Abstract
A 15-year-old Yorkshire terrier dog was presented after ingesting 1 capsule of an over-the-counter cold medication containing pseudoephedrine (120 mg/capsule) and cetirizine (5 mg/capsule). Treatment was initiated with acepromazine and carvedilol. The dog responded well to treatment. This is the first known case report using carvedilol to control pseudoephedrine toxicosis.
Résumé
Utilisation du carvédilol chez un chien avec une tachycardie induite par une toxicose à la pseudoéphédrine. Un chien Terrier du Yorkshire âgé de 15 ans a été présenté suite à l’ingestion d’une capsule d’un médicament contre le rhume en vente libre contenant de la pseudoéphédrine (120 mg/capsule) et de la cétirizine (5 mg/capsule). Le traitement a été entamé avec de l’acépromazine et du carvédilol. Le chien a bien réagi au traitement. Il s’agit du premier cas connu d’utilisation du carvédilol pour contrôler une toxicose à la pseudoéphédrine.
(Traduit par Isabelle Vallières)
Pseudoephedrine-containing cold medications are toxic to the cardiovascular and central nervous systems, due to both alpha- and beta-sympathomimetic effects, by causing the release of stored norepinephrine (1,2). In the human literature, pseudoephedrine and other over-the-counter cold medications have been associated with infant deaths (3,4). Naturally occurring pseudoephedrine toxicosis has not been well-documented in the veterinary literature. In dogs and cats, clinical signs can occur at 5 to 6 mg/kg body weight (BW) and life-threatening symptoms may occur at 10 to 12 mg/kg BW (1). The most common clinical signs are tachycardia, hypertension, sinus arrhythmias, agitation, hyperactivity, mydriasis, and vomiting (1,5). Treatments have focused on symptomatic care of the cardiovascular system and the central nervous system (CNS). Adverse effects on the CNS can be controlled with acepromazine, chlorpromazine, or phenobarbital, and tachycardia can be controlled with beta-blockers such as propranolol (1,2).
This case report describes pseudoephedrine toxicosis in a dog that accidentally ingested an over-the-counter cold medication, and successful management of the toxicosis using acepromazine and carvedilol. To the authors’ knowledge, this is the first case report of the use of carvedilol to control pseudoephedrine toxicosis.
Case description
A 15-year-old intact female Yorkshire terrier dog weighing 2.2 kg was referred for acute vomiting, hyperactivity, and agitation. The dog had accidentally ingested 1 capsule of the over-the-counter cold medication SSinose® (Kwang dong Pharm, Seoul, Korea), which contains pseudoephedrine (120 mg/capsule) and cetirizine (5 mg/capsule). On admission, the dog displayed restlessness and agitation. A physical examination revealed dilated non-reactive pupils, hyperthermia (39.5°C), tachypnea (panting), tachycardia (204 beats/min), and systemic hypertension (167 mmHg) (Cardell Model 9401; Sharn Veterinary, Tampa, Florida, USA). Neurological examination revealed a conscious and awake state. However, the dog would aimlessly wander, bark, and pace. The hemogram showed mild leukocytosis [21.80 × 103/μL; reference interval (RI): 6 to 17 × 103/μL] with a stress leukogram. Serum chemistry profiles showed hyperproteinemia (82 g/L;RI: 54 to 74 g/L), elevated blood urea nitrogen (BUN; 20.5 mmol/L, RI: 2.9 to 9.3 mmol/L), and elevated amylase (879 U/L, RI: 185 to 700 U/L). Urinalysis was unremarkable except for aciduria (pH 5.0).
