Abstract
Numerous in vitro studies argue for quercetin’s chemopreventive potential in colon cancer; however experimental studies in rodents are limited. Macrophages play a role in tumorigenesis, but the effects of quercetin on macrophage infiltration in colon cancer is unknown. We examined the effects of quercetin on intestinal polyp multiplicity and macrophage number in ApcMin/+ mice. ApcMin/+ mice were assigned to placebo or quercetin (n=8/group) groups. Mice were given a placebo or quercetin (0.02%) diet from 4–20 wks of age after which intestines were analyzed for polyp number and size in the small intestine (sections 1–4) and colon (section 5) and for macrophage number in the small intestine (section 1 and 3). Spleen weight was determined as a marker of systemic inflammation. Quercetin decreased total intestinal polyps by 67% (p<0.05). Specifically, quercetin reduced intestinal polyps in categories >2 mm (69%) and 1–2 mm (79%) (P<0.05), and in sections 2 (75%), 3 (80%) and 4 (79%) (P<0.05). Quercetin also decreased macrophage number in sections 1 (57%) and 3 (81%), and spleen weight (P<0.05). These data suggest that quercetin can reduce polyp number and size distribution in the ApcMin/+ mouse and that these effects may be related to a reduction in macrophage infiltration.
INTRODUCTION
Bioactive food components offer exciting possibilities for chemoprevention due to their potential to target many factors associated with the development and progression of cancer (1, 2). One important plant-derived dietary component that has exhibited a diverse range of biological properties, including anti-carcinogenic activity, is quercetin (3, 4). Quercetin (3,3’,4’,5,7-pentahydroxylflavone) is a typical flavonoid, the most well defined group of polyphenolic compounds, and is abundant in many commonly consumed fruits and vegetables, particularly apples, cranberries, blueberries, and onions (4). Studies of its effects on cancer have been largely analyzed in cell culture models, where it has been shown that it can inhibit carcinogenesis via anti-mutagenic activity, antioxidant activity, anti-inflammatory mechanisms, modulation of signal transduction pathways, and apoptosis-inducing and anti-proliferative activity (3, 5, 6). While good in vitro evidence supports a beneficial role of quercetin in cancer, in vivo animal studies of carcinogenesis are limited, and there are no experimental studies in humans.
Recent evidence has emerged for macrophages playing an important role in tumor cell invasion into surrounding normal tissues, proliferation and survival, and metastasis to local distant sites (7–10). The mutual interaction of macrophages with cancer cells enhances production of inflammatory cytokines, chemokines, proteases, prostanoids, growth factors and angiogenesis-related factors (10). These substances can transform the tumor microenvironment so that it favors the survival, growth and motility of cancer cells. The anti-carcinogenic effects of quercetin have at least in part been attributed to its ability to modify the macrophage-induced inflammatory response (11, 12). However, there are no studies that have examined the effects of quercetin on macrophage infiltration in any cancer model. Given the important role of macrophages in tumorigenesis and the ability of quercetin to alter macrophage-induced inflammation, it is certainly possible that macrophages may be a target of quercetin in cancer.
Among all cancers the evidence for a relationship between inflammation and colon cancer risk is the strongest (13, 14). The ApcMin/+ mouse model is the most widely used genetically engineered mouse model for cancer studies that involve the gastrointestinal tract (15, 16). It has been shown to have a mutated Apc gene similar to that in patients with familial adenomatous polyposis and in many sporadic cancers. The loss of Apc function has been proven to play a necessary role in colon carcinogenesis. One drawback of this model is that the tumors occur predominately in the small intestine and not the colon, nonetheless the development of polyps in this model is thought to closely mimic the human disease. This mouse model has been shown to be responsive to treatment with anti-inflammatory agents, including both anti-inflammatory dietary supplements as well as non-steroidal anti-inflammatory drugs (NSAIDs) (15, 16). However, there are no studies that have reported a beneficial effect of the anti-inflammatory dietary component quercetin on tumorigenesis in this model or on macrophage infiltration in any cancer model.
The purpose of this study was to examine the effects of quercetin on polyp multiplicity and macrophage number in the ApcMin/+ mouse model of intestinal tumorigenesis. Based on previous in vitro evidence on quercetin’s anti-carcinogenic properties and its ability to modify many of the protumoral processes of macrophages in vitro we hypothesized that quercetin would decrease polyp number and size, and macrophage number in the intestines.
