as secondary outcome measure to examine whether ratings of Sedation decreased when ratings of Euphoria, Energy and Stimulation increased. Amphetamine produced the expected effects on the outcome measures with dose-dependent increases in Euphoria, Energy, Stimulation and decreases in Sedation (drug main effect from two-way ANOVA/ANCOVA, Euphoria: p<0.001, F(2, 320)=64.36, Energy: p<0.001, F(2, 320)=60.55, Stimulation: p<0.001, F(2, 322)=83.23, Sedation: p<0.001, F(2, 322)=44.94). Amphetamine also had dose-dependent effects increasing blood pressure and heart rate (drug main effect from two-way ANOVA/ANCOVA; systolic blood pressure: p<0.001, F(2, 328)=159.03, diastolic blood pressure: p<0.001, F(2, 326)=81.28, heart rate: p<0.001, F(2, 326)=40.25). For most measures the effect of capsule ingestion was significant by 60 min post-administration and peaked between 90 and 120 minutes post-administration. Males scored significantly higher on Energy than females (main effect of sex from two-way ANOVA: p<0.05, F(1,160)=5.92). Therefore, sex was used as a covariate and included as a between-subject factor in further analyses. Further demographic measures did not affect the self-reported outcome measures. Baseline scores did not differ between genotype groups.
OPRM1 gene polymorphisms and ARCI scales
To reduce variability within and between subjects peak change scores within each session for the primary outcome measures Euphoria, Energy, Stimulation and the secondary outcome measure Sedation (ARCI) were used and calculated by subtracting the predrug baseline scores from the measured scores after drug ingestion. Significant associations between SNPs in OPRM1 and the peak change scores for Euphoria, Energy, Stimulation and Sedation after amphetamine ingestion are reported in Table 3. Genotype A/A of rs510769 was associated with lower Stimulation and Euphoria scores after amphetamine (two-way ANOVA/ANCOVA, Euphoria: p<0.05, F(4, 318)=2.69; Stimulation: p<0,05, F(4,318)=2.99). Figure 2 shows peak change scores of Euphoria and Stimulation (ARCI) between the three genotype groups of the rs510769 polymorphism. Post hoc comparisons using peak change scores of each session respectively revealed that genotype groups scored significantly different on the Stimulation scale in response to the 10 mg dose (one-way ANOVA, p=0,01, F(2, 162)=4.74). A similar trend was apparent for Euphoria (one-way ANOVA, p<0.1, F(2, 162)=2.59) after the 10 mg dose.
Table 3.
Results of ANOVA’s for responses to d-amphetamine for individual OPRM1 gene polymorphisms.
| rs510769A/G | rs2281617C/T | rs1998220A/G | |
|---|---|---|---|
| Euphoria | |||
| F-value (DF)2 | 2.69 (4) | 4.73 (2) | 0.89 (4) |
| p-value | 0.0314 | 0.0104 | 0.468 |
| Post hoc (10 mg): | |||
| F-value (DF)3 | 2.59 (2) | 15.33 (1) | --- |
| p-value | 0.078 | 0.0001 | --- |
| Energy1 | |||
| F-value (DF)2 | 1.33 (4) | 4.92 (2) | 2.91 (4) |
| p-value | 0.2574 | 0.0084 | 0.022 |
| Post hoc (10 mg): | |||
| F-value (DF)3 | --- | 12.88 (1) | 1.32 (2) |
| p-value | --- | 0.0003 | 0.271 |
| Stimulation | |||
| F-value (DF)2 | 2.99 (4) | 2.28 (2) | 2.67 (4) |
| p-value | 0.019 | 0.1044 | 0.0324 |
| Post hoc (10 mg): | |||
| F-value (DF)3 | 4.74 (2) | --- | 1.35 (2) |
| p-value | 0.010 | --- | 0.2635 |
| Sedation | |||
| F-value (DF)2 | 0.80 (4) | 2.76 (2) | 0.89 (4) |
| p-value | 0.525 | 0.0654 | 0.469 |
| Post hoc (10 mg): | |||
| F-value (DF)3 | --- | --- | --- |
| p-value | --- | --- | --- |
Energy adjusted for gender (included as between subject covariate in statistical Analyses)
Drug by genotype interactions: p-values assessed by two-way ANOVA/ANCOVA
Post hoc analysis: p-values assessed by one way ANOVA/ANCOVA (10mg dose)
Greenhouse-Geisser Correction
Figure 2.
