Abstract
Programmed death ligand 1 (PDL1, or B7-H1) is expressed constitutively or is induced by IFN-γ on the cell surface of most human cancer cells and acts as a “molecular shield” by protecting tumor cells from T cell-mediated destruction. Using seven cell lines representing four histologically distinct solid tumors (lung adenocarcinoma, mammary carcinoma, cutaneous melanoma, and uveal melanoma), we demonstrate that transfection of human tumor cells with the gene encoding the costimulatory molecule CD80 prevents PDL1-mediated immune suppression by tumor cells and restores T cell activation. Mechanistically, CD80 mediates its effects through its extracellular domain, which blocks the cell surface expression of PDL1 but does not prevent intracellular expression of PDL1 protein. These studies demonstrate a new role for CD80 in facilitating antitumor immunity and suggest new therapeutic avenues for preventing tumor cell PDL1-induced immune suppression.
Tumor-induced immune suppression is a major obstacle for therapies aimed at activating an individual’s immune system to eliminate autologous cancer cells. This immune suppression can be mediated by nonmalignant host cells such as T regulatory cells (1) and myeloid-derived suppressor cells (2) that are driven by tumor-secreted factors, as well as by malignant cells that express immune inhibitory molecules. Programmed death ligand 1 (PDL1, also known as B7 homolog 1 [B7-H1] or CD274) is such an inhibitory molecule that is either induced or constitutively expressed by many malignant cells (3–6). Tumor cell-expressed PDL1 facilitates tumor progression by at least two mechanisms: (i) tumor-reactive T cells are tolerized by PDL1 binding to its receptor programmed death 1 (PD1; CD279) on T cells (3, 7); and (ii) tumor cells are rendered resistant to T cell-mediated and FasL-mediated lysis by PD1 signaling through tumor cell-expressed PDL1 (8). PDL1 may also tolerize tumor-reactive T cells by reverse signaling through CD80 on T cells, as has been shown in in vitro T cell activation systems (9) and in vivo in an oral tolerance system (10). Regardless of the mechanism by which tumor cell-expressed PDL1 promotes tumor growth, blocking PDL1–PD1 interactions with anti-PDL1 or PD1 Abs improves activation of tumor-reactive T cells and reduces tumor progression (4, 11–13), confirming that tumor cell-expressed PDL1 is a major obstacle for cancer immunotherapies.
Many cancer immunotherapies are designed to stimulate the production of cytokines and chemokines that mobilize tumor-reactive, cell-mediated effector cells such as T lymphocytes, NK cells, and M1 macrophages (14–17). A key cytokine in this process is IFN-γ, which polarizes macrophages toward an M1 phenotype (18), activates NK cells (19), and facilitates the development of type 1 helper CD4+ T cells and cytotoxic CD8+ T cells (20). However, IFN-γ is also a potent inducer of PDL1 (21). Therefore, cancer immunotherapies that optimally activate desirable effector cells may concomitantly upregulate tumor cell expression of PDL1 and thereby limit their own effectiveness.
We have generated tumor cell-based cancer vaccines that induce high levels of IFN-γ and are potent activators of tumor-reactive T cells. Our “MHC class II” vaccines were designed to activate tumor-reactive type 1 CD4+ Th cells and consist of tumor cells transfected/transduced with genes encoding the costimulatory molecule CD80 and syngeneic HLA-DR molecules. In vitro studies with three human solid tumor cell lines demonstrated that the MHC class II (MHC II) vaccines are potent activators of a diverse repertoire of tumor-reactive and tumor-specific CD4+ T cells (22–25), and in vivo studies with three mouse tumor systems confirmed that the vaccines prolong survival of mice with established primary and/or metastatic disease (26). In contrast to accepted dogma (27, 28), T cell activation and tumor rejection required vaccine cell expression of CD80 in the boost stage, suggesting that CD80 was playing a role other than as a costimulatory molecule to prime naive T cells. We now report that CD80 also prevents constitutive and IFN-γ–induced tumor cell expression of PDL1 and thereby facilitates tumor immunity by inhibiting PDL1-mediated immune suppression.
Materials and Methods
Cell lines
Primary uveal melanoma cell lines MEL202 and MEL270, metastatic uveal melanoma line OMM2.3 (29), and cutaneous melanoma cell lines MEL1011 (30), C8161 (31), and 624MEL (32) were obtained from the cited sources except for 624MEL and C8161, which were obtained from F. Marincola (National Cancer Institute, National Institutes of Health) and E. Seftor (Children’s Memorial Research Center, Chicago, IL), respectively. These lines were cultured in RPMI 1640 (BioSource, Rockville, MD) supplemented with 10% heat-inactivated FCS (Hyclone, Logan, UT), 10 mM HEPES (Invitrogen, Grand Island, NY), 1% penicillin/streptomycin (BioSource, Rockville, MD), 1% 2-mercaptoethanol (VWR, West Chester, PA), 2 mM Glutamax (BRL/Life Sciences, Grand Island, NY), 0.1% gentamicin (BioSource), and 5 µg/ml Prophylactic Plasmocin (InvivoGen, San Diego, CA). MCF10CA1 (hereafter called MCF10) (33) mammary carcinoma and bronchioloalveolar adenocarcinoma H358 (34) cell lines were obtained from the American Type Culture Collection and cultured as described (25, 35) except 5 µg/ml Prophylactic Plasmocin was included in the media. Human mature dendritic cells (mDCs) were generated as described (36) from PBMCs derived from apheresis of healthy donors, except media was supplemented with 10% human AB serum instead of autologous serum. Cell lines and procedures with human materials were approved by the institutional review boards of the participating institutions.
IFN-γ treatment
Cells (3 × 105/8 ml/T25 flask) were incubated at 37°C for 48 h in their culture medium supplemented with 50–100 U/ml recombinant human IFN-γ (Pierce Biotechnology, Rockford, IL) and then washed with excess culture medium.
Plasmids, transfections, and transfectants
The human CD80 molecule (accession no. P33681; www.uniprot.org/uniprot/P33681) contains 288 aa of which the C-terminal ~21 aa constitute the cytoplasmic domain (37–39) (structure prediction programs at http://ca.expasy.org/tools/). The CD80tr construct was generated from the pLHCX/CD80 plasmid (22) construct by PCR using the primers 5′-AAAAGGATCCTATGGGCCACACACGGAGG-3′ and 5′-AGCGAAGCTTTTATGGGGCAAAGCAGTA-3′, which amplified all but the 19 aa of the C-terminal cytoplasmic domain. The final construct was confirmed by DNA sequence analysis. To facilitate construction of additional C-terminal variants of CD80, the CD80tr construct was modified to include a BsmI site overlapping the terminal proline codon. The HLA-DRα–chain cytoplasmic region was added to this modified construct by ligation of the double-stranded oligonucleotide adapter 5′-GGAGTGCGCAAAAGCAATGCAGCAGAACGCAGGGGGCCTCTGTAA-3′ and 5′-AGCTTTACAGAGGCCCCCTGCGTTCTGCTGCATTGCTTTTGCGCACTCCCG-3′ to a BsmI-digested CD80tr construct. The resulting fusion molecule (CD80DR) contains the C-terminal sequence NH2-GVRKSNAAERRGPL-COOH and was confirmed by DNA sequence analysis. The pCMV6-AC-PDL1-GFP (PDL1-GFP) plasmid was obtained from Origene (Rockville, MD).