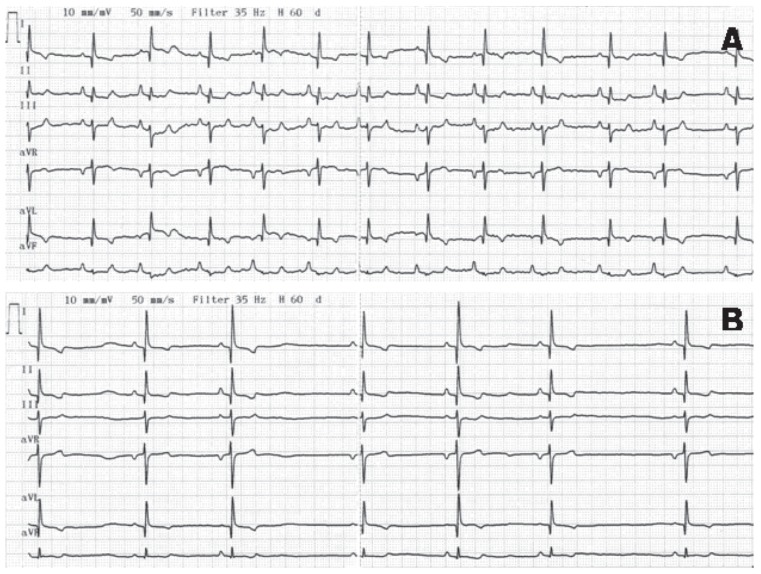
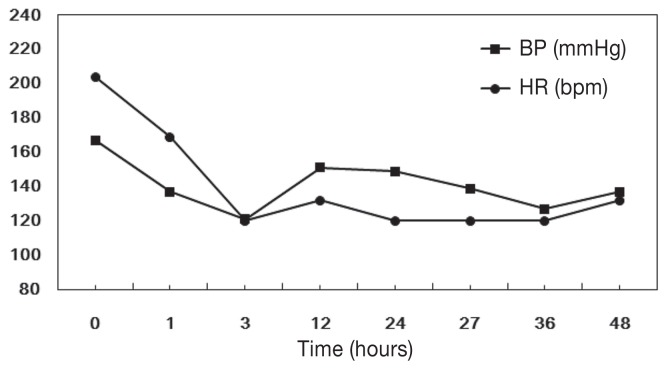
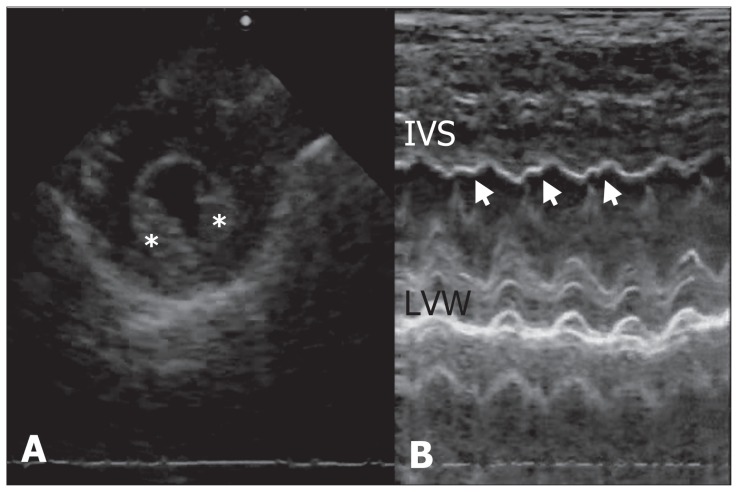
The dog was initially treated with continuous oxygen supplementation and intravenous Hartmann’s solution (JW Pharmaceutical, Seoul, Korea). Acepromazine (Sedaject™; Samu Median, Seoul, Korea), 0.05 mg/kg BW, intramuscularly was administered to control agitation and hyperactivity, and carvedilol (Dilatrend™; Chong Kun Dang Pharm, Seoul, Korea), 0.5 mg/kg BW, q12h, PO was used to treat tachycardia. One hour after presentation, systolic blood pressure returned to normal (137 mmHg) and heart rate decreased to 169 bpm. On electrocardiography, sinus tachycardia and low QRS voltage were identified (Figure 1A). The dog was observed with 24-h Holter monitoring (Televet 100 Version 4.1.1; Frankfurt am Main, Germany) to detect heart rate and arrhythmia, as well as to monitor the dog’s response to therapy. No abnormal rhythms were observed during the 24-hour period and the heart rate decreased gradually (Figure 2). On the next day of hospitalization, the dog’s vital signs had stabilized (temperature: 38.6°C, respiratory rate: 25 breaths/min, heart rate: 120 beats per min). Sinus arrhythmia with improved QRS voltage was identified on an ECG (Figure 1B). Echocardiography showed no pericardial effusion. Left ventricular systolic function was normal (fractional shortening, 35%) and no ventricular dilation was noted. However, hyperechoic endocardium was visible in the left ventricle (Figure 3A) and abnormal interventricular septal motion was demonstrated with M-mode echocardiography (Figure 3B). The dog was discharged 2 d later. At follow-up 1 wk later, the dog had no clinical symptoms and medications were discontinued. No recurrence was noticed 1 mo later.
Figure 1.
Electrocardiogram of a dog after ingestion of pseudoephedrine. (A) Low-voltage QRS complexes were demonstrated. The R-waves were < 0.5 mV amplitude and heart rate (HR) was 169 beats per min (bpm). (B) HR decreased to 81 bpm and improved R wave amplitude with sinus arrhythmia was demonstrated 1 d after the carvedilol treatment.
Figure 2.
Blood pressure and heart rate changes following carvedilol treatment in a dog with pseudoephedrine toxicosis induced tachycardia. Time represents hours after carvedilol application in this dog. BP — blood pressure, HR — heart rate.
