Abstract
Background
The few genetically informative studies to examine post-traumatic stress disorder (PTSD) and alcohol dependence (AD), all of which are based on a male veteran sample, suggest that the co-morbidity between PTSD and AD may be attributable in part to overlapping genetic influences, but this issue has yet to be addressed in females.
Method
Data were derived from an all-female twin sample (n=3768) ranging in age from 18 to 29 years. A trivariate genetic model that included trauma exposure as a separate phenotype was fitted to estimate genetic and environmental contributions to PTSD and the degree to which they overlap with those that contribute to AD, after accounting for potential confounding effects of heritable influences on trauma exposure.
Results
Additive genetic influences (A) accounted for 72 % of the variance in PTSD ; individual-specific environmental (E) factors accounted for the remainder. An AE model also provided the best fit for AD, for which heritability was estimated to be 71 %. The genetic correlation between PTSD and AD was 0.54.
Conclusions
The heritability estimate for PTSD in our sample is higher than estimates reported in earlier studies based almost exclusively on an all-male sample in which combat exposure was the precipitating traumatic event. However, our findings are consistent with the absence of evidence for shared environmental influences on PTSD and, most importantly, the substantial overlap in genetic influences on PTSD and AD reported in these investigations. Additional research addressing potential distinctions by gender in the relative contributions of genetic and environmental influences on PTSD is merited.
Keywords: Alcohol, genetics, post-traumatic stress disorder, women
Introduction
The lifetime prevalence of post-traumatic stress disorder (PTSD) in women is approximately 10–13%, nearly twice that in men (Resnick et al. 1993; Kessler et al. 1995; Breslau et al. 1998) and alcohol abuse and dependence occur at much higher rates in women with PTSD than in women from the general population (Breslau et al. 1997; Kessler et al. 1997; Perkonigg et al. 2000; Danielson et al. 2009). According to National Comorbidity Survey (NCS) data, 27.9 % of women with PTSD meet diagnostic criteria for alcohol use disorders (AUDs), compared to only 13.5% without PTSD (Kessler et al. 1995). Although their cooccurrence is well documented, the mechanisms underlying the association between PTSD and AUDs have not been clearly established. One plausible link is a common source of genetic risk.
The heritability of alcohol dependence (AD) is 50–60% (Reed et al. 1996; True et al. 1996; van den Bree et al. 1998), with the remainder of variance accounted for by individual-specific (i.e. non-shared) environmental influences. Twin studies of PTSD indicate that PTSD is attributable to a greater extent to individual-specific environmental sources of influence (with no substantial shared environmental influences), but genetic contributions are consistently found, typically accounting for about 30 % of variance in risk for the disorder (True et al. 1993; Stein et al. 2002; Koenen et al. 2008). Unlike AD, which has been studied extensively in females, producing nearly identical heritability estimates to those found for males (Kendler et al. 1994; Heath et al. 1997; Prescott et al. 1999), genetic influences on PTSD in women have been examined in only one known twin study (Stein et al. 2002). The vast majority of genetically informative research on PTSD is based on twins from the all-male Vietnam Era Twin Registry (VETR).
Potential common genetic contributions to PTSD and AD have been tested in studies using the VETR sample. Xian et al. (2000) examined PTSD in combination with AD and illicit drug dependence (DD) and reported that 15.3 % of variance in PTSD was accounted for by heritable influences common to AD and DD and 55.7 % of variance in AD was attributable to genetic sources of influence shared by PTSD and DD. In Scherrer et al.’s (2008) study of combat exposure, PTSD and AD in VETR twins, a link found between combat exposure and AD was accounted for by genetic influences shared with PTSD. By contrast, in Koenen et al.’s (2003) co-twin control design study, rates of AD in monozygotic (MZ) co-twins of PTSD-positive twins did not differ from MZ co-twins of combat-exposed twins who did not develop PTSD, indicating that genetic liability to PTSD did not confer additional risk for AD.
All of these VETR studies accounted for possible heritable contributions to trauma exposure, which may otherwise be misattributed to PTSD (Seedat et al. 2001; Afifi et al. 2010). Lyons et al. (1993) estimated heritability of combat exposure to be 47% in the VETR sample and Stein et al. (2002) found evidence for genetic contributions to assaultive (but not nonassaultive) trauma in their general population-based twin sample. An individual’s genetic composition clearly does not directly influence the likelihood of experiencing trauma, but may do so indirectly through heritable personality traits. Jang et al. (2003), for example, found a substantial genetic correlation between exposure to assaultive trauma and both juvenile antisociality and openness to new experiences. Thus, to accurately characterize the relative genetic and environmental influences on PTSD and the overlap of these influences on those that contribute to AD, trauma exposure must also be modeled.