MEL202, MEL202/DR1, MEL202/CD80, MEL202/DR1/CD80, MCF10, MCF10/DR7/CD80, H358, H358/CD80, H358/DR7, and H358/DR7/CD80 were described previously (22, 23, 25, 35). Stable C8161/CD80 and 624MEL/CD80 transfectants were generated by Amaxa nucleofection with the pLHCX/CD80 plasmid (22) and were selected and maintained in media supplemented with 400 µg/ml hygromycin (Calbiochem, San Diego, CA). C8161/CD80tr, C8161/CD80DR, and MEL202/DR1/CD80/PDL1-GFP transient transfectants were generated as per stable transfectants but were not drug selected and were used 48 h after transfection.
Abs and flow cytometry
Flow cytometry Abs CD80–FITC (clone L307.4), PD1–PE (clone MIH4), PDL1–PE (clone MIH1), mouse IgG1–FITC (clone X40), and mouse IgG1–PE (clone MOPC-21) mAbs were from BD Pharmingen (San Diego, CA). PDL1–allophycocyanin (clone 29E.2A3), PDL1–PE (clone 27A2), and mouse IgG2b–allophycocyanin were from BioLegend (San Diego, CA), Medical & Biological Laboratories (Woburn, MA), and eBioscience (San Diego, CA), respectively. Cells were stained for cell surface and intracellular expression by flow cytometry as described (22, 24) and analyzed using a Beckman Coulter Cyan ADP flow cytometer and Summit V4.3.02 software.
PCR
For RT-PCR, total RNA was isolated using an RNeasy Mini kit according to the manufacturer’s directions (Qiagen), and 1.3 µg RNA/sample was amplified using a one-step RT-PCR kit (Qiagen). PDL1 forward primer, 5′-AAACAATTAGACCTGGCTG-3′, and PDL1 reverse primer, 5′-TCTTACCACTCAGGACTTG-3′. cDNA was amplified in a PTC-200 Peltier Thermal Cycler (MJ Research) under the following conditions: reverse transcription for 30 min at 50°C, denature for 15 min at 95°C, 40 cycles of 94°C for 30 s, 50°C for 30 s, 72°C for 1 min, followed by a final extension at 72°C for 10 min. PCR products were analyzed on a 1.0% agarose gel stained with ethidium bromide (BioRad, Hercules, CA).
For quantitative PCR (qPCR), 1.0 µg RNA/sample was amplified using the Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies, Santa Clara, CA) in a Step One Real Time PCR System (Applied Biosystems, Foster City, CA). PDL1 forward primer, 5′-TATGGTGGTGCCGACTACAA-3′, and PDL1 reverse primer, 5′-TGGCTCCCAGAATTACCAAG-3′; 18S forward primer, 5′-ACCGATTGGATGGTTTAGTGAG-3′, and 18S reverse primer, 5′-CCTACGGAAACCTTGTTACGAC-3′ (Metabion, Martinsried, Germany). Samples were denatured for 15 min at 95°C, subsequently 40 cycles including denaturation at 95°C for 5 s and annealing at 60°C for 15 s, followed by continuous fluorescence measurement during heating from 60°C to 90°C (0.1°C/s). 18S to PDL1 signal ratios are presented.
Western blots
Western blots were performed as described (22) with the following modifications. Cell lysates were resuspended in sample buffer without 2-mercaptoethanol. After electrophoresis on 10% SDS-PAGE gels, proteins were transferred to polyvinylidene difluoride membranes (Amersham, Piscataway, NJ) using a Bio-Rad PowerPac HC (100 V for 70 min) and blocked with 4% nonfat dry milk in TBST. PDL1 and actin were detected using 1 µg/ml PDL1 mAb (clone MIH1; eBioscience) and 0.05 µg/ml β-actin mAb, respectively, followed by 1:5000 dilution of goat anti-mouse HRP (BD Biosciences).
PBMC activation
PBMCs were obtained and then primed and boosted with vaccine cells as described (22, 23) except they were expanded for 7 d with IL-15 (20 ng/ml; PeproTech, Rocky Hill, NJ). Alternatively, PBMCs (1 × 105/200 µl) were cocultured with 5 µg/ml PHA (Sigma-Aldrich, St. Louis, MO) and irradiated tumor cells (C8161 and C8161/CD80, or MEL202 and MEL202/CD80, 5000 and 10,000 rads, respectively) at 37°C, 5% CO2 for 72 h. Recombinant human and mouse PD1–Fc fusion proteins (R&D Systems) were added to some coculture experiments. IFN-γ production was measured by ELISA (23).
Microscopy
Tumor cells were cultured in 6-well plates for 48 h after transfection, then washed with excess PBS containing 2% FCS (2% PBS), labeled with CD80-PE*Cy7 (clone 2D10) for 30 min, and again washed with excess 2% PBS. Live fluorescence images were captured with an Olympus IX-81 (Olympus, Center Valley, PA) microscope using Slidebook software (Intelligent Imaging Innovations, Denver, CO). A minimum of 150 cells/sample was counted.
Statistical analysis
SD and Student t test were calculated using Microsoft Excel version 2008. Mann–Whitney U test was performed using http://faculty.vassar.edu/lowry/VassarStats.html.
Results
Tumor cell expression of CD80 facilitates the boosting of primed T cells
Priming of naive T cells by professional APCs requires two signals, an Ag-specific signal delivered by a peptide–MHC complex and a costimulatory signal. Boosting of primed T cells by professional APCs requires an Ag-specific signal but does not require a costimulatory signal (40). To determine if costimulation is optional in the boosting phase of CD4+ T cells when tumor cells are the APCs, we used uveal melanoma (MEL202) and lung adenocarcinoma (H358) tumor cells transfected with genes encoding HLADR and the costimulatory molecule CD80 (B7.1; MEL202/DR1/CD80 and H358/DR7/CD80, respectively). Because of their ability to activate tumor-reactive CD4+ T cells that do not react with nonmalignant tissue, we have called these genetically modified tumor cells “MHC II vaccines” (22, 25, 41). Priming and boosting HLA-DR7+ or HLA-DR1+ PBMCs with HLA-DR syngeneic MHC II lung adenocarcinoma (Fig. 1A) or uveal melanoma vaccine cells (Fig. 1B), respectively, yielded high levels of IFN-γ. In contrast, priming with syngeneic HLA-DR+CD80+ tumor cells and boosting with HLA-DR+ tumor cells lacking CD80 induced significantly less IFN-γ. Therefore, if APCs are tumor cells, then costimulation in the boosting phase is essential for optimal T cell activation.