METHODS
Animals
ApcMin/+ male mice on a C57BL/6 background (Jackson Laboratories) were purchased and bred with female C57BL/6 mice in the University of South Carolina's Center for Colon Cancer Research (CCCR). Offspring were genotyped as heterozygotes by RT-PCR for the Apc gene by taking tail snips at weaning. The primer sequences were sense: 5'-TGAGAAAGACAGAAGTTA-3'; and antisense: 5'-TTCCACTTTGGCATAAGGC-3'. Mice were maintained on a 12:12 h light-dark cycle in a low-stress environment (22°C, 50% humidity and low noise) and provided food and water ad libitum. All animal experimentation was approved by the University of South Carolina's Institutional Animal Care and Use Committee.
Quercetin Treatment
Male and female ApcMin/+ mice were randomly assigned to either quercetin or placebo treatment (n=8/group; 4 males and 4 females). The experiment was not set up to allow for comparison across gender; that was not a purpose of this study. Quercetin (Herbal Extracts Plus; Croydon, PA) was incorporated into a grain based diet (Rodent BLTs™ product no. F05072) by Bioserv (Frenchtown, NJ) at a concentration of 0.02%. The dose of quercetin used in this experiment is lower than doses used in earlier studies in rodent models of colon cancer. This dose was selected based on previous evidence from our laboratory involving other effects of quercetin in rodents (17, 18) and clinical trials (19, 20), including increased sirtuin1 (SIRT1) and decreased inflammation, both of which have been linked to a reduction in colon cancer. Placebo mice received an identical diet but without the quercetin. Quercetin feedings began at 4 wks of age and continued for a period of 16 wks, until animals reached 20 wks of age at which time they were sacrificed. Food intake and body weight were measured weekly throughout the treatment period.
Tissue collection
Mice were sacrificed at 20 wks of age for tissue collection. The small intestine was carefully dissected distally to the stomach and proximal to the cecum. The large intestine (section 5) was removed from the distal end of the cecum to the anus. Mesentary tissue was removed with tweezers, and the small intestine was cut into four equal sections (sections 1–4). All intestinal sections were flushed with PBS, opened longitudinally, and flattened with a cotton swab. All sections were fixed in 10% buffered formalin (Fisher Scientific, Pittsburg, PA) for 24 h. Spleens were also harvested and weighed as an indirect measure of inflammation.
Polyp counts
Formalin-fixed intestinal sections from all animals were rinsed in deionized water, briefly stained in 0.1% methylene blue, and counted by the same investigator who was blinded to the treatments. Polyps in each section were counted under a dissecting microscope, using tweezers to pick through the intestinal villi and identify polyps. Polyps were categorized as >2 mm (large), 1–2 mm (medium), and <1 mm (small).
Immunohistochemistry
Formalin-fixed paraffin-embedded intestinal sections (1 and 3) were Swiss-rolled and cut on a microtime in 4-μm sections. Sections were deparrafinized in xylene and rinsed in 100% ethanol. A cell and tissue staining kit (R&D Systems, Minneapolis MN) was used to stain sections with F4/80 antibody (Serotec, Raleigh NC). The kit used the Avidin-Biotin Complex (ABC) to detect F4/80 and 3,3’ diaminobenzidine (DAB) to visualize macrophages. Briefly, peroxidase activity was squelched with 3% H2O2 for 5 min. Sections were blocked for 15 min in rat serum. Slides were incubated with the Avidin and Biotin blocking reagents for 15 min each, then 1:50 with F4/80 for 2 hr. Slides were rinsed in PBS and incubated with anti-rat secondary biotinylated antibody for 30 min. Sections were incubated with high sensitivity streptavidin conjugated to HRP for 30 min and then stained with DAB chromagen for 2 min. Sections were then stained with hematoxylin for 90 sec and rinsed for 20 sec. For analyses, F4/80 positive cells were counted in 10 villi per animal and averaged for each animal.
Statistical Analysis
Data were analyzed using a one-way ANOVA with Student-Neuman-Keuls post hoc analysis. All data were analyzed using commercial software (SigmaStat, SPSS, Chicago, IL). Statistical significance was set with an alpha value of P<0.05. Data are presented as mean (±SEM).