The current study was guided by two primary aims. The first was to estimate heritability of PTSD in young women, using a large (n=3768) community-based sample. This is itself a unique contribution to the literature, as the one known twin study addressing PTSD that included women was based on only 812 twins (25 % of whom were male) and examined symptoms rather than the full PTSD diagnosis (Stein et al. 2002). Our second aim was to test for common genetic influences on PTSD and AD in women after accounting for possible heritable influences on trauma exposure. Given the gender differences in rates of exposure to various types of trauma, conditional probability of developing PTSD following trauma exposure (Resnick et al. 1993; Breslau et al. 1998; Hapke et al. 2006), and prevalence of AD (Holdcraft & Iacono, 2002; Hasin et al. 2007; Keyes et al. 2008), patterns of heritable influences may differ considerably from what has been observed in combat-exposed males.
Method
Participants
The sample was composed of 3768 female twins: 964 MZ pairs, 808 dizygotic (DZ) pairs, and 243 singletons from the Missouri Adolescent Female Twin Study (MOAFTS). The MOAFTS is a longitudinal study of alcohol-related problems and associated psychopathology in female adolescents and young adults using twins born in Missouri between 1975 and 1985. Twins were identified through birth records and recruited from 1995 to 1999 using a cohort-sequential design. Cohorts of 13-, 15-, 17- and 19-year-old female twin pairs and their families were ascertained in the first 2 years. New cohorts of 13-year-old twins and their families were added in the subsequent 2 years. Parental diagnostic interviews were completed by at least one parent in 78 % of eligible families. [See Heath et al. (2002) for additional information on ascertainment.] Wave 3 retest interviews were conducted with a subset of Wave 1 participants 2 years after Wave 1 was completed. (Data were not drawn from Wave 2 assessments as they did not cover all domains of interest for this study and referenced experiences from only the previous 24 months.) All twins from the target cohort (excluding those who had asked not be recontacted) were contacted for Wave 4 interviews, which were conducted from 2002 to 2005. (Approximately 83 % of Wave 1 participants took part in Wave 4. Attrition was not related to history of childhood trauma.) PTSD diagnoses were collected only at Wave 4, but trauma exposure and AD diagnoses were also assessed in Waves 1 and 3. Lifetime trauma and AD status were established using data from all available waves of data collection (78% of the sample had participated in Wave 1 and/or Wave 3). Mean age at the time of Wave 4 interviews was 21.7 years (s.d.=2.7, range=18–29). Eighty-five per cent of the sample self-identified as Caucasian, 15 % as African-American.
Procedure
Data were collected by telephone by trained interviewers. At baseline, a screening to determine zygosity of the twin pair was conducted with one of the twins’ parents. Parental diagnostic interviews were then scheduled and conducted after verbal consent was obtained. Interviews with the twins were conducted after obtaining verbal consent (and parental consent for those under the age of 18). The same protocol was followed in Waves 3 and 4. An interview modified for telephone administration from the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA/SSAGA-II ; Bucholz et al. 1994; Hesselbrock et al. 1999) was used to assess trauma history, early childhood home environment, and DSM-IV diagnoses, including PTSD and AD.
A standard trauma checklist (adapted from the checklist used in the NCS) was used to query history of traumatic experiences. In an effort to capture as many cases of trauma exposure as possible, child maltreatment and sexual assault questions were also included in other sections of the interview under different wording.
Trauma checklist
Respondents were asked if they had experienced any of the following events : (1) fire, flood, or natural disaster ; (2) life-threatening accident ; (3) witnessing someone being badly injured or killed; (4) rape; (5) sexual molestation ; (6) physical abuse as a child ; (7) serious physical attack or assault; (8) serious neglect as a child ; and (9) being threatened with a weapon, held captive, or kidnapped.