FIGURE 1.
Tumor cell expression of CD80 facilitates the boosting of primed T cells. A, PBMCs from an HLA-DR7+ healthy donor were primed in vitro with irradiated H358 lung adenocarcinoma cells transduced with HLA-DR7 and CD80 (H358/DR7/CD80) and boosted with either H358/DR7/CD80, single gene transductants (H358/DR7 or H358/CD80), or parental cells. B, PBMCs from an HLA-DR1+ healthy donor were primed with irradiated uveal melanoma cells transduced with HLA-DR1 and CD80 (MEL202/DR1/CD80) and boosted with either MEL202/DR1/CD80, single gene transductants (MEL202/DR1 or MEL202/CD80), or parental cells. IFN-γ production was measured by ELISA. Data are representative of three independent experiments.
Expression of PDL1 on the cell surface of tumor cells is reduced by transfection of HLA-DR and CD80
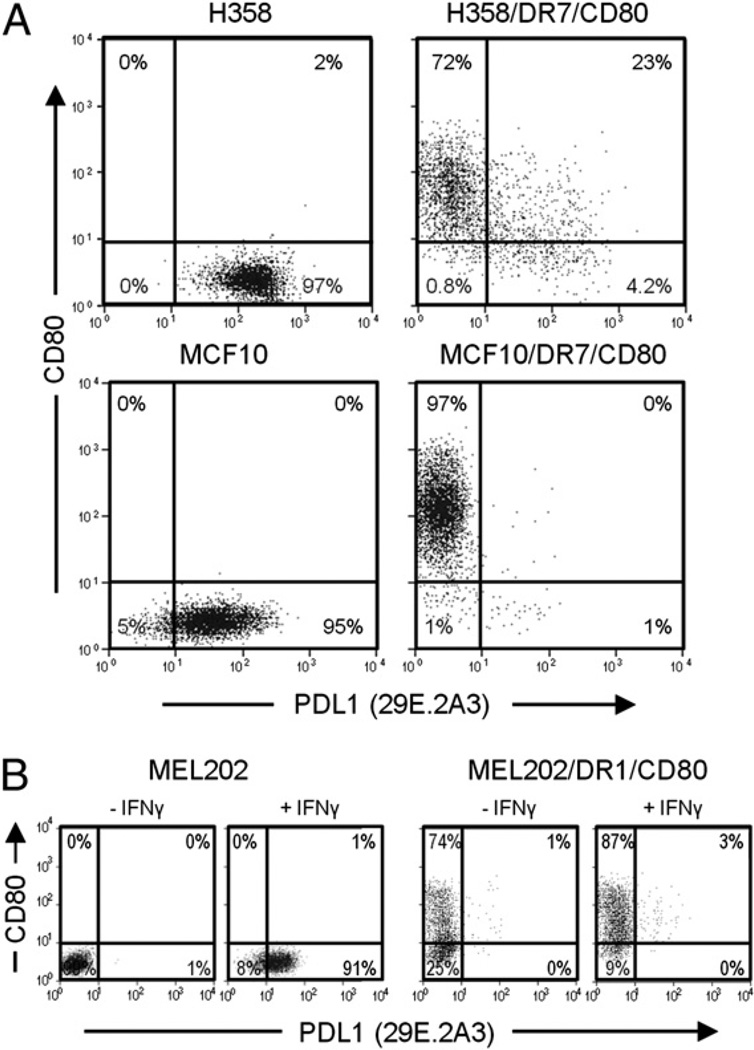
Many tumor cells constitutively express or are induced by IFN-γ to express PDL1 on their cell surface (21), raising the possibility that MHC II vaccines may induce T cell apoptosis due to PDL1 expression. To assess this possibility, H358/DR7/CD80 and a mammary carcinoma MHC II vaccine (MCF10/DR7/CD80) (24, 35) and their parental cells were stained for CD80 and PDL1 (mAb 29E.2A3) and analyzed by flow cytometry (Fig. 2A). Parental H358 and MCF10 cells constitutively express PDL1; however, H358/DR7/CD80 and MCF10/DR7/CD80 vaccine cells did not have detectable PDL1. To confirm that the lack of PDL1 on the vaccine cells was not a peculiarity of the 29E.2A3 PDL1 mAb, H358 and H358/DR7/CD80 cells were stained with two other PDL1 mAbs (mAbs MIH1 and 27A2) (Supplemental Fig. 1A). As for the MIH1 and 27A2 mAbs, most vaccine cells did not express PDL1, whereas most parental cells constitutively expressed PDL1. To determine if MHC II vaccine cells were refractory to IFN-γ induction of PDL1, uveal melanoma MEL202 and its corresponding vaccine (MEL202/DR1/CD80) were cultured in IFN-γ and subsequently stained for CD80 and PDL1 (mAb 29E.2A3) (Fig. 2B). IFN-γ treatment induced cell surface expression of PDL1 on MEL202 cells, whereas MEL202/DR1/CD80 cells did not have detectable cell surface PDL1. To ascertain that the absence of cell surface PDL1 on IFN-γ–treated MEL202/DR1/CD80 cells was not a peculiarity of the 29E.2A3 mAb, parental and vaccine cells were also stained with the MIH1 and 27A2 mAbs to PDL1 (Supplemental Fig. 1B). In agreement with Fig. 2B, cell surface PDL1 was not detected. PDL1 was similarly induced on the cell surface of uveal melanoma MEL270 and its metastatic derivative OMM2.3 but not detected by the MIH1 mAb if the tumor cells were transfected with CD80 and HLA-DR (data not shown). Therefore, human tumor cells transfected with CD80 and HLA-DR do not constitutively express, nor are they induced by IFN-γ to express, cell surface PDL1.
FIGURE 2.
Expression of PDL1 at the cell surface of tumor cells is reduced by transfection of HLA-DR and CD80. A, Expression of HLA-DR and CD80 blocks constitutive expression of PDL1. Parental H358 (adenocarcinoma) and MCF10 (mammary adenocarcinoma) cells and their HLA-DR7 and CD80 transductants were stained for cell surface CD80 and PDL1 (mAb 29E.2A3) and analyzed by flow cytometry. B, Tumor cells expressing HLA-DR and CD80 are not induced by IFN-γ to express PDL1. Parental MEL202 (uveal melanoma) cells and their HLA-DR1 and CD80 transductants were either untreated or cocultured in IFN-γ for 48 h, stained for cell surface CD80 and PDL1 (mAb 29E.2A3), and analyzed by flow cytometry. Data are representative of four independent experiments.