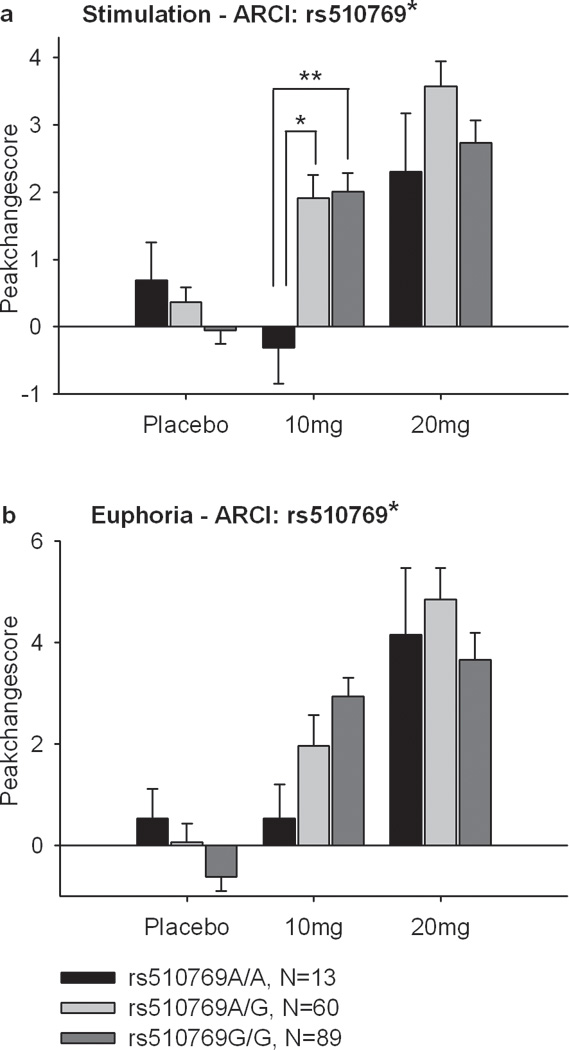
Mean±SEM peak change scores on Euphoria and Stimulation (ARCI) in the three genotype groups at rs510769 after Placebo, 10 and 20 mg of amphetamine. Amphetamine (10 mg) increased Euphoria and Stimulation more in the rs510769A/G and G/G group than in the 510769A/A group. (*Significant drug × genotype interaction, Two-way ANOVA, not significant after adjustment for multiple testing). **Post hoc one-way ANOVA multiple comparisons between genotypes with Bonferroni correction p<0.05).
Subjects with genotype C/C of rs2281617 felt significantly more Euphoric and Energetic after amphetamine (10 mg) compared to participants with genotypes C/T and T/T (Genotype × Drug interaction on two-way ANOVA/ANCOVA, Euphoria: p=0.01, F(2, 320)=4.73; Energy: p<0.01, F(2, 316)=4.92). Figure 3 shows peak change scores of Euphoria and Energy (ARCI) between the genotype groups. Post hoc analyses (one-way ANOVAs or ANCOVAs, Euphoria p<0.001, F(1, 163)=15.33; Energy p<0.001, F(1, 163)=12,88) revealed that genotype groups significantly differed in Euphoria and Energy after 10 mg amphetamine. To clarify the source of peak change scores, time courses of Euphoria, Energy, Stimulation and Sedation, are descriptively shown in Figure 4 as changes from baseline for the genotype groups after the 10 mg dose. Locus rs1998220 was in strong linkage disequilibrium with rs2281617 (D’=1.0) and revealed to be also significantly associated with response to amphetamine. Subjects with genotype A/A at rs1998220 had significantly higher Energy levels and felt more stimulated than subjects with alleles A/G or G/G at rs1998220 (Genotype × Drug interaction on two-way ANOVA/ ANCOVA, Energy: p<0.05, F(4, 312)=2.91; Stimulation: p<0.05, F(4, 318)=2.67), but there was no significant association between rs1998220 and Energy in Post hoc analyses (one-way ANOVA/ANCOVA).
Figure 3.
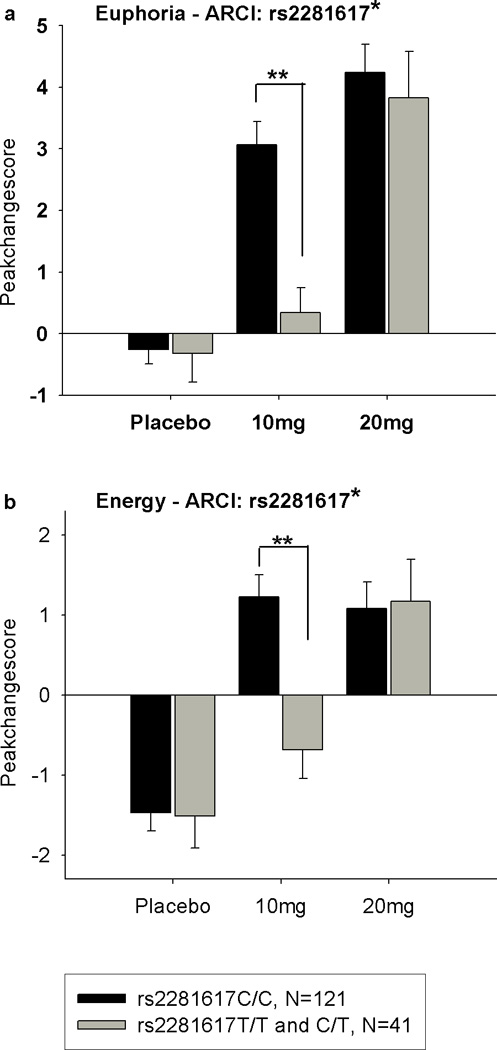
Mean±SEM peak change scores on Euphoria and Energy (ARCI) in the two genotype groups at rs2281617 (C/C: N=121; C/T and T/T: N=41) after Placebo, 10 and 20 mg of amphetamine. After 10mg of amphetamine (10 mg) the rs2281617C/C group reported greater increases in Euphoria, Energy and Stimulation and lower levels of Sedation compared to the 2281617C/T and T/T group. (*Significant drug × genotype interaction, two-way ANOVA, not significant after adjustment for multiple testing). **Post hoc one-way ANOVA multiple comparisons between genotypes with Bonferroni correction p<0.05).
Figure 4.