Additional child maltreatment and sexual assault questions
Childhood physical abuse and neglect (CPAN) were queried in the early home environment section, which covered experiences that occurred from 6 to 13 years of age. Participants were coded positive for CPAN if they reported either of the following experiences as occurring often : (1) ‘hit with a belt or stick or something like that ’ or (2) ‘physically punished so hard that you hurt the next day’ (with mother figure and father figure queried separately) ; or if they endorsed (3) ‘being physically injured or hurt on purpose by any adult. ’ CPAN criteria could also be met by endorsing (4) ‘non-physical, harsh (lock in closet, deprive of food) ’ or ‘physical harsh (use weapon, punch, whip) ’ as the usual means of punishment by either the mother figure or father figure (queried separately). Sexual molestation was assessed in the home environment section by asking whether before age 16 there was any forced sexual contact with (1) a family member or (2) anyone 5 or more years older. History of rape was assessed in the sexual maturation with the question, ‘Has anyone ever forced you to have sexual intercourse ? ’
Trauma status
Individuals who endorsed criteria at any wave of data collection were coded as positive for that traumatic event. A three-level variable was used to represent trauma status, with no trauma (i.e. none of the items on the traumatic event checklist endorsed and neither child maltreatment nor sexual assault criteria met) as ‘0’, non-assaultive trauma (fire, flood, or natural disaster, life-threatening accident, or witnessing someone being badly injured or killed endorsed on the traumatic event checklist) only as ‘1 ’, and assaultive trauma (child maltreatment or sexual assault criteria met or rape, sexual molestation, physical abuse, neglect, serious physical attack or assault, or being threatened with a weapon, held captive, or kidnapped endorsed on the traumatic event checklist) as ‘2 ’. A total of 43.9% of the sample did not report any trauma, 22.1 % reported non-assaultive trauma only, and 34.0 % reported assaultive trauma. The ranking of assaultive trauma above non-assaultive trauma with respect to risk for PTSD was based on prior literature (Resnick et al. 1993; Breslau et al. 1998; Hapke et al. 2006) and on empirical findings in our data. Participants who experienced assaultive trauma had more than 19 times the odds of developing PTSD than those exposed only to non-assaultive trauma [odds ratio (OR) 19.18, confidence interval (CI) 7.83–47.01] (10.4% developed PTSD compared to 0.6% of women exposed only to non-assaultive trauma). [Prevalence and conditional probability of developing PTSD are reported for each qualifying event in McCutcheon et al. (in press).]
The conditional nature of the PTSD diagnosis (i.e. its contingency on exposure to trauma) necessitated the use of a three-level trauma variable when modeling trauma and PTSD in combination. Correct model specification required that more than one category of trauma exposure had the potential to be associated with positive PTSD status. We allowed for this possibility by classifying trauma as assaultive, nonassaultive, and absent rather than simply present or absent. [See Heath et al. (2004) for this approach to structural equation modeling with contingent phenotypes.]
PTSD
All respondents endorsing one or more traumatic events in the trauma checklist administered at Wave 4 (n=1684; 44.5% of the sample) were asked which of the events they experienced was the most disturbing. They were then asked if they had experienced ‘intense fear, helplessness, or horror ’ following that event (DSM-IV Criterion A). For those who responded ‘yes’, this became the index event for which PTSD symptoms were assessed. For Criteria B, C and D, diagnostic questions were administered only if the preceding criterion was met. Criterion B was met if 1 or more of the five possible re-experiencing symptoms were endorsed. Meeting Criterion C required endorsement of three or more of the seven possible avoidance symptoms. Criterion D was met if two or more of the five arousal symptoms were endorsed. A diagnosis of PTSD was given if Criteria A–D were met and respondents reported clinically significant distress or impairment and persistence of symptoms for ≥1 month. The prevalence of PTSD in the sample was 3.7% (n=138). As a PTSD diagnosis is contingent on trauma exposure, participants who did not endorse any traumatic experiences were coded as missing for PTSD.
AD
AD was also defined according to DSM-IV, as three or more of the seven possible AD symptoms occurring during the same 12-month period. Individuals who endorsed full AD criteria at any wave of data collection were coded positive for lifetime AD.
Data analysis
Twin modeling
Genetic analyses conducted with twins reared together are used to decompose the variance of a phenotype into additive genetic sources (A), shared environmental sources (C; influences that make members of a twin pair more alike, such as family and school) and environmental influences that contribute to within-pair differences (E). Genetic influence is suggested by higher correlations in MZ than DZ twin pairs for the phenotype ; shared environmental influence is indicated by DZ twin correlations exceeding half of MZ twin correlations. Non-additive sources of genetic influence (D) can be estimated in place of C when the correlation between members of DZ twin pairs is less than half of that between their MZ counterparts. However, as C and D cannot be estimated jointly when data from twins alone are used, the choice of an ACE versus an ADE model is made based on results of twin correlations. In our sample, the DZ correlations were greater than half the MZ correlations for trauma exposure (rDZ=0.51, rMZ= 0.60) and AD (rDZ=0.45, rMZ=0.71). In the case of PTSD, the twin correlation for DZs was less than half that for MZs (rDZ=0.19, rMZ=0.69). We therefore tested an ADE model in the univariate analysis. D could be dropped without a significant change in model fit, so we fitted an ACE model to represent genetic and environmental influence on the three phenotypes in the trivariate analysis.