CD80 inhibits the constitutive and IFN-g–induced expression of cell surface PDL1 on tumor cells
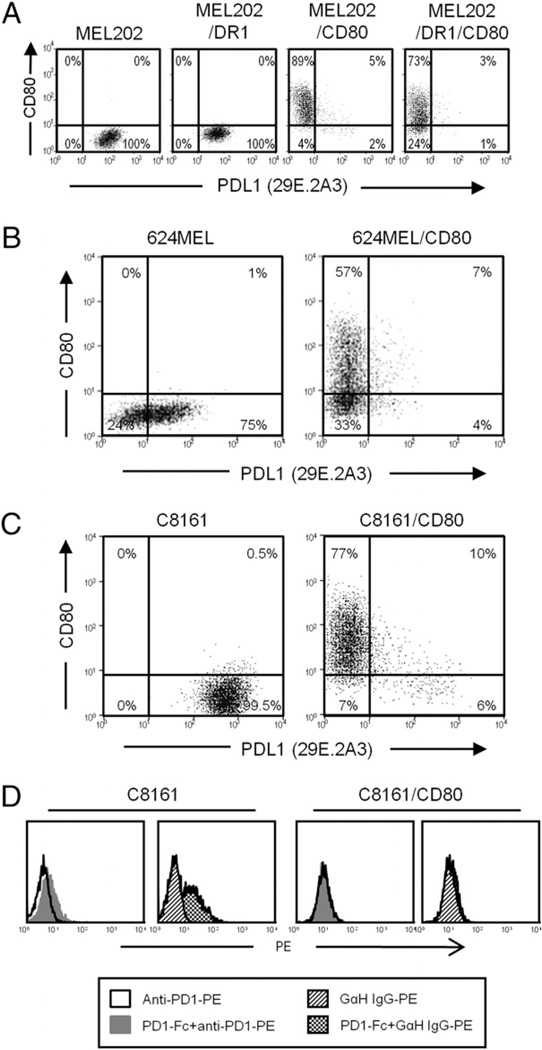
The absence of detectable PDL1 on the surface of DR+CD80+ vaccine cells could be mediated by either HLA-DR or CD80 or could be the result of the transfection, transduction, or drug selection. To distinguish these possibilities, MEL202 cells expressing HLA-DR1 or CD80 were treated with IFN-γ and subsequently labeled with mAbs to CD80 and PDL1 (29E.2A3 mAb) (Fig. 3A). MEL202/DR1 cells had detectable PDL1, whereas MEL202/CD80 cells did not; indicating that IFN-γ–induced cell surface expression of PDL1 was inhibited by coexpression of CD80 and was not due to drug selection or the transfection or transduction process. The absence of detectable PDL1 on IFN-γ–treated MEL202/CD80 cells was confirmed by staining with the two other PDL1 mAbs (Supplemental Fig. 2A, MIH1 and 27A2 mAbs). To confirm that the effect of CD80 was not limited to uveal melanoma cells, parental and CD80-transfected 624MEL cutaneous melanoma cells were treated with IFN-γ and analyzed for expression of cell surface PDL1 (Fig. 3B; mAb 29E.2A3). Similar to MEL202/CD80, 624MEL/CD80 cells did not have detectable cell surface PDL1 after IFN-γ stimulation, whereas parental 624MEL cells did. Another cutaneous melanoma, C8161, which constitutively expresses PDL1, was also negative for cell surface PDL1 when transfected with CD80 (Fig. 3C). Likewise, two other PDL1 mAbs (MIH1 and 27A2) failed to detect cell surface PDL1 on IFN-γ–treated 624MEL/CD80 (Supplemental Fig. 2B) and C8161/CD80 cells (Supplemental Fig. 2C) cells. Therefore, coexpression or pre-expression of CD80 inhibits the constitutive and IFN-γ–induced cell surface expression of PDL1 on human tumor cells.
FIGURE 3.
CD80 inhibits the constitutive and IFN-γ–induced expression of cell surface PDL1 on tumor cells. A, CD80 is sufficient to prevent cell surface expression of PDL1 on uveal melanoma cells. MEL202 transductants expressing HLA-DR1, CD80, or DR1 plus CD80 were cultured with IFN-γ for 48 h, stained for cell surface CD80 and PDL1 (mAb 29E.2A3), and analyzed by flow cytometry. B, CD80 inhibits IFN-γ–induced expression of PDL1. IFN-γ–treated 624MEL (cutaneous melanoma) parental cells and CD80 transfectants (624MEL/CD80) were stained for cell surface CD80 and PDL1 (mAb 29E.2A3) and analyzed by flow cytometry. C, Expression of CD80 blocks constitutive expression of PDL1. C8161 (cutaneous melanoma) parental cells and CD80 transfectants were stained for cell surface CD80 and PDL1 (mAb 29E.2A3). D, Expression of CD80 prevents PDL1 binding by PD1. C8161 and C8161/CD80 cells were incubated with or without recombinant human PD1–Fc fusion protein followed by staining with either goat anti-human IgG–PE (GαH IgG–PE) or PE-labeled mAb to PD1 (anti-PD1–PE) and analyzed by flow cytometry. Data are representative of two, three, four, and three independent experiments for A, B, C, and D, respectively.
CD80 binds to PDL1 (9, 42) raising the possibility that PDL1 may be sterically blocked from binding PDL1 Abs but retain the ability to function and bind PD1. To assess this possibility, C8161 and C8161/CD80 cells were incubated with recombinant human PD1–Fc fusion protein. Binding of the fusion protein was detected by staining with fluorescently labeled anti-PD1 mAb or goat anti-human IgG and analysis by flow cytometry (Fig. 3D). Parental C8161 cells, but not C8161/CD80 cells, bound PD1–Fc. Therefore, coexpression of CD80 not only blocks Ab-mediated detection of PDL1 but also prevents binding of PDL1 to its receptor PD1.
CD80 blocks PDL1 suppressive activity and restores T cell activation
The preceding experiments demonstrate that CD80 prevents detection of PDL1 but do not establish if tumor cell expression of CD80 overcomes PDL1-mediated immune suppression. To test functionality, we compared IFN-γ production by PHA-activated PBMCs with and without various numbers of MEL202, MEL202/CD80, C8161, or C8161/CD80 cells (Fig. 4A). PBMCs cocultured with CD80 transfected tumor cells produced more IFN-γ, consistent with the concept that CD80 prevented PDL1-mediated suppression. To confirm that CD80 increased PBMC activation by inhibiting PDL1-mediated suppression, we compared IFN-γ production by PHA-activated PBMCs cocultured with C8161 or C8161/CD80 in the presence of increasing quantities of recombinant human PD1–Fc (hPD1–Fc) fusion protein (Fig. 4B). Recombinant mouse PD1–Fc (mPD1–Fc) was used as a negative control. Inclusion of hPD1–Fc, but not mPD1–Fc, significantly increased IFN-γ production in cultures with C8161 cells. In contrast, there was no significant difference in IFN-γ production in cultures containing C8161/CD80 cells with hPD1–Fc versus mPD1–Fc. Therefore, blocking PDL1 has the same effect as CD80 expression, consistent with the concept that CD80 facilitates T cell activation by inhibiting PDL1–PD1 interactions.