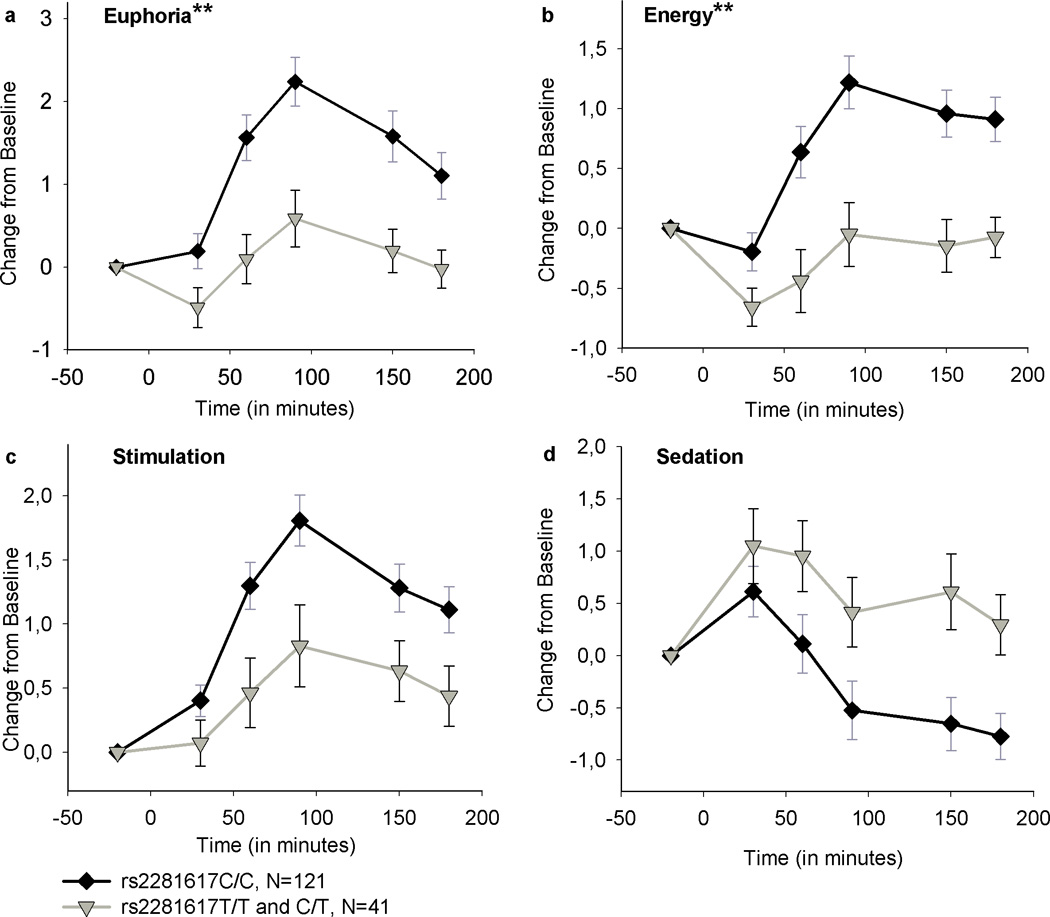
Time courses of Euphoria, Energy, Stimulation and Sedation (ARCI) after 10 mg of amphetamine administration for the rs2281617 groups. The rs2281617C/C group reported a significantly greater increase in Euphoria and Energy compared to the T/T and C/T group in post hoc analyses (one-way ANOVA/ANCOVA). Post hoc analyses were not carried out for Stimulation and Sedation, as both scales were not significantly associated with rs2281617 genotype groups in primary analyses (two-way ANOVA/ANCOVA). Data are mean (SEM) ratings as change from predrug baseline. The groups did not significantly differ on baseline scores. (**p<0.01).
However, taking into account the number of comparisons performed, none of the results remained significant after adjustment for multiple testing.
There were no significant Genotype × Drug interactions on the outcome measures for the genotype groups at rs1799971, rs660756, rs1918760 and rs9371781. No significant associations were found between any of the seven investigated SNPs and placebo or the 20 mg amphetamine condition.
OPRM1 haplotypes and ARCI scales
Linkage disequilibrium measures (D’-values) between the seven investigated polymorphisms are shown in Fig 1 (Haploview software version: 4.0). Because high pairwise LD measures (D’=1.0) were found between rs1799971 and rs510769 as well as between rs1918760, rs2281617 and rs1998220, haplotype pairs from the linked SNPs were estimated for each individual. The probability of all haplotype estimates was ≥.99, except for one subject, that had an undetermined genotype at locus rs1799971. This subject was excluded from the relevant haplotype analyses. Seven reconstructed haplotypes were assessed for association with the ARCI scales by performing a two-way ANOVA/ANCOVA using peak change scores for Euphoria, Energy, Stimulation and Sedation as within subject factors. Post hoc analyses were carried out conducting one-way ANOVAs/ANCOVAs using peak change scores for each session. Results are shown in Table 4. Group peak change score means and standard error of means (SEM) for the haplotype groups after the 10 mg dose are shown in Table S1.
Table 4.