Trivariate Cholesky
A trivariate genetic Cholesky decomposition was fitted to assess the degree of overlap in genetic and environmental influences between trauma exposure, PTSD and AD. Models were fitted in Mx (Neale et al. 2003) using raw categorical data. Thresholds were adjusted for age at time of report (divided into three approximately equal groups based on age distributions) using two dummy variables for each pheno-type: 13–18 and 22–29, with 19–21 as the comparison group for trauma exposure; 18–19 and 25–29, with 20–24 as the comparison group for PTSD (because all participants were 18 or older when PTSD was assessed); and 13–19 and 23–29, with 20–22 as the comparison group for AD. For those individuals who did not endorse a given condition/diagnosis, age at time of report was coded as age at Wave 4 interview, that is, age at the time the absence of that condition/ diagnosis was reported. A series of submodels examining the statistical significance of A, C and E were compared to the full model to derive the best-fitting trivariate model. Submodels were tested by calculating the difference between the −2 log likelihood fit of the full model and the nested submodel, which is distributed as χ2 for the given degrees of freedom.
Results
Table 1 displays the rates of AD by non-assaultive and assaultive trauma status separately for those who met criteria for PTSD and those who did not (and also for women who did not report any traumatic experiences). As expected, a greater proportion of women with versus without lifetime PTSD diagnoses were alcohol dependent: one in three compared to only one in nine. Rates of AD did not differ significantly by non-assaultive versus assaultive trauma status among women with PTSD (although this finding should be interpreted with caution, given the low prevalence of PTSD in women who experienced only non-assaultive trauma). However, increasing rates of AD from no trauma (7.3%) to non-assaultive trauma (12.4 %) to assaultive trauma (16.4%) were observed in women without PTSD [χ2(2)=59.3, p<0.001].
Table 1.
Rates of lifetime DSM-IV AD by trauma and lifetime DSM-IV PTSD status
| PTSD |
No PTSD |
|||
|---|---|---|---|---|
| n | Prevalence of AD (%) |
n | Prevalence of AD (%) |
|
| No trauma | – | – | 1649 | 7.3 |
| Non-assaultive trauma |
5 | 40.0 | 825 | 12.4 |
| Assaultive trauma | 133 | 33.1 | 1144 | 16.6 |
| Totala | 138 | 33.3 | 3618 | 11.3 |
PTSD, Post-traumatic stress disorder ; AD, alcohol dependence.
Thirty-one cases missing trauma status.
Within-twin cross-trait polychoric correlations revealed modest associations between the phenotypes. For trauma exposure and AD, r=0.27, and for PTSD and AD, r=0.30.
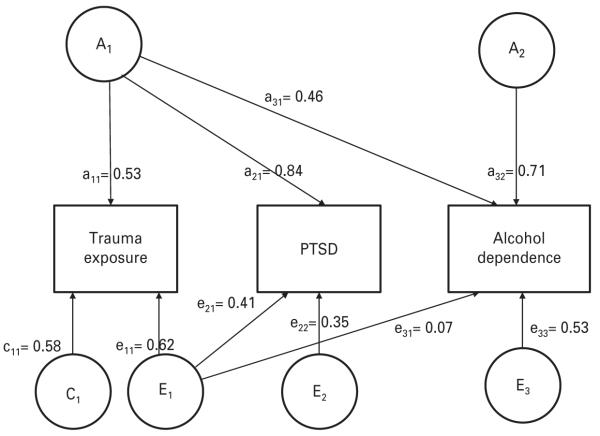
The best-fitting trivariate Cholesky is shown with unstandardized path coefficients in Fig. 1 and summarized with standardized coefficients and 95 % confidence limits in Table 2. The final model was derived by conducting a series of tests. First, for each pheno-type we dropped the A and C variance components with values below 0.40 (A for trauma exposure and C for PTSD and AD) one at a time and compared the resulting submodels to the full model. Dropping the A pathway from trauma exposure resulted in a significant deterioration in model fit [Δχ2(3)=8.50, p=0.04], but removal of neither C pathway significantly impacted model fit. [For PTSD, Δχ2(3)=2.78, p=0.43 and for AD, Δχ2(3)=5.11, p=0.16.] In the second step, the C pathways were dropped simultaneously from PTSD and AD and the reduced model was compared to the full model. No deterioration in fit was observed [Δχ2(5)=4.44, p=0.49], so the reduced model was accepted. Next, we tested this reduced model against a model in which a single factor accounted for genetic influences on trauma exposure and PTSD and found no significant change in model fit [Δχ2(3)=0.16, p=0.92]. Variance in these three phenotypes was best represented with an ACE model for trauma exposure and AE models for PTSD and AD, with one genetic factor accounting for heritable influences on trauma exposure and PTSD and a second accounting for heritable influences on AD.