RESULTS
Body weight and food intake
Food intake and body weight were measured weekly throughout the treatment period. There was no difference in body weight or food intake between the placebo and quercetin group which suggests that the 0.02% quercetin diet was well tolerated. Initial body weights (4wks) were 12.6 ± 0.5 and 13.3 ± 0.5g and final body weights (20wks) were 28.6 ± 2.2 and 28.3 ± 3.6g for placebo and quercetin, respectively. Food consumption at 4wks was 3.4 ± 0.2 and 3.1 ± 0.4g and at 20 wks was 4.0 ± 0.1 and 4.3 ± 0.1g in placebo and quercetin groups, respectively. The approximate daily consumption of quercetin was 0.6 – 0.9 mg.
Polyp incidence
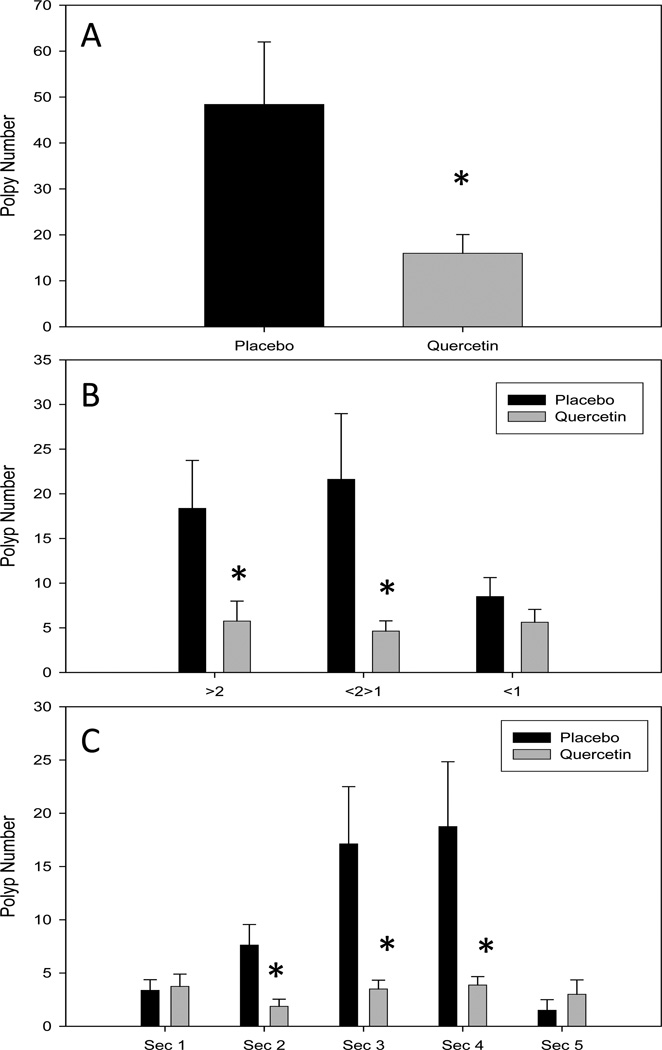
Quercetin feedings for 16 wks (age 4 wks to 20 wks) resulted in a 67% decrease in total polyp number compared with mice fed a placebo diet (48.4 ± 13.6 versus 16 ± 4.1; P <0.05) (Fig 1A). Specifically, quercetin feedings significantly reduced polyp size in categories >2 mm (large) and 1–2 mm (medium) (P<0.05) but not in <1 mm (small) (Fig 1B). Large polyps (>2-mm diameter) were reduced by 69% (18.4 ± 5.4 versus 5.8 ± 2.2; P <0.05) and medium polyps (<2 >1-mm diameter) were reduced by 79% (21.6 ± 7.3 versus 4.6 ± 1.1; P <0.05). Small polyps (<1-mm diameter) were reduced by 34% (8.5 ± 2.1 versus 5.6 ± 1.4), however this did not reach statistical significance. Polyp numbers were also analyzed across intestinal section. Quercetin significantly reduced polyp number in sections 2, 3 and 4 but not in sections 1 and 5 (Fig 1C). Section 2 polyps were reduced by 75% (7.6 ± 1.9 versus 1.9 ± 0.7; P <0.05), section 3 polyps by 80% (17.1 ± 5.4 versus 3.5 ± 0.8; P <0.05) and section 4 polyps by 79% (18.8 ± 6.1 versus 3.9 ± 0.8; P <0.05).