FIGURE 4.
CD80 blocks PDL1 suppressive activity and restores T cell activation. A, T cell suppression is reversed by CD80 inhibition of PDL1 expression. PBMCs were activated with PHA in the presence or absence of varying numbers of irradiated MEL202, MEL202/CD80, C8161, or C8161/CD80 cells, and IFN-γ production was measured by ELISA. B, PBMCs were activated with PHA and analyzed for IFN-γ production as in A in the presence or absence of 1.5–6 µg/ml human or mouse recombinant PD1–Fc fusion protein and irradiated C8161 or C8161/CD80 cells. PBMC/tumor cell ratio is 1:0.5. Data are representative of three and two independent experiments for A and B, respectively. *p < 0.009.
CD80 does not inhibit intracellular expression of PDL1
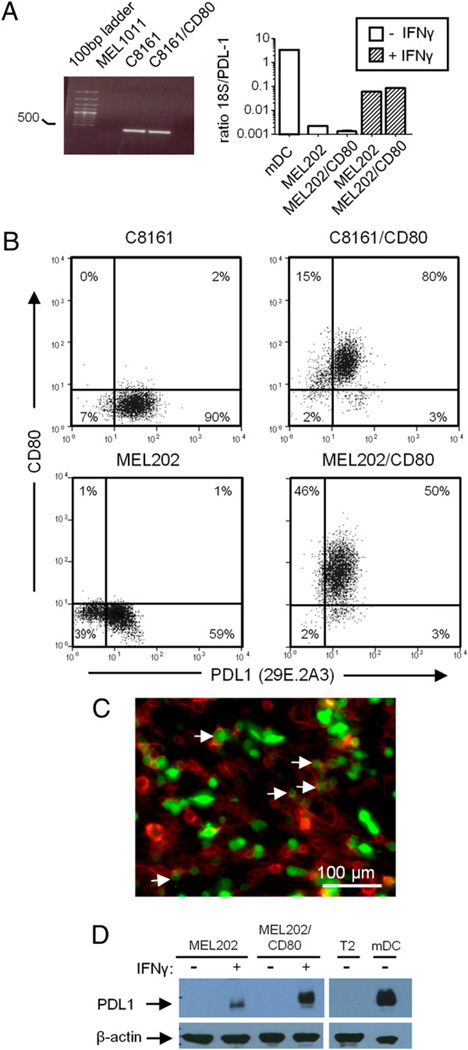
To determine if CD80 inhibits detection and function of PDL1 by blocking PDL1 transcription, we performed RT-PCR and qPCR on tumor cells that constitutively express PDL1 (C8161) or are induced by IFN-γ to express PDL1 (MEL202). MEL1011 cells, which neither constitutively express nor are induced to express PDL1, and mDCs, which constitutively express PDL1, served as negative and positive controls, respectively (Fig. 5A). PDL1 mRNA was present in constitutive expressers and after IFN-γ induction regardless of CD80 expression, indicating that CD80 does not prevent transcription of PDL1.
FIGURE 5.
CD80 does not inhibit intracellular expression of PDL1. A, RNA from MEL1011 (cutaneous melanoma), C8161, and C8161/CD80 cells was subjected to RT-PCR using PDL1 primers (left). RNA from mDCs, MEL202, MEL202/CD80, and IFN-γ–treated MEL202 and MEL202/CD80 cells was subjected to qPCR using 18S and PDL1 primers (right). B, Fixed and permeabilized C8161, C8161/CD80, MEL202, and MEL202/CD80 cells were internally stained for CD80 and PDL1 (mAb 29E.2A3) and analyzed by flow cytometry. MEL202 and MEL202/CD80 cells were treated with IFN-γ for 48 h prior to fixation, permeabilization, and mAb labeling. C, MEL202/DR1/CD80 cells were transiently transfected with a PDL1–GFP plasmid, plated in 6-well plates, stained with CD80–PE–Cy7 (mAb 2D10), and analyzed by fluorescence microscopy. CD80 is false-colored red. Of 167 cells counted, 18% were CD80+PDL1+, 54% were CD80+PDL1−, and 28% were CD80−PDL1+. D, Western blots of untreated (−) or IFN-γ–treated (+) MEL202 and MEL202/CD80 cells probed for PDL1 (mAb MIH1) and β-actin. mDCs and T2 cells served as PDL1+ and PDL1− controls, respectively. Data are representative of three independent experiments.
To determine if CD80 inhibits translation of PDL1 mRNA, C8161, MEL202, and their CD80 transfectants were fixed, permeabilized, and stained for CD80 and PDL1 (mAb 29E.2A3) to visualize intracellular PDL1 protein (Fig. 5B). In contrast to the absence of PDL1 at the cell surface, C8161/CD80 and IFN-γ–induced MEL202/CD80 contained intracellular PDL1, and there was no difference in the level of intracellular PDL1 in CD80+ versus CD80− cells. Staining with the MIH1 and 27A2 PDL1 mAbs confirmed intracellular PDL1 in C8161/CD80 and IFN-γ–treated MEL202/CD80 cells (Supplemental Fig. 3A, 3B, respectively). To examine further intracellular expression of PDL1 in CD80+ tumor cells, MEL202/DR1/CD80 cells were transiently transfected with a retroviral PDL1–GFP construct and 48 h later stained for cell surface CD80 and analyzed by fluorescence microscopy (Fig. 5C). PDL1 was present intracellularly and on the cell surface of CD80− cells. In contrast, CD80+ cells contained lower levels of intracellular PDL1 and no PDL1 at the cell surface. In agreement with the intracellular staining and fluorescence microscopy, Western blot analysis revealed PDL1 protein in IFN-γ–treated MEL202 and MEL202/CD80 cells (Fig. 5D). These results demonstrate that although CD80+ tumor cells do not have detectable cell surface PDL1, they contain intracellular PDL1 protein.