Fig. 1.
Trivariate Cholesky decomposition : genetic and environmental influences on trauma exposure, post-traumatic stress disorder (PTSD) and alcohol dependence (AD). Unstandardized pathways are shown. A, additive genetic ; C, shared environmental ; and E, non-shared environmental influences.
Table 2.
Magnitude of additive genetic (A), shared environmental (C), and non-shared environmental (E) influences on trauma exposure, PTSD and AD : best-fitting trivariate model
| Proportion of total variance attributable to A, C and E |
|||
|---|---|---|---|
| Phenotype | A | C | E |
| Trauma exposure | A1 0.28 (95% CI 0.16–0.33) |
0.33 (95% CI 0.21–0.36) |
E1 0.39 (95% CI 0.31–0.44) |
| PTSD | 0.71 (95% CI 0.41–0.85) |
– | E2 0.29 (95% CI 0.12–0.40) |
| AD | A2 0.72 (95% CI 0.66–0.74) |
– | E3 0.28 (95% CI 0.21–0.38) |
PTSD, Post-traumatic stress disorder ; AD, alcohol dependence ; CI, confidence interval.
In the final model, over two-thirds of the variance in PTSD (71 %) and AD (72 %) were attributable to additive genetic effects, with the remainder accounted for by individual-specific environmental influences. By contrast, only 28 % of variance in trauma exposure was accounted for by genetic factors. Unlike either PTSD or AD, to which there was no evidence of significant shared environmental contributions, 33 % of variance in trauma exposure was attributable to C. A considerable degree of overlap was observed between the two genetic factors, with rA1–A2=0.54, CI 0.31–0.66. Individual-specific environmental influences on trauma exposure were highly correlated with those contributing to PTSD (rE1–E2=0.75, CI 0.29–0.82) and modestly correlated with those contributing to AD (rE1–E3=0.13, CI 0.04–0.38), but CIs for covariance in E between AD and PTSD included 0, so this pathway was not included in the final model.
Discussion
The current investigation extends our understanding of the co-morbidity of PTSD and AD in women by characterizing common genetic and environmental contributions to the two disorders using a large, all-female community-based sample. Ours is the first known study to quantify the degree of overlap in the heritable and environmental influences on PTSD and AD in women and one of only a few to estimate heritability of PTSD in a sample that includes females. The existing literature is based primarily on an all-male twin sample in which combat was the traumatic event that led to PTSD. Our examination of PTSD and AD in an all-female population-based sample allows us both to uncover potential differences by gender in the relative role of genetics versus environment and to study the inter-relatedness of these heritable and environmental influences in the context of a broader range of trauma exposures.
Consistent with the existing literature, phenotypic analyses revealed elevated rates of lifetime AD diagnoses in women who had experienced traumatic events (Fergusson et al. 1996; Kilpatrick et al. 1997; Wilsnack et al. 1997), with an even more pronounced elevation in women meeting lifetime criteria for PTSD (Kessler et al. 1995; Breslau et al. 1997; Danielson et al. 2009). We also found that, among women who reported traumatic events but had no history of PTSD, the likelihood of meeting AD criteria was higher in those who experienced assaultive versus only nonassaultive traumas (paralleling the elevated rates of PTSD in victims of assaultive events). Genetic analyses produced heritability estimates of 28%, 72% and 71% for trauma exposure, PTSD and AD respectively, and indicated that trauma exposure and PTSD were attributable to the same source of genetic variance. Whereas only additive genetic and individual-specific environmental influences were evident for PTSD and AD, shared environmental factors were also found to play a role in trauma exposure, accounting for 33% of variance. Of greatest relevance to our primary research question, we found that the genetic factors that contribute to trauma exposure and PTSD were correlated at 0.54 with those that contribute to AD, thus accounting for just under 30 % of the genetic variance in AD. Although we did not test a causal model of the PTSD–AD association (Chilcoat & Breslau, 1998), our findings have implications for such models, specifically that they need to take into account the contribution of common genetic influences to the two disorders.