Figure 1.
Quercetin reduces (A) total intestinal polyps, (B) large and medium polyps and (C) region specific polyps (sections 2–4) in the ApcMin/+ mouse model of intestinal tumorigenesis (n=8/group). Mice were fed a placebo or quercetin (0.02%) diet from 4 wks to 20 wks of age. Following the treatment period mice were sacrificed and polyps were counted. * Significantly different from Placebo P<0.05.
Macrophage Number
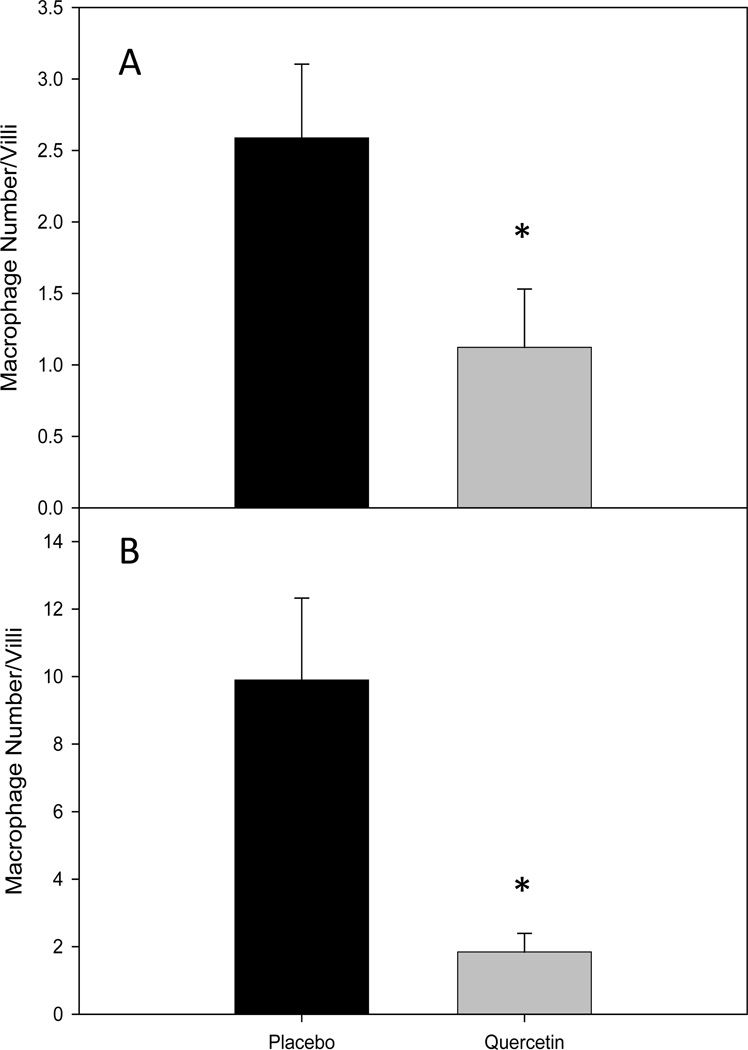
The effect of quercetin feedings on macrophage number in the intestinal villi was identified by F4/80 immunohistochemistry in sections 1 and 3 of the small intestine. Regions 1 and 3 were chosen so as to represent a section of the intestine with low and high polyp incidence, respectively. Quercetin feedings significantly reduced macrophage number in intestinal villi in both regions 1 (2.6 ± 0.5 versus 1.1 ± 0.4; P <0.05) (Fig 2A) and 3 (9.9 ± 2.4 versus 1.8 ± 0.5; P <0.05 (Fig 2B) of the small intestine.
Figure 2.
Quercetin reduces macrophage number in intestinal villi in sections (A) 1 and (B) 3 of the small intestine in the ApcMin/+ mouse model of intestinal tumorigenesis (n=8/group). Mice were fed a placebo or quercetin diet from 4 wks to 20 wks of age. Following the treatment period mice were sacrificed and intestinal sections 1 and 3 were stained for macrophages using F4/80 Ab. * Significantly different from Placebo P<0.05.