Figure 3.
Echocardiograms from a dog with pseudoephedrine toxicosis. (A) M-mode echocardiogram at the level of the left ventricle, showing the hyperechogenic left ventricular endocardium (asterisks). The endocardium was brighter than the underlying myocardium. (B) Abnormal interventricular septal motion was demonstrated on left ventricular M-mode (arrows). IVS — interventricular septum, LVW — left ventricular wall.
Discussion
Ingestion of herbal supplements containing guarana and ma huang may induce ephedrine toxicosis (6). Most affected dogs display hyperactivity, tremors, seizures, behavioral changes, and other signs including vomiting, tachycardia, and hyperthermia (6). In this case, the dog ingested approximately 50 mg/kg BW of pseudoephedrine, which is a potentially lethal dose. Mydriasis, hyperthermia, tachycardia, and tachypnea were apparent at presentation. Based on history and clinical signs, this dog was diagnosed with pseudoephedrine toxicosis.
Clinical signs started 8 h after ingesting the drugs and persisted for 2 d. However, the symptoms were not life-threatening, even though the ingested dosage was potentially lethal to this dog. Pseudoephedrine is excreted through the kidneys and acidic urine accelerates renal excretion (7). Urinalysis revealed aciduria, which can decrease the half-life of the pseudoephedrine. The dog’s owner added meat to the diet to enhance the palatability. The elevatd protein in the diet could have induced aciduria. The resulting accelerated excretion rates may have helped reduce the toxic effects of the pseudoephedrine. The dog was presented with severe tachycardia. Low QRS amplitude and hyperechoic endocardium were demonstrated through ECG and echocardiography, respectively. Decreased ventricular filling due to tachycardia could result in displacement of the heart. The left ventricle would more closely approach the precordial electrodes, which would lower the QRS amplitude. An area of hyperechoic endocardium suggested myocardial ischemia, which could also be related to a rapid heart rate.
Clinical signs of this dog were consistent with the alpha- and beta-sympathomimetic effects of pseudoephedrine. Chronically increased sympathetic tone results in tachycardia-induced cardiomyopathy, which usually shows left and right ventricular dilation along with systolic dysfunction (8,9). Therefore, it is important to recognize tachycardia and normalize the heart rate early. A non-selective beta-blocker, such as propranolol, has been used to control pseudoephedrine toxicosis in dogs (1,2). In this case, we used carvedilol instead of propranolol to treat the pseudo-ephedrine toxicosis, due to the alpha- and beta-sympathomimetic effects of pseudoephedrine. Carvedilol, a multi-functional neurohormonal antagonist, exhibits non-selective beta-blockade as well as vasodilation via α1-blockade and potent antioxidant activity (10,11). When compared to propranolol, carvedilol has less adverse drug reactions and exhibits vasodilation and more cardioprotective activity (12–15). In veterinary medicine, some studies have evaluated the effects of carvedilol. The improvement of echocardiographic cardiac function was poor (16,17), and reduction in blood pressure and heart rate was variable depending on the dose of carvedilol (16–18). Carvedilol can decrease the heart rate and blood pressure in a dose-dependent manner (14), but orally administered carvedilol has limited bioavailability, leading to individual variability (18). The dose of carvedilol used in other studies has varied from 0.2 to 1.5 mg/kg BW (14,15–18). There were no significant adverse effects in dogs that had received 0.3 mg/kg BW carvedilol for heart failure. In this case, we chose a higher dose than the treatment dosage for heart failure, and lower than the highest dosage previously used. Treatment with carvedilol successfully controlled the tachycardia and no adverse reactions were noted.
This case report describes pseudoephedrine toxicosis due to accidental ingestion of an over-the-counter cold medication in a dog. Adequate heart rate control using carvedilol was a successful treatment that prevented further deterioration. We used carvedilol because pseudoephedrine has both alpha- and beta-sympathomimetic effects, and carvedilol exhibits both non-selective beta-blockade and α1-blockade actions. To the authors’ knowledge, this is the first report of the use of carvedilol to control pseudoephedrine toxicosis.
Acknowledgments
This work was supported by the Brain Korea 21 and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 20100018275). CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Means C. Decongestants. In: Plumlee KH, editor. Clinical Veterinary Toxicology. St. Louis, Missouri: Mosby; 2003. pp. 309–311. [Google Scholar]
- 2.Meadow I, Gwaltney-Brant S. Toxicology brief: The 10 most common toxicoses in dogs. [Last accessed May 2, 2012]. Available from http://veterinarymedicine.dvm360.com/vetmed/article/articleDetail.jsp?id=314007.