The relative contributions of genetic and environmental factors to AD observed in our sample are very similar to those reported in earlier studies, with additive genetic factors accounting for just over half of the variance in the disorder and individual-specific, but not shared, environmental influences accounting for just under half (Kendler et al. 1994; van den Bree, 1998; Prescott et al. 1999). By contrast, the heritability estimate for PTSD in our sample is approximately twice that reported in earlier investigations. Xian et al.’s (2000) study using the VETR sample, which is among the few to examine the full lifetime PTSD diagnosis, reported the heritability of PTSD to be 35%. Although not as directly comparable to our findings, similar values have been reported for PTSD symptoms. Stein et al. (2002), for example, found that 38 % of variance in PTSD symptoms was accounted for by genetic sources. Discrepancies between our findings and earlier reports cannot be attributed to the possible confounding effects of genetic contributions to trauma exposure itself, as we modeled trauma as a separate phenotype in our analyses. They are also not likely to be due purely to our exclusive focus on females or to the wider range of traumas represented in our sample, as the sample used by Stein et al. (2002) was nonmilitary and 75 % female. However, given that Stein and colleagues did not test for gender differences in heritability estimates, it is possible that modest distinctions by gender in the relative contribution of genetic factors were present but not identified. Another factor to consider is that the prevalence of PTSD in our sample is lower than that reported for women in previous studies (Resnick et al. 1993; Kessler et al. 1995; Breslau et al. 1998). If this reflects under-reporting, we may have identified only the most severe cases of PTSD. To the extent that severity is associated with heritability, categorizing only cases at the high end of the severity continuum as positive for PTSD could inflate the heritability estimate. Importantly, although we found a greater relative contribution of additive genetic influences to PTSD than has been reported previously, the support in our sample for an AE model is consistent with all prior genetically informative studies of PTSD.
Although intended to be a statistical control rather than a focus of the current study, the results of genetic modeling of the trauma exposure phenotype merit comment. We found that 28 % of variance in trauma exposure could be accounted for by genetic sources. In the VETR sample, Lyons et al. (1993) reported 47% heritability for combat exposure. Stein et al. (2002) found no evidence for genetic influences on nonassaultive trauma, but estimated heritability for assaultive trauma at 20%. Our finding that 33 % of variance in trauma exposure was accounted for by shared environmental factors is closely aligned with Stein et al.’s estimates of 21 % and 39 % for assaultive and non-assaultive trauma respectively, but contrasts sharply with the absence of shared environmental influences reported in the VETR sample. With so few studies against which to compare our results, it is difficult to draw any definitive conclusions, but the greater similarity of our results to those of Stein et al. (2002) versus Lyons et al. (1993) suggests that the relative contribution of genetic and shared environmental influences may vary by gender and/or nature of the trauma. Again, although rarely reported in other studies, the high genetic correlation we found between trauma exposure and PTSD has also been documented previously (Stein et al. 2002).
Despite our substantially higher heritability estimate for PTSD, the associations observed in our sample between genetic and environmental factors that influence PTSD and those that influence AD are highly comparable to results from the VETR sample (the only other sample in which genetic overlap between the disorders has been studied). In Xian et al.’s (2000) study examining PTSD and AD in combination with drug dependence and also in Scherrer et al.’s (2008) study examining PTSD and AD with combat exposure, common genetic contributions to PTSD and AD were evident. Also consistent with our findings, Scherrer et al. (2008) found genetic overlap between combat exposure and PTSD.