Spleen Weight
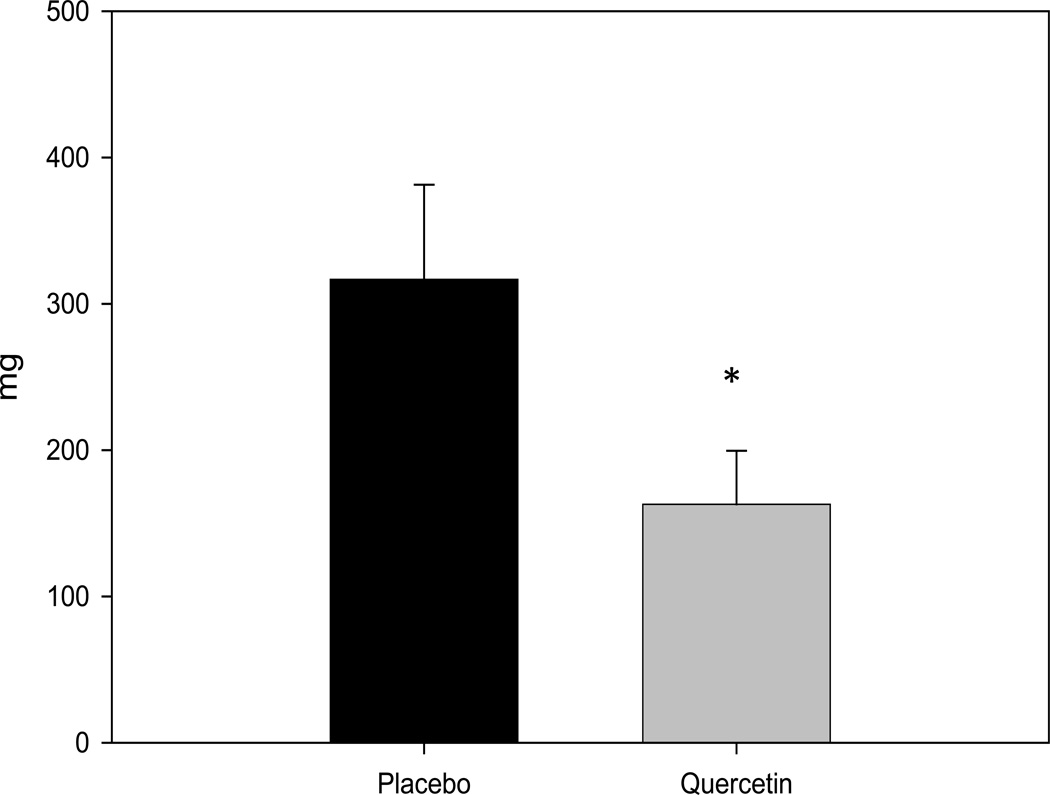
The effect of quercetin on spleen weight was also determined as an indirect measure of inflammation; spleen weight has been reported to be increased following infection and inflammation. We have previously reported that a reduction in spleen weight was associated with reduced IL-6 in male ApcMin/+ mice following exercise (21). Our findings indicate that quercetin feedings can decrease spleen weight in ApcMin/+ mice (P<0.05); placebo mice had an average spleen weight of 317 ± 65 mg whereas spleens from mice fed quercetin weighed only 163 ± 36 mg.