PDL1 expression is predominately regulated by the extracellular domains of CD80
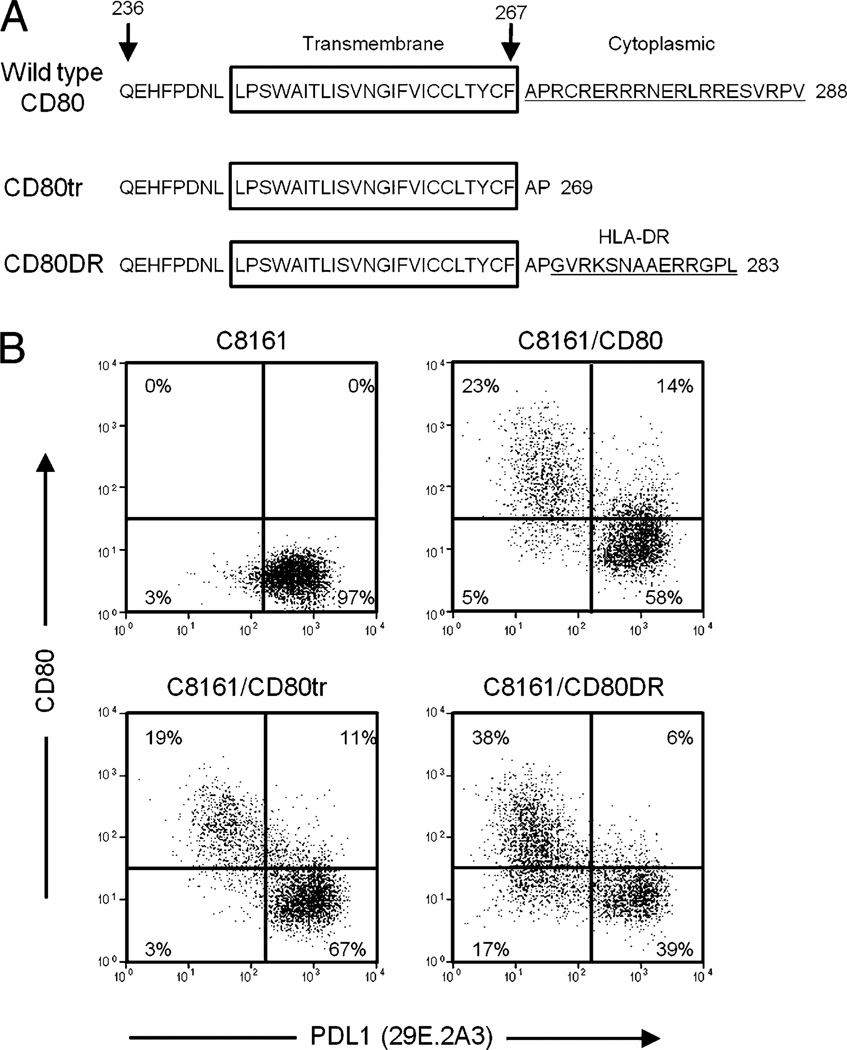
CD80 signals intracellularly to modulate dendritic cell (43) and T cell (44, 45) function raising the possibility that CD80 may downregulate PDL1 cell surface expression in tumor cells via its cytoplasmic domain. To test this possibility, two retroviral constructs were made (Fig. 6A). One construct encoded CD80 truncated for 19 of the 21 aa of the C-terminal end cytoplasmic domain (CD80 truncated, or CD80 tr). The second construct encoded the truncated CD80 gene ligated to a DNA fragment encoding the C-terminal 14 aa of the cytoplasmic domain of HLA-DRα-chain (CD80DR). Constitutive PDL1-expressing C8161 cells were transiently transfected with these constructs and 48 h later stained for CD80 and PDL1 (mAb 29E.2A3) (Fig. 6B). CD80 tr transfectants expressed less cell surface CD80 and a lower percentage of cells were transfected compared with C8161 cells transfected with the wild-type CD80 construct. This decreased expression is most likely due to the known role of the cytoplasmic domain of CD80 in regulating plasma membrane localization of CD80 (38). However, C8161 cells expressing the CD80tr and CD80DR proteins were significantly downregulated for cell surface PDL1, with maximum downregulation observed in the transfectants expressing the highest quantity of CD80. Similar results were obtained using the MIH1 mAb (Supplemental Fig. 4). In contrast, C8161 cells transiently transfected with the same vector containing a CD80 gene mutated in its N-terminal extracellular domain were not downregulated for PDL1 expression (data not shown). These results demonstrate that the extracellular domains and not the cytoplasmic region of CD80 predominantly regulate cell surface expression of PDL1.
FIGURE 6.
PDL1 expression is regulated by the extracellular domains of CD80. A, Amino acid sequence and predicted transmembrane and cytoplasmic domains of wild-type human CD80, CD80 with the truncated cytoplasmic domain (CD80tr), and CD80 with the cytoplasmic domain of HLA-DRα–chain (CD80DR). Numbers indicate the amino acid position, with the first amino acid of the structural protein being position 1. B, C8161 cells were transiently transfected with plasmids encoding wild-type CD80, CD80tr, or CD80DR. Forty-eight hours after transfection, the cells were stained for cell surface CD80 and PDL1 (mAb 29E.2A3) and analyzed by flow cytometry. Data are representative of two independent experiments.
Discussion
PDL1 is expressed constitutively or is induced by IFN-γ on the cell surface of most human cancer cells and is credited with protecting tumor cells against T cell-mediated elimination through its delivery of apoptotic signals to tumor-reactive T cells (3). The results reported in this article using seven cell lines representing four histologically distinct solid tumors (lung, breast, cutaneous melanoma, and uveal melanoma) demonstrate that human tumor cell coexpression of CD80 reverses PDL1-mediated immune suppression, enables tumor cell boosting of primed tumor-specific T cells, and restores T cell activation during priming of tumor-reactive T cells. CD80 mediates this effect by preventing the expression of PDL1 protein at the cell surface of the tumor cells and without altering PDL1 transcription or intracellular expression.
Many cells contain PDL1 mRNA but do not contain PDL1 protein (46). Gong and colleagues (47) have shown that PDL1 protein can be regulated by microRNA-513 (miRNA-513). miRNA-513 inhibits translation of PDL1 mRNA and is downregulated by IFN-γ, consistent with the ability of IFN-γ to upregulate PDL1 expression. miRNA-513, however, is unlikely to play a role in CD80-mediated regulation of PDL1 because CD80-transfected tumor cells that constitutively express PDL1 or are IFN-γ–treated to express PDL1 contain intracellular PDL1 protein.
Our studies demonstrate that mechanistically, the extracellular domains of CD80 regulate PDL1 expression. CD80 and PD1 bind to the same region of PDL1 (9, 42), raising the possibility that PDL1 is present on the cell surface, but the extracellular domains of CD80 sterically block binding of anti-PDL1 mAb and PD1. The dissociation constant (KD) for CD80–PDL1 binding (~1.4 µM) is higher than the KD for PD1–PDL1 binding (~0.77 µM) (42), indicating that PD1 could compete with CD80 for binding to PDL1. However, PD1–Fc fusion protein did not bind to CD80-expressing cells, and CD80+ cells facilitated T cell boosting, suggesting that PDL1 is physically absent from the cell membrane. Furthermore, if CD80 is mediating its effect by steric interference, then it is unclear why CD80 does not also obscure PDL1 detection in the cytoplasm of CD80+ cells. If CD80 is not sterically blocking PDL1 at the cell surface, it may mediate its effect by restricting trafficking of PDL1 to the cell surface or by facilitating the rapid turnover of cell surface PDL1.