Limitations
Certain limitations should be kept in mind when interpreting the results from the current investigation. First, although the higher rate of PTSD in women who experienced assaultive versus only non-assaultive traumas supports the validity of this distinction as a crude severity indicator, we expect that a more direct measure of severity (e.g. subjective ratings of distress) would be more accurate. Second, our use of retrospective reports introduced potential retrospective reporting biases, although the adjustments made for age at time of report for all three phenotypes likely reduced their impact. Third, although integrating data from earlier waves of data collection and other sections of the interview with the Wave 4 trauma checklist enhanced the accuracy in establishing trauma status, only individuals who endorsed events on the trauma checklist (which, unlike the questions from early home environment and sexual maturation questions, were not worded behaviorally) were assessed for PTSD. As a result, PTSD diagnoses were missing for this subset of the sample. Fourth, although highly comparable to the lifetime prevalence of trauma exposure reported in studies using similar definitions, the rate of trauma exposure in our sample is lower than that reported in studies using broader definitions (e.g. learning about a loved one experiencing a traumatic event). Fifth, most, but not all, participants had passed the age of risk for AD. Similarly, some women in our study who had not yet been exposed to trauma or developed PTSD are likely to do so in the future, so we cannot assume that our findings are equally valid in older women.
Future directions
Given the scarcity of the literature in this area, the first priority in this line of research is to attempt to replicate our findings in another genetically informative female sample. The next logical step is to test for gender differences in heritability of PTSD and its genetic overlap with AD in a civilian sample of men and women. Studies in this area are of particular interest in light of the absence of evidence of gender differences for other anxiety disorders (Hettema et al. 2005; Kendler et al. 2003) and mixed evidence for depression (Bierut et al. 1999; Franic et al. 2010). It is also important to address potential distinctions in both the link with AD and the relative contribution of genetic factors to childhood versus adulthood trauma exposure and PTSD onset. With respect to identifying populations at high risk for substance-related problems, it is also important to determine the extent to which our findings generalize to other substances of abuse.
Acknowledgments
This study was funded by grants AA009022, AA007728, AA017010, AA017688, AA018146 and AA011998 from the National Institute on Alcohol Abuse and Alcoholism, grant HD049024 from the National Institute of Child Health and Human Development, and grants DA014363, DA018267, DA027995, DA012854 and DA017305 from the National Institute on Drug Abuse.
Footnotes
Declaration of Interest None.
References
- Afifi TO, Asmundson GJG, Taylor S, Jang KL. The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms : a review of twin studies. Clinical Psychology Review. 2010;30:101–112. doi: 10.1016/j.cpr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Heath AC, Bucholz KK, Dinwiddie SH, Madden PAF, Statham DJ, Dunne MP, Martin NG. Major depressive disorder in a community-based twin sample : are there different genetic and environmental contributions for men and women ? Archives of General Psychiatry. 1999;56:557–562. doi: 10.1001/archpsyc.56.6.557. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Archives of General Psychiatry. 1997;54:81–87. doi: 10.1001/archpsyc.1997.01830130087016. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community. Archives of General Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr., Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies : a report of the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N. Investigations of causal pathways between PTSD and drug use disorders. Addictive Behaviors. 1998;23:827–840. doi: 10.1016/s0306-4603(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Danielson CK, Amstandter AB, Dangelmaier RE, Resnick HE, Saunders BE, Kilpatrick DG. Trauma-related risk factors for substance abuse among male versus female young adults. Addictive Behaviors. 2009;34:395–399. doi: 10.1016/j.addbeh.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Childhood sexual abuse and psychiatric disorder in young adulthood : II. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1365–1374. doi: 10.1097/00004583-199610000-00024. [DOI] [PubMed] [Google Scholar]