DISCUSSION
Bioactive food components, such as the dietary flavonoid quercetin, have received increased interest in recent years due to their ability to target multiple factors involved in the development and progression of tumorigenesis (1–3, 5, 6). One such property of quercetin is its ability to reduce the inflammatory response of macrophages (11, 12), a function that contributes largely to the protumoral processes of this cell. However, there have been few in vivo rodent studies that have examined the effects of quercetin on tumorigenesis in a colon cancer model and those that are available report inconsistent findings (22–25). Further, there have been no studies that have specifically examined the effects of quercetin on macrophage number in a rodent model of colon cancer. This study used a well established mouse model of intestinal tumorigenesis to examine the effects of the anti-inflammatory dietary flavonoid quercetin on polyp multiplicity, and macrophage number in the intestines. Our findings indicate that a 0.02% quercetin diet can reduce polyp number and size as well as macrophage number in the intestines of the ApcMin/+ mouse
While good in vitro evidence supports a beneficial role of quercetin in cancer, in vivo animal studies of colon carcinogenesis are limited. Further, there are some inconsistencies among the available findings. It was reported that a 3% quercetin diet resulted in a 4-fold reduction in aberrant crypt foci (ACF) in an azoxymethane (AOM) rat colon cancer model that was consistent with an induction of apoptosis (25). Similarly, Deschner et al. reported that a 2% quercetin diet inhibited AOM-induced hyperproliferation in mice and reduced colonic tumor incidence by 76% and tumor multiplicity by 48% (24). To date there has only been one reported study that has examined the effects of quercetin on intestinal tumorigenesis in ApcMin/+ mice (22). Mahmoud et al. fed ApcMin/+ mice a 2% quercetin diet for a duration of 10 wks and reported no beneficial effects on tumor number or distribution (22). This is inconsistent with the findings of our study that show a 59% reduction in intestinal polyps in the ApcMin/+ mouse following a much lower dose of quercetin (0.02%). Our data also indicate that quercetin can decrease the overall number of large (>2mm) and medium (<2>1mm) polyps as well as polyp number in intestinal regions 2–4. There was no effect of quercetin in regions 1 and 5, however this is not surprising as these are regions of low polyp multiplicity. It is possible that the low polyp incidence in these regions may preclude any observable benefit of quercetin on polyp number. This inconsistency between our findings and that of Mahmoud et al. may be due to several factors including the dose of quercetin (0.02% versus 2%), timing of administration (feedings from 4–20 wks of age versus 5–15 wks of age), gender used (males and females versus females only) and overall tumor burden reported in the placebo groups (48 ± 14 versus 33 ± 5). This highlights the need for dose response studies to further evaluate the most effective dose of quercetin and appropriate timing of administration in this model.
The exact mechanism whereby quercetin exerts its chemopreventive potential is not yet known. However, it is likely to involve macrophages, at least in part. Macrophages play a pivotal role in the initiation and promotion of tumorigenesis and the progression of malignant cells (7, 26, 27). They are key effector cells in the tumor microenvironment, producing growth stimulators and inhibitors, proteolytic enzymes, and cytokines that modify the extracellular matrix and regulate angiogenesis (28). These macrophages are referred to as tumor-associated macrophages (TAMs), most of which are derived from peripheral blood monocytes recruited into the tumor mass from circulation (29). In vitro studies support a role for quercetin on macrophages. For example De Stefano et al., has shown that quercetin can inhibit NFkB activation in the RAW 264.7 macrophage cell line (11). Similarly, quercetin has been reported to inhibit LPS-induced pro-inflammatory cytokine expression in RAW 264.7 cells via NFkB mediated mechanisms (30). It has also been shown that macrophages may be a target for quercetin; Kawai et al. reported that quercetin metabolites accumulate in macrophages where they exert their biological activity (31). Our data show that quercetin feedings can result in a reduction in macrophage number in the intestinal villi. However, the decrease in macrophage number in section 1 was not associated with decreased tumorigenesis; given the low incidence of polyps (~ 3) that generally occur in section 1 this result is not surprising. To our knowledge these are the first data to show a quercetin-induced reduction in macrophage number in this cancer model or any other cancer model. These findings are consistent with Camuesco et al. that show a reduction in macrophage number and associated inflammation in a rat model of inflammatory bowl disease (IBD) following treatment with a glycoside formed from quercetin (quercetrin) (32).
Although quercetin is likely to at least in part mediate its chemopreventive effects through macrophages, it is important to point out that given its wide range of biological properties, its anti-carcinogenic effects are likely to involve multiple bioactive targets (4). In addition to its ability to reduce macrophage-induced inflammation in vitro, evidence has shown that quercetin can inhibit carcinogenesis via anti-mutagenic activity, antioxidant activity, modulation of signal transduction pathways, and apoptosis-inducing and anti-proliferative activity. Quercetin’s anti-mutagenic activity has been shown against aflatoxin B1 (AFB1) and 2-acetamido-flurene (2-AAF)-induced mutagenesis (33). Its potent antioxidant activity is likely to reduce reactive oxygen species (ROS) produced in cancer cells which has been linked to genomic instability and cancer initiation, progression and maintenance (4). Further, quercetin has been reported to induce apoptotic cell death through the mitochondrial pathway (34) and down-regulate the expression of various oncogenes (35). Therefore it is not appropriate to ascribe a role of macrophages independent of these other properties.