Association of ARCI scores Euphoria, Energy, Stimulation and Sedation after amphetamine with OPRM1 gene haplotypes.
| Haplotype rs1799971-A rs510769-G |
Haplotype rs1799971-A rs510769-A |
Haplotype rs1799971-G rs510769-G |
Haplotype rs1918760-A rs2281617-C rs1998820-G |
Haplotype rs1918760-A rs2281617-C rs1998220-A |
Haplotype rs1918760-A rs2281617-T rs1998220-A |
Haplotype rs1918760-G rs2281617-C rs1998220-G |
|
|---|---|---|---|---|---|---|---|
| Frequencies | |||||||
| Number of copies | 2: N=60 1: N=74 0: N=26 |
2: N=13 1: N=59 0: N=88 |
2: N=4 1: N=32 0: N=124 |
2: N=0 1: N=12 0: N=149 |
2: N=30 1: N=80 0: N=51 |
2: N=3 1: N=38 0: N=120 |
2: N=23 1: N=78 0: N=60 |
| Euphoria | |||||||
| F-value (DF)2 | 2.23 (4) | 3.34 (4) | 0.21 (4) | 0.03 (2) | 1.62 (4) | 3.47 (4) | 0.68 (4) |
| p-value | 0.066 | 0.0124 | 0.932 | 0.970 | 0.169 | 0.0094 | 0.610 |
| Post hoc (10 mg): | --- | ||||||
| F-value (DF)3 | --- | 3.26 (2) | --- | --- | --- | 8.17 (2) | --- |
| p-value | --- | 0.041 | --- | --- | --- | 0.000 | |
| Energy1 | |||||||
| F-value (DF)2 | 1.50 (4) | 1.41 (4) | 0.56 (4) | 0.15 (2) | 0.54 (4) | 3.18 (4) | 2.78 (4) |
| p-value | 0.201 | 0.230 | 0.691 | 0.859 | 0.709 | 0.014 | 0.027 |
| Post hoc (10 mg): | |||||||
| F-value (DF)3 | --- | --- | --- | --- | --- | 7.01 (2) | 2.21 (2) |
| p-value | --- | --- | --- | --- | --- | 0.001 | 0.113 |
| Stimulation | |||||||
| F-value (DF)2 | 2.90 (4) | 3 38 (4) | 0.73 (4) | 0.25 (2) | 1.52 (4) | 2.34 (4) | 1.73 (4) |
| p-value | 0.022 | 0.010 | 0.571 | 0.781 | 0.195 | 0.0544 | 0.142 |
| Post hoc (10 mg): | |||||||
| F-value (DF)3 | 5.36 (2) | 4.76 (2) | --- | --- | --- | --- | --- |
| p-value | 0.006 | 0.010 | --- | --- | --- | --- | --- |
| Sedation | |||||||
| F-value (DF)2 | 0.88 (4) | 0.92 (4) | 0.48 (4) | 0.14 (2) | 0.44 (4) | 0.48 (4) | 1.56 (4) |
| p-value | 0.484 | 0.450 | 0.751 | 0.874 | 0.780 | 0.0604 | 0.1844 |
| Post hoc (10 mg): | |||||||
| F-value (DF)3 | --- | --- | --- | --- | --- | --- | --- |
| p-value | --- | --- | --- | --- | --- | --- | --- |
Energy adjusted for gender (included as between subject covariate in statistical Analyses)
Drug by genotype interactions: p-values assessed by two-way ANOVA/ANCOVA,
Post hoc analysis: p-values assessed by one way ANOVA/ANCOVA (10mg dose)
Greenhouse-Geisser Correction
Haplotype AA from rs1799971 and rs510769 was found to be significantly associated with levels of Euphoria and Stimulation (two-way ANOVA, Euphoria: p<0.05, F(4, 310)=3.34; Stimulation: p=0.01, F(4, 310)=3.38) and haplotype AG from rs1799971 and rs510769 was associated with Stimulation scores (two-way ANOVA, p<0.05, F(4, 310)=2.90). Both of these findings were confined to the 10 mg dose condition (one-way ANOVA, p<0.05, F(2, 157)=5.36). Subjects with 0 copies of haplotype AG scored lower on the associated ARCI scale than subjects with one or two haplotype copies. Participants with two copies of haplotype AA felt less euphoric and stimulated after 10mg of amphetamine than subjects with one or zero haplotype copies (one-way ANOVA, Euphoria: p<.0.05, F(2, 157)=3.26; Stimulation: p=0.01, F(2, 157)=4.76).
Haplotype ATA rs1918760, rs2281617 and rs1998220 was significantly associated with Euphoria and Energy (two-way ANOVA/ANCOVA, Euphoria: p<0.01, F(4, 312)=3.47; Energy: p<0.05, F(4 312), =3.18) after the 10 mg dose (one-way ANOVA/ANCOVA, Euphoria: p<0.001, F(2, 158)=8.17; Energy: p=0.001, F(2, 155)=7.01). People having zero copies of haplotype ATA from rs1918760, rs2281617 and rs1998220 felt significantly more energetic and euphoric after 10 mg amphetamine than participants with one or two copies. Along the same lines the haplotype GCG was significantly associated with increased Energy (two-way ANCOVA, p<0.05, F(4, 306)=2.78) after 10 mg amphetamine; however this effect was not significant in any of the post-hoc analyses.
There were few associations between the haplotypes and baseline scores. Haplotype ATA groups from rs1918760, rs2281617 and rs1998220 differed at baseline on the Euphoria and Stimulation scales (two-way ANOVA, Euphoria: p<0.05, F(4, 312)=2.69; Stimulation: p<0.01, F(4, 312)=3.77). Subjects with two copies of haplotype ATA scored higher on Euphoria and Stimulation at baseline at the 10 mg session, which was significant in post hoc analyses for Euphoria only (one-way ANOVA, p: p<0.05, F(2, 158)=3.37). Haplotype GCG groups from rs1918760, rs2281617 and rs1998220 differed at baseline on the Sedation scale (two-way ANOVAs, p<0.05, F(4, 312)=2.85). Subjects with 2 copies of haplotype GCG appeared to score higher on Sedation at baseline of the 10 and 20 mg amphetamine session. However, these differences were not significant in post-hoc analyses.