- Franic S, Middledorp CM, Dolan CV, Ligthart L, Boomsma DI. Childhood and adolescent anxiety and depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:820–829. doi: 10.1016/j.jaac.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Hapke U, Schumann A, Rumpf H, John U, Meyer C. Post-traumatic stress disorder : the role of trauma, pre-existing psychiatric disorders, and gender. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:299–306. doi: 10.1007/s00406-006-0654-6. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States : results from the National Epidemiological Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut RJ, Statham DJ, Dunne NP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample : consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Howells W, Bucholz KK, Glowinski AL, Nelson EC, Madden PA. Ascertainment of a midwestern U.S. female adolescent twin cohort for alcohol studies : assessment of sample representativeness using birth record data. Twin Research. 2002;5:107–112. doi: 10.1375/1369052022974. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PAF. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Research. 2004;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA : a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Archives of General Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cohort effects on gender differences in alcohol dependence. Addiction. 2002;97:1025–1036. doi: 10.1046/j.1360-0443.2002.00142.x. [DOI] [PubMed] [Google Scholar]
- Jang KL, Stein MB, Taylor S, Asmundson GJG, Livesley WJ. Exposure to traumatic events and experiences : aetiological relationships with personality function. Psychiatry Research. 2003;120:61–19. doi: 10.1016/s0165-1781(03)00172-0. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LC. A twin-family study of alcoholism in women. American Journal of Psychiatry. 1994;151:707–715. doi: 10.1176/ajp.151.5.707. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug and Alcohol Dependence. 2008;93:21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Acierno R, Resnick HS, Saunders BE, Best CL. A 2-year longitudinal analysis of the relationship between violent assault and substance use in women. Journal of Consulting and Clinical Psychology. 1997;65:834–847. doi: 10.1037//0022-006x.65.5.834. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Fu QJ, Ertel K, Lyons MJ, Eisen SA, True WR, Goldberg J, Tsuang MT. Common genetic liability to major depression and posttraumatic stress disorder in men. Journal of Affective Disorders. 2008;105:109–115. doi: 10.1016/j.jad.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Lyons MJ, Goldberg J, Simpson J, Williams WM, Toomey R, Eisen SA, True WR, Cloitre M, Wolfe J, Tsuang M. A high risk study of combat-related PTSD comorbidity. Twin Research. 2003;6:218–226. doi: 10.1375/136905203765693870. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Goldberg J, Eisen SA, True W, Tsuang MT, Meyer JM, Henderson WG. Do genes influence exposure to trauma ? A twin study of combat. American Journal of Medical Genetics (Neuropsychiatric Genetics) 1993;8:22–27. doi: 10.1002/ajmg.1320480107. [DOI] [PubMed] [Google Scholar]
- McCutcheon VV, Sartor CE, Pommer NE, Bucholz KK, Nelson EC, Madden PAF, Heath AC. Age at trauma exposure and PTSD risk in a young adult female sample. Journal of Traumatic Stress. doi: 10.1002/jts.20577. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx : Statistical Modeling. 6th edn Virginia Commonwealth University; Richmond, VA: 2003. [Google Scholar]
- Perkonigg A, Kessler RC, Storz S, Wittchen H. Traumatic events and post-traumatic stress disorder in the community : prevalence, risk factors and comorbidity. Acta Psychiatrica Scandinavica. 2000;101:46–59. doi: 10.1034/j.1600-0447.2000.101001046.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcoholism : Clinical and Experimental Research. 1999;23:1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Reed T, Page WF, Viken RJ, Christian JC. Genetic predisposition to organ-specific endpoints of alcoholism. Alcoholism : Clinical and Experimental Research. 1996;20:1528–1533. doi: 10.1111/j.1530-0277.1996.tb01695.x. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. Journal of Consulting and Clinical Psychology. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Scherrer JF, Xian H, Lyons MJ, Goldberg J, Eisen SA, True WR, Tsuang M, Bucholz KK, Koenen KC. Posttraumatic stress disorder ; combat exposure ; and nicotine dependence, alcohol dependence, and major depression in male twins. Comprehensive Psychiatry. 2008;49:297–304. doi: 10.1016/j.comppsych.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedat S, Niehaus DJ, Stein DJ. The roles of genes and family in trauma exposure and posttraumatic stress disorder. Molecular Psychiatry. 2001;6:360–362. doi: 10.1038/sj.mp.4000867. [DOI] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms : a twin study. American Journal of Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- True WR, Heath AC, Bucholz KK, Slutske W, Romeis JC, Scherrer JF, Lin N, Eisen SA, Goldberg J, Lyons MJ, Tsuang MT. Models of treatment seeking for alcoholism : the role of genes and environment. Alcoholism : Clinical and Experimental Research. 1996;20:1577–1581. doi: 10.1111/j.1530-0277.1996.tb01702.x. [DOI] [PubMed] [Google Scholar]
- True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Archives of General Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- van den Bree MBM, Johnson EO, Neale MC, Svikis DS, McGue M, Pickens RW. Genetic analysis of diagnostic systems of alcoholism in males. Biological Psychiatry. 1998;43:139–145. doi: 10.1016/S0006-3223(97)00225-4. [DOI] [PubMed] [Google Scholar]
- Wilsnack SC, Vogeltanz ND, Klassen AD, Harris TR. Childhood sexual abuse and women’s substance abuse : national survey findings. Journal of Studies on Alcohol. 1997;58:264–271. doi: 10.15288/jsa.1997.58.264. [DOI] [PubMed] [Google Scholar]
- Xian H, Chantarujikapong SI, Scherrer JF, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug and Alcohol Dependence. 2000;61:95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]