- 3.Wingert WE, Mundy LA, Collins GL, Chmara ES. Possible role of pseudoephedrine and other over-the-counter cold medications in the deaths of very young children. J Forensic Sci. 2007;52:487–490. doi: 10.1111/j.1556-4029.2007.00391.x. [DOI] [PubMed] [Google Scholar]
- 4.Rimsza ME, Newberry S. Unexpected infant deaths associated with use of cough and cold medications. Pediatrics. 2008;122:e318–e322. doi: 10.1542/peds.2007-3813. [DOI] [PubMed] [Google Scholar]
- 5.Roberge RJ, Hirani KH, Rowland PL, 3rd, Berkeley R, Krenzelok EP. Dextromethorphan- and pseudoephedrine-induced agitated psychosis and ataxia: Case report. J Emerg Med. 1999;17:285–288. doi: 10.1016/s0736-4679(98)00193-0. [DOI] [PubMed] [Google Scholar]
- 6.Oomas TG, Khan SA, Means C. Suspected caffeine and ephedrine toxicosis resulting from ingestion of an herbal supplement containing guarana and ma huang in dogs: 47 cases (1997–1999) J Am Vet Med Assoc. 2001;218:225–229. doi: 10.2460/javma.2001.218.225. [DOI] [PubMed] [Google Scholar]
- 7.Pentel P. Toxicity of over-the-counter stimulants. JAMA. 1984;252:1898–1903. [PubMed] [Google Scholar]
- 8.Zupan I, Rakovec P, Budihna N, Brecelj A, Kozelj M. Tachycardia induced cardiomyopathy in dogs; relation between chronic supraventricular and chronic ventricular tachycardia. Int J Cardiol. 1996;56:75–81. doi: 10.1016/0167-5273(96)02728-3. [DOI] [PubMed] [Google Scholar]
- 9.Foster SF, Hunt GB, Thomas SP, Ross DL, Pearson MR, Malik R. Tachycardia-induced cardiomyopathy in a young Boxer dog with supraventricular tachycardia due to an accessory pathway. Aust Vet J. 2006;84:326–331. doi: 10.1111/j.1751-0813.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer WH, Politowski J, Hwang B, et al. Metabolism of carvedilol in dogs, rats, and mice. Drug Metab Dispos. 1998;26:958–969. [PubMed] [Google Scholar]
- 11.Oliveira PJ, Rolo AP, Sardão VA, et al. Advantages in the use of carvedilol versus propranolol for the protection of cardiac mitochondrial function. Rev Port Cardiol. 2004;23:1291–1298. [PubMed] [Google Scholar]
- 12.Stoschitzky K, Koshucharova G, Zweiker R, et al. Different effects of propranolol, bisoprolol, carvedilol and doxazosin on heart rate, blood pressure, and plasma concentrations of epinephrine and norepinephrine. J Clin Basic Cardiol. 2003;6:69–72. [Google Scholar]
- 13.Abbott JA, Broadstone RV, Ward DL, Pyle RL. Hemodynamic effects of orally administered carvedilol in healthy conscious dogs. Am J Vet Res. 2005;66:637–641. doi: 10.2460/ajvr.2005.66.637. [DOI] [PubMed] [Google Scholar]
- 14.Uechi M, Sasaki T, Ueno K, Yamamoto T, Ishikawa Y. Cardiovascular and renal effects of carvedilol in dogs with heart failure. J Vet Med Sci. 2002;64:469–475. doi: 10.1292/jvms.64.469. [DOI] [PubMed] [Google Scholar]
- 15.Marcondes-Santos M, Tarasoutchi F, Mansur AP, Strunz CM. Effects of carvedilol treatment in dogs with chronic mitral valvular disease. J Vet Intern Med. 2007;21:996–1001. doi: 10.1892/0891-6640(2007)21[996:eoctid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Oyama MA, Sisson DD, Prosek R, Bulmer BJ, Leuthy MW, Fuentes VL. Carvedilol in dogs with dilated cardiomyopathy. J Vet Intern Med. 2007;21:1272–1279. doi: 10.1892/07-034.1. [DOI] [PubMed] [Google Scholar]
- 17.Gordon SG, Arsenault WG, Longnecker M, Boothe DM, Miller MW, Chalkley J. Pharmacodynamics of carvedilol in conscious, healthy dogs. J Vet Intern Med. 2006;20:297–304. doi: 10.1892/0891-6640(2006)20[297:pocich]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Beddies G, Fox PR, Papich MD, Boothe DM, Miller MW, Chalkley J. Comparison of the pharmacokinetic properties of bisoprolol and carvedilol in healthy dogs. Am J Vet Res. 2008;69:1659–1663. doi: 10.2460/ajvr.69.12.1659. [DOI] [PubMed] [Google Scholar]