The beneficial effects of a bioactive food component like quercetin are largely dependent on bioavailability, absorption, distribution, metabolism, and excretion following oral administration. We have recently reported that quercetin can be detected in human plasma within 15–30 min of ingesting of a 250 or 500 mg dose, reaching a peak concentration at approximately 120–180 min, returning to baseline levels at 24 hr (36). Our results are consistent with others that have measured quercetin absorption in the plasma following ingestion of the pure quercetin aglycone as well as various gluconated forms contained in foods (37). Quercetin has also been shown to reach and accumulate in various tissues including colon, kidney, liver, lung, muscle and brain (38). While it is unlikely that the equivalent human dose used in these experiments (~1000mg/day) would be consumed in the diet (e.g. one apple contains ~10mg of quercetin) this is certainly a reasonable dose for supplemental administration and has been determined to be safe (4).
In summary, the dietary flavonoid quercetin has well documented anti-carcinogenic effects in vitro. However, to our knowledge these are the first data to show a benefit of the quercetin on polyp number and size distribution in the ApcMin/+ mouse model of intestinal tumorigenesis. Further, these are the first data in any cancer model to report a quercetin-induced reduction in macrophage number in the intestinal villi as a potential mechanism for its effects on tumorigenesis. However, additional dose response studies are necessary to truly evaluate the potential benefit of dietary quercetin in colon cancer and to further understand its specific effects on macrophages in this model.
Figure 3.
Quercetin decreases spleen weight in the ApcMin/+ mouse. Mice were fed a placebo or quercetin diet from 4 wks to 20 wks of age. Following the treatment period mice were sacrificed and spleens were harvested and weighed. * Significantly different from Placebo P<0.05.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Cancer Institute (R21 CA135377). We would like to thank Dr. Franklin Berger, Director at the Center for Colon Cancer Research at the University of South Carolina for kindly providing the ApcMin/+ mice used in this study.
Footnotes
No portion of the work has is currently under consideration for publication elsewhere.
REFERENCES
- 1.Kim YS, Young MR, Bobe G, Colburn NH, Milner JA. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prev Res (Phila Pa) 2009 Mar;2:200–208. doi: 10.1158/1940-6207.CAPR-08-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Tharakan ST, Sung B, Anand P. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008 Oct;74:1560–1569. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- 3.Gerhauser C. Cancer chemopreventive potential of apples, apple juice, and apple components. Planta Med. 2008 Oct;74:1608–1624. doi: 10.1055/s-0028-1088300. [DOI] [PubMed] [Google Scholar]
- 4.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007 Nov;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Gerhauser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, Knauft J, Liu GY, Sitthimonchai S, Frank N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat Res. 2003 Feb-Mar;523–524:163–172. doi: 10.1016/s0027-5107(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 6.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008 Oct 8;269:315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007 May;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of pathology. 2002 Mar;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 9.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000 Aug;6:3282–3289. [PubMed] [Google Scholar]
- 10.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008 Aug;99:1501–1506. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Stefano D, Maiuri MC, Simeon V, Grassia G, Soscia A, Cinelli MP, Carnuccio R. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-gamma. European journal of pharmacology. 2007 Jul 2;566:192–199. doi: 10.1016/j.ejphar.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 12.Nam NH. Naturally occurring NF-kappaB inhibitors. Mini reviews in medicinal chemistry. 2006 Aug;6:945–951. doi: 10.2174/138955706777934937. [DOI] [PubMed] [Google Scholar]
- 13.Fantini MC, Pallone F. Cytokines: from gut inflammation to colorectal cancer. Curr Drug Targets. 2008 May;9:375–380. doi: 10.2174/138945008784221206. [DOI] [PubMed] [Google Scholar]
- 14.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008 Jan 21;14:378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corpet DE, Pierre F. Point: From animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003 May;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 16.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, Williams ML, Steward WP, Gescher AJ. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002 Jun;11:535–540. [PubMed] [Google Scholar]