T cell activation is also inhibited by the binding of CD80 to the negative regulator CTLA-4 (48). CTLA-4 and PDL1 share a partially overlapping binding site on CD80 (42). As a result of this common binding site, CD80 can bind either CTLA-4 or PDL1, but not both molecules concurrently. Therefore, if CD80 is bound to PDL1, then it may not be able to suppress via CTLA-4. Our data showing MHC II activation of T cells from healthy donors and cancer patients is consistent with the concept that the vaccines are potent T cell activators because they deliver activation signals and do not suppress via either PDL1–PD1 or CD80–CTLA-4 pathways.
The cytoplasmic domain of CD80 is required for CD80 costimulation through CD28 (38) and is needed for correct subcellular localization of CD80 and resultant signaling to CD28 (37). It is also needed for PDL1 reverse signaling and resulting T cell suppression through CD80 (10). However, the CD80 cytoplasmic domain is not required for the downregulation of PDL1 on the cell surface of tumor cells. This discrepancy raises the possibility that CD80-mediated downregulation of PDL1 could be functionally separated from the costimulatory effects of CD80 and therefore might be exploited therapeutically to inhibit PDL1-mediated tumor cell-induced immune suppression.
CD80-mediated blocking of PDL1 at the cell surface appears to be a tumor-specific effect because nonmalignant cells such as dendritic cells and macrophages simultaneously express both molecules (S. Haile, J. Bosch, and S. Ostrand-Rosenberg, unpublished observations, and Refs. 49, 50). PDL1 downregulation is also proportional to the level of CD80. Quantity of CD80 may also explain why some human leukemias with low-level expression of CD80 have serologically detectable PDL1 (51).
Regardless of how CD80 deters PDL1 expression, PDL1 is not serologically detectable and, more importantly, is functionally absent from the cell surface of tumor cells transfected with CD80. This inhibition of PDL1 is likely responsible for the ability of the MHC II vaccines to efficiently activate and maintain tumor-specific effector T cells and suggests new therapeutic avenues for preventing tumor cell PDL1-induced immune suppression.
Supplementary Material
Acknowledgments
We thank Miguel A. Acosta for assistance with the microscopy, Dr. P.W. Chen for helpful discussions, and Virginia Clements and Barbara Bock for technical support.
This work was supported by National Institutes of Health Grants R01CA115880, R01CA84232 (to S.O.-R.), and R01EY016486 (to B.R.K.). S.T.H. was partially supported by a Graduate Assistance in Areas of National Need predoctoral fellowship and by the National Institutes of Health (T32 GM066706 and R25-GM55036). J.J.B. was partially supported by Rotterdamse Vereniging Blindenbelangen, Stichting Blindenhulp, Stichting Blinden-Penning, Stichting Dondersfonds, Stichting Nelly Reef Fund, Gratama Stichting, Stichting Admiraal van Kinsbergen Fonds, and Foundation “De Drie Lichten” fellowships and by Deutsche Forschungsgemeinschaft DFG-SFB643 (C8).
Abbreviations used in this article
- CD80DR
CD80tr with the 14 terminal aa of HLA-DRα–chain at its C-terminal end
- CD80tr
CD80 truncated for its C-terminal 19 aa
- hPD1–Fc
human PD1–Fc fusion protein
- mDC
mature dendritic cell
- MHC II
MHC class II
- miRNA-513
microRNA-513
- mPD1–Fc
mouse PD1–Fc fusion protein
- PD1
programmed death 1
- PDL1
programmed death ligand 1
- qPCR
quantitative PCR
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 4.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 5.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 6.Yang W, Li H, Chen PW, Alizadeh H, He Y, Hogan RN, Niederkorn JY. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Invest. Ophthalmol. Vis. Sci. 2009;50:273–280. doi: 10.1167/iovs.08-2397. [DOI] [PubMed] [Google Scholar]
- 7.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 12.Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R, Mackensen A. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int. J. Cancer. 2006;119:317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 13.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 14.Zhu X, Fallert-Junecko BA, Fujita M, Ueda R, Kohanbash G, Kastenhuber ER, McDonald HA, Liu Y, Kalinski P, Reinhart TA, et al. Poly-ICLC promotes the infiltration of effector T cells into intracranial gliomas via induction of CXCL10 in IFN-alpha and IFN-gamma dependent manners. Cancer Immunol. Immunother. 2010;59:1401–1409. doi: 10.1007/s00262-010-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkie S, Burbridge SE, Chiapero-Stanke L, Pereira AC, Cleary S, van der Stegen SJ, Spicer JF, Davies DM, Maher J. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J. Biol. Chem. 2010;285:25538–25544. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, Rigatos G, Papamichail M, Perez SA. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol. Immunother. 2010;59:1781–1789. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumorassociated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J. Leukoc. Biol. 2002;72:101–106. [PubMed] [Google Scholar]
- 19.Itoh K, Shiiba K, Shimizu Y, Suzuki R, Kumagai K. Generation of activated killer (AK) cells by recombinant interleukin 2 (rIL 2) in collaboration with interferon-gamma (IFN-gamma) J. Immunol. 1985;134:3124–3129. [PubMed] [Google Scholar]
- 20.Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J. Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 21.Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, Oh S, Shin JG, Yao S, Chen L, Choi IH. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 22.Dissanayake SK, Thompson JA, Bosch JJ, Clements VK, Chen PW, Ksander BR, Ostrand-Rosenberg S. Activation of tumor-specific CD4(+) T lymphocytes by major histocompatibility complex class II tumor cell vaccines: a novel cell-based immunotherapy. Cancer Res. 2004;64:1867–1874. doi: 10.1158/0008-5472.can-03-2634. [DOI] [PubMed] [Google Scholar]
- 23.Bosch JJ, Thompson JA, Srivastava MK, Iheagwara UK, Murray TG, Lotem M, Ksander BR, Ostrand-Rosenberg S. MHC class II-transduced tumor cells originating in the immune-privileged eye prime and boost CD4(+) T lymphocytes that cross-react with primary and metastatic uveal melanoma cells. Cancer Res. 2007;67:4499–4506. doi: 10.1158/0008-5472.CAN-06-3770. [DOI] [PubMed] [Google Scholar]