OPRM1 gene polymorphisms, haplotypes and physiological measures
Association analyses were performed between peak change scores of heart rate, systolic and diastolic blood pressure at the different doses and the investigated SNPs and haplotypes (two-way ANOVA/ANCOVA). Because subjects with a higher BMI had significantly higher diastolic blood pressure BMI was included as a covariate in analyses involving this measure.
The SNP rs2281617 and one haplotype were associated with some of the physiological measures (Table S2). Specifically, genotype C/C of rs2281617 was associated with higher diastolic blood pressure after 10 mg amphetamine (two-way ANCOVA, p<0.05, F(2, 310)=3.84, post hoc analysis: one-way ANCOVA, 10 mg: p<0.05, F(1, 159)=5.76). Increases in diastolic blood pressure after 10 mg amphetamine were also significantly associated with haplotype ATA from rs1918760, rs2281617 and rs1998220 (two-way ANCOVA, p<0.05, F(4, 298)=3.07 post hoc analysis: one-way ANCOVA, 10mg: p<0.05, F(2, 153)=3.45) and haplotype GCG (two-way ANCOVA, p=0.05, F(4, 296)=3.27, post hoc analysis: one-way ANCOVA, 10 mg, p<0.05, F(2, 153)=4.58). There was a significant Drug×Genotype interaction between haplotype AA from rs1799971 and rs510769 and diastolic blood pressure (two-way ANOVA, p<0.05, F(2, 296)=2.89), but post hoc analyses revealed that haplotype groups significantly differed after placebo only (one-way ANOVA, p F(2, 155)=3.79). Other polymorphisms and haplotypes did not differ at baseline, after placebo, 10 mg or 20 mg dose for any of the investigated physiological measures.
DISCUSSION
The main finding of the study is that subjects with genotype C/C at rs2281617 scored significantly higher on Euphoria and Energy than subjects with genotypes C/T and T/T after 10 mg amphetamine, and trends were also observed with measures of Stimulation and (lower) Sedation. Volunteers with genotypes A/G and G/G at rs510769 felt significantly more stimulated and euphoric after 10 mg amphetamine than participants with genotype A/A at rs510769, which is in strong LD with the functional exonic variant rs1799971. These primary findings were extended in the haplotype analysis (Table 3). Haplotypes AA and AG from rs1799971 and rs510769 as well as haplotypes ATA and GCG from rs1918760, rs2281617 and rs1998220 were significantly related to feelings of Euphoria, Energy and Stimulation in the expected directions (Table 3). But, as noted previously, it has to be considered that none of these results remained significant after adjustment for multiple testing.
Interestingly, we found several associations between genotypes and blood pressure responses. First, the associated genotype C/C at locus rs2281617 and haplotype ATA from rs1918760, rs2281617 and rs1998220 were significantly associated with increases in diastolic blood pressure after 10 mg (Table S2). Participants with the C/C genotype at rs2281617 or ATA haplotype exhibited greater blood pressure responses after amphetamine than other genotypic groups, which was consistent with the associations observed with subjective responses. Second, we found a significant interaction between haplotype AA from rs1799971 and rs510769 and diastolic blood pressure, but only on the placebo session. This association was not related to the number of haplotype copies: Subjects with two haplotype copies had the lowest diastolic blood pressure whereas subjects with one haplotype copy had the highest and subjects with zero copies were in between. These modest associations with amphetamine-induced changes in blood pressure, and possibly in the drug free state, suggest that opioid mechanisms may also be involved in the cardiovascular response to amphetamine.
The present results add to a growing literature on the genetic influences of OPRM1 on differences in sensitivity or rewarding effects of different drugs (Ide et al., 2006; Levran et al., 2008; Ray et al., 2004; Schinka et al., 2002). Our findings have implications for individual differences in susceptibility to substance abuse. In studies with rodents, individual differences in sensitivity to stimulant drugs are predictive of future self-administration (Piazza et al., 1989). Adolescents who report positive reactions to early use of cannabis are at increased risk of later cannabis dependence (Fergusson et al., 2003) and drug users report that their initial subjective responses to psychostimulants and opioids predicted their future use (Haertzen et al., 1983). It is possible that people carrying the common genotype C/C at rs2281617 or genotypes A/G and G/G at rs510769 might be more likely to progress to excessive use of amphetamine because they experience more positive effects from the drug. Recent studies have shown that the opioid receptor antagonist naltrexone attenuates reinstatement of amphetamine drug-seeking in the rat and Javaram-Lindstroem et al (2004a, b) demonstrated the efficacy of naltrexone in reducing amphetamine use in amphetamine-dependent humans in placebo-controlled clinical trials. Our finding showing that OPRM1 significantly influences response to amphetamine suggests that opioid antagonists may be of value in the treatment of stimulant addiction. However, we found that OPRM1 polymorphisms only influenced response to the lower 10 mg dose and not for the 20 mg dose, while drug abusers typically use high doses of drug. We previously reported a similar finding (Veenstra-VanderWeele et al, 2006), in which associations with subjective responses to amphetamine were observed for the lower dose (10 mg) but not the higher dose (20 mg). This suggests that certain genotype effects on response to amphetamine are apparent only at lower, marginally effective doses, and that the genetic differences are overcome by higher doses of the drug.