- 17.Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009 Feb 11; doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- 18.Davis JM, Murphy EA, McClellan JL, Carmichael MD, Gangemi JD. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am J Physiol Regul Integr Comp Physiol. 2008 Aug;295:R505–R509. doi: 10.1152/ajpregu.90319.2008. [DOI] [PubMed] [Google Scholar]
- 19.Davis JM, Carlstedt CJ, Chen S, Carmichael MD, Murphy EA. The dietary flavonoid quercetin increases VO(2max) and endurance capacity. Int J Sport Nutr Exerc Metab. Feb;20:56–62. doi: 10.1123/ijsnem.20.1.56. [DOI] [PubMed] [Google Scholar]
- 20.Nieman DC, Henson DA, Davis JM, Angela Murphy E, Jenkins DP, Gross SJ, Carmichael MD, Quindry JC, Dumke CL, et al. Quercetin's influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J Appl Physiol. 2007 Nov;103:1728–1735. doi: 10.1152/japplphysiol.00707.2007. [DOI] [PubMed] [Google Scholar]
- 21.Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol. 2005 Jun;98:2219–2225. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 22.Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, Newmark HL, Bertagnolli MM. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000 May;21:921–927. doi: 10.1093/carcin/21.5.921. [DOI] [PubMed] [Google Scholar]
- 23.Femia AP, Caderni G, Ianni M, Salvadori M, Schijlen E, Collins G, Bovy A, Dolara P. Effect of diets fortified with tomatoes or onions with variable quercetin-glycoside content on azoxymethane-induced aberrant crypt foci in the colon of rats. Eur J Nutr. 2003 Dec;42:346–352. doi: 10.1007/s00394-003-0431-5. [DOI] [PubMed] [Google Scholar]
- 24.Deschner EE, Ruperto J, Wong G, Newmark HL. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis. 1991 Jul;12:1193–1196. doi: 10.1093/carcin/12.7.1193. [DOI] [PubMed] [Google Scholar]
- 25.Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin) Carcinogenesis. 2005 Aug;26:1450–1456. doi: 10.1093/carcin/bgi089. [DOI] [PubMed] [Google Scholar]
- 26.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004 Jan;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006 Jun;97:439–447. doi: 10.1111/j.1349-7006.2006.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer research. 2004 Oct 1;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 29.Stephens TC, Currie GA, Peacock JH. Repopulation of gamma-irradiated Lewis lung carcinoma by malignant cells and host macrophage progenitors. British journal of cancer. 1978 Nov;38:573–582. doi: 10.1038/bjc.1978.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, Jeong WI, Ryu SY, Do SH, et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Molecular and cellular biochemistry. 2003 Jan;243:153–160. doi: 10.1023/a:1021624520740. [DOI] [PubMed] [Google Scholar]
- 31.Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, Kobayashi M, Kanayama M, Uchida K, Terao J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. The Journal of biological chemistry. 2008 Apr 4;283:9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- 32.Camuesco D, Comalada M, Rodriguez-Cabezas ME, Nieto A, Lorente MD, Concha A, Zarzuelo A, Galvez J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol. 2004 Dec;143:908–918. doi: 10.1038/sj.bjp.0705941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snijman PW, Swanevelder S, Joubert E, Green IR, Gelderblom WC. The antimutagenic activity of the major flavonoids of rooibos (Aspalathus linearis): some dose-response effects on mutagen activation-flavonoid interactions. Mutation research. 2007 Jul 28;631:111–123. doi: 10.1016/j.mrgentox.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Granado-Serrano AB, Martin MA, Bravo L, Goya L, Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2) J Nutr. 2006 Nov;136:2715–2721. doi: 10.1093/jn/136.11.2715. [DOI] [PubMed] [Google Scholar]
- 35.Ranelletti FO, Maggiano N, Serra FG, Ricci R, Larocca LM, Lanza P, Scambia G, Fattorossi A, Capelli A, Piantelli M. Quercetin inhibits p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int J Cancer. 2000 Feb 1;85:438–445. [PubMed] [Google Scholar]
- 36.Davis JM, Murphy EA, Carmichael MD. Effects of the dietary flavonoid quercetin upon performance and health. Curr Sports Med Rep. 2009 Jul-Aug;8:206–213. doi: 10.1249/JSR.0b013e3181ae8959. [DOI] [PubMed] [Google Scholar]
- 37.Egert S, Wolffram S, Bosy-Westphal A, Boesch-Saadatmandi C, Wagner AE, Frank J, Rimbach G, Mueller MJ. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J Nutr. 2008 Sep;138:1615–1621. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- 38.de Boer VC, Dihal AA, van der Woude H, Arts IC, Wolffram S, Alink GM, Rietjens IM, Keijer J, Hollman PC. Tissue distribution of quercetin in rats and pigs. J Nutr. 2005 Jul;135:1718–1725. doi: 10.1093/jn/135.7.1718. [DOI] [PubMed] [Google Scholar]