- 24.Thompson JA, Srivastava MK, Bosch JJ, Clements VK, Ksander BR, Ostrand-Rosenberg S. The absence of invariant chain in MHC II cancer vaccines enhances the activation of tumor-reactive type 1 CD4+ T lymphocytes. Cancer Immunol. Immunother. 2008;57:389–398. doi: 10.1007/s00262-007-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients’ CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol. Immunother. 2008;57:1493–1504. doi: 10.1007/s00262-008-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostrand-Rosenberg S, Pulaski BA, Clements VK, Qi L, Pipeling MR, Hanyok LA. Cell-based vaccines for the stimulation of immunity to metastatic cancers. Immunol. Rev. 1999;170:101–114. doi: 10.1111/j.1600-065x.1999.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 27.Schweitzer AN, Sharpe AH. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J. Immunol. 1998;161:2762–2771. [PubMed] [Google Scholar]
- 28.van Rijt LS, Vos N, Willart M, Kleinjan A, Coyle AJ, Hoogsteden HC, Lambrecht BN. Essential role of dendritic cell CD80/CD86 costimulation in the induction, but not reactivation, of TH2 effector responses in a mouse model of asthma. J. Allergy Clin. Immunol. 2004;114:166–173. doi: 10.1016/j.jaci.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 29.Verbik DJ, Murray TG, Tran JM, Ksander BR. Melanomas that develop within the eye inhibit lymphocyte proliferation. Int. J. Cancer. 1997;73:470–478. doi: 10.1002/(sici)1097-0215(19971114)73:4<470::aid-ijc3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 30.Marincola FM, Shamamian P, Simonis TB, Abati A, Hackett J, O’Dea T, Fetsch P, Yannelli J, Restifo NP, Mulé JJ, et al. Locus-specific analysis of human leukocyte antigen class I expression in melanoma cell lines. J. Immunother. Emphasis Tumor Immunol. 1994;16:13–23. doi: 10.1097/00002371-199407000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welch DR, Bisi JE, Miller BE, Conaway D, Seftor EA, Yohem KH, Gilmore LB, Seftor RE, Nakajima M, Hendrix MJ. Characterization of a highly invasive and spontaneously metastatic human malignant melanoma cell line. Int. J. Cancer. 1991;47:227–237. doi: 10.1002/ijc.2910470211. [DOI] [PubMed] [Google Scholar]
- 32.Wang RF, Johnston SL, Zeng G, Topalian SL, Schwartzentruber DJ, Rosenberg SA. A breast and melanoma-shared tumor antigen: T cell responses to antigenic peptides translated from different open reading frames. J. Immunol. 1998;161:3598–3606. [PubMed] [Google Scholar]
- 33.Pauley RJ, Soule HD, Tait L, Miller FR, Wolman SR, Dawson PJ, Heppner GH. The MCF10 family of spontaneously immortalized human breast epithelial cell lines: models of neoplastic progression. Eur. J. Cancer Prev. 1993;2(Suppl 3):67–76. [PubMed] [Google Scholar]
- 34.Brower M, Carney DN, Oie HK, Gazdar AF, Minna JD. Growth of cell lines and clinical specimens of human non-small cell lung cancer in a serum-free defined medium. Cancer Res. 1986;46:798–806. [PubMed] [Google Scholar]
- 35.Thompson JA, Dissanayake SK, Ksander BR, Knutson KL, Disis ML, Ostrand-Rosenberg S. Tumor cells transduced with the MHC class II transactivator and CD80 activate tumor-specific CD4+ T cells whether or not they are silenced for invariant chain. Cancer Res. 2006;66:1147–1154. doi: 10.1158/0008-5472.CAN-05-2289. [DOI] [PubMed] [Google Scholar]
- 36.Schaft N, Dörrie J, Thumann P, Beck VE, Müller I, Schultz ES, Kämpgen E, Dieckmann D, Schuler G. Generation of an optimized polyvalent monocyte-derived dendritic cell vaccine by transfecting defined RNAs after rather than before maturation. J. Immunol. 2005;174:3087–3097. doi: 10.4049/jimmunol.174.5.3087. [DOI] [PubMed] [Google Scholar]
- 37.Doty RT, Clark EA. Subcellular localization of CD80 receptors is dependent on an intact cytoplasmic tail and is required for CD28-dependent T cell costimulation. J. Immunol. 1996;157:3270–3279. [PubMed] [Google Scholar]
- 38.Doty RT, Clark EA. Two regions in the CD80 cytoplasmic tail regulate CD80 redistribution and T cell costimulation. J. Immunol. 1998;161:2700–2707. [PubMed] [Google Scholar]
- 39.Tseng SY, Liu M, Dustin ML. CD80 cytoplasmic domain controls localization of CD28, CTLA-4, and protein kinase Ctheta in the immunological synapse. J. Immunol. 2005;175:7829–7836. doi: 10.4049/jimmunol.175.12.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sagerström CG, Kerr EM, Allison JP, Davis MM. Activation and differentiation requirements of primary T cells in vitro. Proc. Natl. Acad. Sci. USA. 1993;90:8987–8991. doi: 10.1073/pnas.90.19.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosch JJ, Iheagwara UK, Reid S, Srivastava MK, Wolf J, Lotem M, Ksander BR, Ostrand-Rosenberg S. Uveal melanoma cell-based vaccines express MHC II molecules that traffic via the endocytic and secretory pathways and activate CD8(+) cytotoxic, tumor-specific T cells. Cancer Immunol. Immunother. 2010;59:103–112. doi: 10.1007/s00262-009-0729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butte MJ, Peña-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Mol. Immunol. 2008;45:3567–3572. doi: 10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, et al. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat. Immunol. 2004;5:1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 44.Hirokawa M, Kitabayashi A, Kuroki J, Miura AB. Signal transduction by B7/BB1 expressed on activated T lymphocytes: cross-linking of B7/ BB1 induces protein tyrosine phosphorylation and synergizes with signalling through T-cell receptor/CD3. Immunology. 1995;86:155–161. [PMC free article] [PubMed] [Google Scholar]
- 45.Hirokawa M, Kuroki J, Kitabayashi A, Miura AB. Transmembrane signaling through CD80 (B7-1) induces growth arrest and cell spreading of human B lymphocytes accompanied by protein tyrosine phosphorylation. Immunol. Lett. 1996;50:95–98. doi: 10.1016/0165-2478(96)02526-6. [DOI] [PubMed] [Google Scholar]
- 46.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 47.Gong AY, Zhou R, Hu G, Li X, Splinter PL, O’Hara SP, LaRusso NF, Soukup GA, Dong H, Chen XM. MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes. J. Immunol. 2009;182:1325–1333. doi: 10.4049/jimmunol.182.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 49.Boasso A, Hardy AW, Landay AL, Martinson JL, Anderson SA, Dolan MJ, Clerici M, Shearer GM. PDL-1 upregulation on monocytes and T cells by HIV via type I interferon: restricted expression of type I interferon receptor by CCR5-expressing leukocytes. Clin. Immunol. 2008;129:132–144. doi: 10.1016/j.clim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meier A, Bagchi A, Sidhu HK, Alter G, Suscovich TJ, Kavanagh DG, Streeck H, Brockman MA, LeGall S, Hellman J, Altfeld M. Upregulation of PD-L1 on monocytes and dendritic cells by HIV-1 derived TLR ligands. AIDS. 2008;22:655–658. doi: 10.1097/QAD.0b013e3282f4de23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge W, Ma X, Li X, Wang Y, Li C, Meng H, Liu X, Yu Z, You S, Qiu L. B7-H1 up-regulation on dendritic-like leukemia cells suppresses T cell immune function through modulation of IL-10/IL-12 production and generation of Treg cells. Leuk. Res. 2009;33:948–957. doi: 10.1016/j.leukres.2009.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.