It is not clear whether rs2281617 itself alters the regulation of ORRM1 by modulating gene-transcription, mRNA stability, processing and splicing or whether it is instead in strong LD with some other functional variant, for example any 3' functional variant that regulates gene transcription rate. Rs2281617 is located in transcription-factor binding sites, according to the TRANSFAC program providing matrix families the identified transcription factor binding sites belong to (MatInspector; Genomatix Software, http://www.Genomatix.de/index.html). The analyses showed that subjects with the associated C allele at rs2281617 have additional predicted transcription factor binding sites from the matrix families V$Hand (Twist subfamily of class B bHLH transcription factors), V$ZBPF (Zinc binding protein factors) and V$GLIF (GLI zinc finger family) compared to subjects carrying the T allele. Matrix families V$ZBPF and V$GLIF are found in the central nervous system. However, to our knowledge in the literature there are no findings about the families of the predicted binding sites that might modulate and enhance transcription rate.
One SNP in our study, rs1799971, is known to cause an amino acid substitution in OPRM1. This SNP was previously reported to result in an increased beta-endorphin binding density and in an impairment of receptor signaling (Mague et al., 2009; Befort et al., 2001). We did not find any significant interaction between the rs1799971 genotype groups and amphetamine response. However, our subjects with genotype G/G (N=4) and A/G (N=32) were pooled into one group, whereas only subjects with genotype G/G are likely to differ in their response to amphetamine compared to participants that are heterozygous or homozygous for the A allele. Genotype G/G at rs1799971 was in strong LD with genotype A/A at rs510769 in our sample (D’=1.0). Thus, significant effects of genotype A/A could reflect the influence of the highly linked but less frequent functional rs1799971G/G genotype. Genotype alleles A/A at rs510769 were associated with a lower response to amphetamine compared to both heterozygous A/G and homozygous G/G groups, who reported very similar drug effects (Figure 2). Overall, Euphoria and Stimulation peak change scores of only G/G subjects (N=4) at rs1799971 were lower than peak change scores of A/G and A/A subjects, suggesting that genotype G/G at rs1799971 might have an influence on amphetamine response similar to genotype A/A at rs510769. Zhang et al. (2005) found the G allele at rs1799971 to have deleterious effects on both mRNA and protein yield in an experimental environment and the rs1799971G variant has been found to be associated with decreased potency of opioids such as morphine, its active metabolite morphine-6-glucoronide and alfentanil (Romberg et al., 2005; Romberg et al., 2003; Skarke et al., 2003; Lötsch et al., 2002; Caraco et al., 2001). In addition, humans carrying the G allele at rs1799971 were found to have decreased clinical responses to the OPRM1 agonist levomethadone (Lötsch et al., 2006); in their analysis, the association was only statistically significant when subjects homozygous for the G allele were included as a separate group. Thus, the effect of the G/G genotype needs to be examined in larger samples. As only subjective primary outcome measures were included in this study, imaging studies would be helpful to further elucidate how and in which brain regions the investigated genetic variations modulate response to amphetamine.
There were minor differences in the demographic characteristics of some of the genotypic groups in our study. People with genotypes G/G and A/G of rs1799971 reported significantly higher marijuana consumption per month than subjects with genotype A/A, and people with genotype C/C allele of rs660756 reported higher lifetime opioid and sedative use compared to subjects with genotypes A/A and A/C. It is unlikely that these differences are related to our primary findings. Separate correlation analyses showed that habitual use of marijuana, opioids and sedatives was not related to responses to amphetamine in the entire sample. Locus rs660756 was not in high LD with any of the other investigated markers (D`<0.21), was not associated with any of the outcome measures and not included in any haplotype analysis. Finally, both of these apparent correlations were of marginal significance.
An important consideration in this and related drug challenge studies concerns the problem of multiple testing. Because we tested several specific hypotheses about the effects of genetic polymorphisms (Dlugos et al., 2009; Dlugos et al., 2010) using this same sample, the most stringent approach would be to set a criteria for significance that limits the chance of a single false positive to 5% in any past, present or future study. However, when sample size and thus the corresponding power is limited, such a stringent threshold for significance greatly increases the likelihood of false negative (type 2) errors, and reduces the likelihood of detecting a real effect. Because we have not used such stringent criteria, our results must be interpreted with appropriate caution until confirmed with larger numbers of subjects. However, despite the limitations related to sample size, we believe that the detailed and well controlled phenotypes collected in this study are extremely valuable for elucidating genetic factors related to individual differences in drug responses.
In summary our study provides support that genetic variations of the OPRM1 are related to quantitative amphetamine response in healthy human volunteers. This finding elucidates possible reasons for inter-individual susceptibility becoming addicted to the drug and potency of opioid antagonists as pharmacological targets in amphetamine treatment.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully thank Dr. Andrew Skol for his invaluable input and technical support. We also thank Ms. Margo Meverden and Ms. Patricia Kriegel for their skillful technical assistance. This work was supported by DA021336, DA02812 and MO RR00055.
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: 1994. [Google Scholar]
- Befort K, Filliol D, Decaillot FM, Gaveriaux-Ruff C, Hoehe MR, Kieffer BL. A single nucleotide polymorphic mutation in the human mu-opioid receptor severely impairs receptor signaling. J Biol Chem. 2001;276:3130–3137. doi: 10.1074/jbc.M006352200. [DOI] [PubMed] [Google Scholar]
- Brauer LH, de Wit H. Subjective responses to d-amphetamine alone and after pimozide pretreatment in normal healthy volunteers. Biol Psychiatry. 1996;39:26–32. doi: 10.1016/0006-3223(95)00110-7. [DOI] [PubMed] [Google Scholar]
- Caraco Y, Maroz Y, Davidson E. Variability in alfentanil analgesia may be attributed to polymorphism in the mu opioid receptor [abstract] Clin Pharmacol Ther. 2001;69:63. [Google Scholar]
- Crabbe JC, Jarvik LF, Liston EH, Jenden DJ. Behavioral responses to amphetamine in identical twins. Acta Genet Med Gemellol (Roma) 1983;32:139–149. doi: 10.1017/s0001566000006425. [DOI] [PubMed] [Google Scholar]
- Dalia A, Uretsky NJ, Wallace LJ. Dopaminergic agonists administered into the nucleus accumbens, effects on extracellular glutamate and on locomotor activity. Brain Res. 1998;30 788(1–2):111–117. doi: 10.1016/s0006-8993(97)01518-7. [DOI] [PubMed] [Google Scholar]
- Derogatis L. SCL-90-R Manual II. Towson, MD: Clinical Psychometric Research; 1983. [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986;196:341–360. doi: 10.1016/0376-8716(86)90068-2. [DOI] [PubMed] [Google Scholar]
- Dlugos A, Freitag C, Hohoff C, McDonald J, Cook EH, Deckert J, de Wit H. Norepinephrine transporter gene variation modulates acute response to D-amphetamine. Biol Psychiatry. 2007;61:1296–1305. doi: 10.1016/j.biopsych.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Dlugos AM, Hamidovic A, Palmer AA, de Wit H. Further evidence of association between amphetamine response and NET-gene variants. Psychopharmacology. 2009;206:501–511. doi: 10.1007/s00213-009-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos AM, Hamidovic A, Hodgkinson CA, Goldman D, Palmer AA, de Wit H. More aroused, less fatigued: FAAH gene polymorphisms influence acute response to amphetamine. Neuropsychopharmacology. 2010;35:613–622. doi: 10.1038/npp.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakenberg K, Nikoshov A, Horvath MC, Fagergren P, Gharibyan A, Saarelainen K, Rahman S, Nylander I, Bakalkin G, Rajs J, Keller E, Hurd YL. Mu opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci U S A. 2006;103:7883–7888. doi: 10.1073/pnas.0600871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Daly E, Chefer V, Sandill S, Shippenberg TS. Modulation of the neurotoxic effects of methamphetamine by the selective kappa-opioid receptor agonist U69593. J Neurochem x. 2004;74:1553–1562. doi: 10.1046/j.1471-4159.2000.0741553.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT, Madden PA. Early reactions to cannabis predict later dependence. Arch Gen Psychiatry. 2003;60:1033–1039. doi: 10.1001/archpsyc.60.10.1033. [DOI] [PubMed] [Google Scholar]
- Florin SM, Kuczenski R, Segal DS. Amphetamine-induced changes in behavior and caudate extracellular acetylcholine. Brain Res. 1998;581:53–58. doi: 10.1016/0006-8993(92)90343-8. [DOI] [PubMed] [Google Scholar]
- Gerrits MA, Lesscher HB, van Ree JM. Drug dependence and the endogenous opioid system. Eur Neuropsychopharmacol. 2003;13:424–434. doi: 10.1016/j.euroneuro.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino ML, Dannals RF, Frost JJ. Brain mu-opioid receptor binding, relationship to relapse to cocaine use after monitored abstinence. Psychopharmacology (Berl) 2008;200:475–486. doi: 10.1007/s00213-008-1225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M, Endres CJ, Dannals RF, Frost JJ. Imaging brain mu-opioid receptors in abstinent cocaine users, time course and relation to cocaine craving. Biol Psychiatry. 2005;57:1573–1582. doi: 10.1016/j.biopsych.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchmann J. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2006;41:26. doi: 10.1097/00004583-200111000-00020. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Kocher TR, Miyasato K. Reinforcements from the first drug experience can predict later drug habits and/or addiction, Results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug Alcohol Depend. 1983;11:147–165. doi: 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- Hall FS, Goeb M, Li XF, Sora I, Uhl GR. Mu-Opioid receptor knockout mice display reduced cocaine conditioned place preference but enhanced sensitization of cocaine-induced locomotion. Brain Res Mol Brain Res. 2004;121:123–130. doi: 10.1016/j.molbrainres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Hishimoto A, Cui H, Mouri K, Nushida H, Ueno Y, Maeda K, Shirakawa O. A functional polymorphism of the micro-opioid receptor gene is associated with completed suicides. J Neural Transm. 2008;115:531–536. doi: 10.1007/s00702-007-0853-y. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D. Addictions biology, haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Jones DN, Justice JB, Jr, Holtzman SG. Naloxone reduces amphetamine-induced stimulation of locomotor activity and in vivo dopamine release in the striatum and nucleus accumbens. Pharmacol Biochem Behav. 2004;42:765–770. doi: 10.1016/0091-3057(92)90027-d. [DOI] [PubMed] [Google Scholar]
- Ide S, Kobayashi H, Ujike H, Ozaki N, Sekine Y, Inada T, Harano M, Komiyama T, Yamada M, Iyo M, Iwata N, Tanaka K, Shen H, Iwahashi Km, Itokawa M, Minami M, Satoh M, Ikeda K, Sora I. Linkage disequilibrium and association with methamphetamine dependence/psychosis of mu-opioid receptor gene polymorphisms. Pharmacogenomics J. 2006;6:179–188. doi: 10.1038/sj.tpj.6500355. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindström N, Wennberg P, Hurd YL, Franck J. Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. Clin Psychopharmacol. 2004a;24:665–669. doi: 10.1097/01.jcp.0000144893.29987.e5. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindström N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2004b;33:1856–1863. doi: 10.1038/sj.npp.1301572. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The deltaopioid receptor, isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci USA. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding polydrug use review of heroin and cocaine co-use. Addiction. 2003;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Levran O, Londono D, O’Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Genetic susceptibility to heroin addiction; a candidate-gene association study. Genes Brain Behav. 2008;7:720–729. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J, Sharke C, Wieting J, Oerrtel BG, Schmidt H, Brockmöller J, Geisslinger G. Modulation of the central nervous effects of levomethadone by genetic polymorphisms potentially affecting its metabolism, distribution, and drug action. Pharmacogenomics J. 2006;6:200–210. doi: 10.1016/j.clpt.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Lötsch J, Skarke C, Grösch S, Darimont J, Schmidt H, Geisslinger G. The polymorphism A118G of the human mu-opioid receptor gene decreases the clinical activity of morphine-6-glucuronide but not that of morphine. Pharmacogenetics. 2002;12:3–9. doi: 10.1097/00008571-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA. Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc Natl Acad Sci USA. 2009;106:10847–10852. doi: 10.1073/pnas.0901800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Mathon DS, Vamderschuren LJ, Ramakeres GM. Reduced psychostimulant effects on dopamine dynamics in the nucleus accumbens of mu-opioid receptor knockout mice. Neuroscience. 2006;141:1679–1684. doi: 10.1016/j.neuroscience.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Gershon ES, Simmons S, Ebert M, Kesller LR, Dibble ED, Jimerson SS, Brown GM, Gold P, Jimerson DC, Guroff JJ, Storch FI. Behavioral, biochemical and neuroendocrine responses to amphetamine in normal twins and “well-state“ bipolar patients. Psychoneuroendocrinology. 1982;7:163–176. doi: 10.1016/0306-4530(82)90009-9. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ. Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm Genome. 2005;16:291–305. doi: 10.1007/s00335-004-2451-8. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H. Links Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:511–513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stevens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchinson KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Romberg RR, Olofsen E, Bijl H, Taschner PE, Teppema LJ, Sarton EY, van Kleef JW, Dahan A. Polymorphism of mu-opioid receptor gene (OPRM1,c.118A_G) does not protect against opioid-induced respiratory depression despite reduced analgesic response. Anesthesiology. 2005;102:522–530. doi: 10.1097/00000542-200503000-00008. [DOI] [PubMed] [Google Scholar]
- Romberg R, Olofsen E, Sarton E, Teppema L, Dahan A. Pharmacodynamic effect of morphine-6-glucuronide versus morphine on hypoxic and hypercapnic breathing in healthy volunteers. Anesthesiology. 2003;99:788–798. doi: 10.1097/00000542-200310000-00008. [DOI] [PubMed] [Google Scholar]
- Schinka JA, Town T, Abdullah L, Crawford FC, Ordorica PI, Francis E, Hughes P, Graves AB, Mortimer JA, Mullan M. A functional polymorphism within the mu-opioid receptor gene and risk for abuse of alcohol and other substances. Mol Psychiatry. 2002;7:224–228. doi: 10.1038/sj.mp.4000951. [DOI] [PubMed] [Google Scholar]
- Schuldiner S, Steiner-Mordoch S, Yelin R, Wall SC, Rudnick G. Amphetamine derivatives interact with both plasma membrane and secretory vesicle biogenic amine transporters. Mol Pharmacol. 1993;44:1227–1231. [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test, The quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Skarke C, Jarrar M, Erb K, Schmidt H, Geisslinger G, Lötsch J. Respiratory and miotic effects of morphine in healthy volunteers when P-glycoprotein is blocked by quinidine. Clin Pharmacol Ther. 2003;74:303–311. doi: 10.1016/S0009-9236(03)00220-0. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Abikoff HB, Connor DF, Biedermann J, Pliszka SR, Boellner S, Read CS, Pratt R. Efficacy and safety of mixed amphetamine salts extended release (adderall XR) in the management of oppositional defiant disorder with or without comorbid attention-deficit/hyperactivity disorder in school-aged children and adolescents, A 4-week, multicenter, randomized, double-blind, parallel-group, placebo-controlled, forced-dose-escalation study. Clin Ther. 2006;28:402–418. doi: 10.1016/j.clinthera.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Wallace LJ, Connell LE. Mechanisms by which amphetamine redistributes dopamine out of vesicles, a computational study. Synapse. 2008;62:370–378. doi: 10.1002/syn.20495. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Metabotropic glutamate receptor agonist increases neuropeptide mRNA expression in rat striatum. Mol Brain Res. 1998;54:262–269. doi: 10.1016/s0169-328x(97)00341-0. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:38888. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 2005;2:1225–1212. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


