Abstract
Maintaining coral reef resilience against increasing anthropogenic disturbance is critical for effective reef management. Resilience is partially determined by how processes, such as herbivory and nutrient supply, affect coral recovery versus macroalgal proliferation following disturbances. However, the relative effects of herbivory versus nutrient enrichment on algal proliferation remain debated. Here, we manipulated herbivory and nutrients on a coral-dominated reef protected from fishing, and on an adjacent macroalgal-dominated reef subject to fishing and riverine discharge, over 152 days. On both reefs, herbivore exclusion increased total and upright macroalgal cover by 9–46 times, upright macroalgal biomass by 23–84 times, and cyanobacteria cover by 0–27 times, but decreased cover of encrusting coralline algae by 46–100% and short turf algae by 14–39%. In contrast, nutrient enrichment had no effect on algal proliferation, but suppressed cover of total macroalgae (by 33–42%) and cyanobacteria (by 71% on the protected reef) when herbivores were excluded. Herbivore exclusion, but not nutrient enrichment, also increased sediment accumulation, suggesting a strong link between herbivory, macroalgal growth, and sediment retention. Growth rates of the corals Porites cylindrica and Acropora millepora were 30–35% greater on the protected versus fished reef, but nutrient and herbivore manipulations within a site did not affect coral growth. Cumulatively, these data suggest that herbivory rather than eutrophication plays the dominant role in mediating macroalgal proliferation, that macroalgae trap sediments that may further suppress herbivory and enhance macroalgal dominance, and that corals are relatively resistant to damage from some macroalgae but are significantly impacted by ambient reef condition.
Keywords: Plant-herbivory, Eutrophication, Fiji, MPA, Overfishing
Introduction
Corals, and the reefs they build, are in rapid global decline due to numerous anthropogenic stresses (Bellwood et al. 2004; Knowlton and Jackson 2008; Hughes et al. 2010). Interactions between climate-induced coral bleaching (Hoegh-Guldberg et al. 2007; Baker et al. 2008), coral disease (Bruno et al. 2003, 2007; Harvell et al. 2007), coastal pollution (Bruno et al. 2003) and the cascading effects of overfishing (Jackson et al. 2001; Bellwood et al. 2004; Raymundo et al. 2009) have led to dramatic losses of coral over large spatial scales (Hughes et al. 2003, 2010; Bellwood et al. 2004). Emerging research suggests that overfishing of reef herbivores at local scales limits the capacity of corals to resist or recover from global-scale disturbance (Hughes et al. 2003, 2007, 2010; Mumby and Steneck 2008); the loss of herbivores from already-disturbed reefs has commonly been followed by macroalgal proliferation (i.e. a “phase-shift”) (Folke et al. 2004; Hughes et al. 2010). Once established, algal-dominated communities limit coral and herbivore recruitment, reduce intensity of herbivory, and thereby reinforce the persistence of algal-dominated communities (Mumby et al. 2007a; Mumby and Steneck 2008; Hughes et al. 2010; Hoey and Bellwood 2011). However, the relative importance of processes mediating macroalgal proliferation and phase shifts on reefs are debated (Lapointe et al. 2004; Burkepile and Hay 2006; Littler et al. 2006a, b; Heck and Valentine 2007; Houk et al. 2010; Smith et al. 2010).
Numerous empirical, theoretical, and meta-analytical studies suggest that the “top–down” process of herbivory plays a critical role in determining the abundance and distribution of macroalgae, and the outcome of coral–algal interactions affecting phase-shifts on reefs (Lewis 1986; Jompa and McCook 2002; Burkepile and Hay 2006; Heck and Valentine 2007; Hughes et al. 2007; Mumby et al. 2007a; Burkepile and Hay 2008; Elmhirst et al. 2009; Rasher and Hay 2010). Manipulations of reef herbivores (Lewis 1986; Hughes et al. 2007; Burkepile and Hay 2008), long-term observations of reef decline (Hughes 1994; Cheal et al. 2010), and monitoring of the consequences of reef protection (Mumby et al. 2007b; Mumby and Harborne 2010) all suggest that herbivores strongly suppress macroalgal colonization and growth, lessen algal damage to corals, and promote coral recruitment and growth. For many of these studies, strong herbivore effects were observed even in the presence of elevated nutrient levels that might stimulate algal growth, indicating that herbivory may buffer against increased macroalgal production associated with nutrient enrichment (Burkepile and Hay 2006; Heck and Valentine 2007). However, a few field studies suggest that the “bottom–up” process of nutrient supply can mediate algal proliferation, even in the presence of herbivory, if threshold nutrient levels are exceeded (Lapointe 1997; Smith et al. 2001; Lapointe et al. 2004; Littler et al. 2006a, b). Other studies demonstrate that nutrient enrichment can impact algal proliferation if herbivory is strongly reduced (Burkepile and Hay 2006, 2009; Smith et al. 2010), and if experiments are conducted over sufficient time-scales for nutrient effects to emerge (Smith et al. 2010). Moreover, small-scale field manipulations may not match large-scale, long-term survey results (Houk et al. 2010) or long-term manipulative studies (Smith et al. 2010), and some authors suggest that results from studies conducted on reefs already dominated by macroalgae may not be typical of reefs that have yet to undergo phase-shifts (Smith et al. 2010). Thus, although the preponderance of data available to date indicates a greater role for top–down than for bottom–up forces, the relative influences of these forces on algal proliferation can be context dependent (Burkepile and Hay 2006; Houk et al. 2010; Smith et al. 2010).
This context-dependent nature of top–down versus bottom–up control of reef community state has created a debate concerning the relative importance of each process, in part due to the limited number of studies that have interactively assessed herbivory and nutrient enrichment, and due to the limited duration and/or scale of most experiments (Burkepile and Hay 2006; Houk et al. 2010; Smith et al. 2010). Additionally, even fewer studies have monitored the cascading effects of these processes on coral recruitment, growth and/or survival (Burkepile and Hay 2009; Sotka and Hay 2009; Houk et al. 2010; Smith et al. 2010). Moreover, studies have rarely assessed the importance of these processes along gradients of environmental stress, such as on fished reefs dominated by macroalgae versus protected reefs dominated by corals or among reefs with varying levels of natural or anthropogenic nutrient input—such studies are needed to better evaluate the context-dependency of bottom–up versus top–down effects (Houk et al. 2010; Smith et al. 2010). Increased knowledge of the cascading effects of herbivore exploitation versus reef eutrophication is critical for the prioritization of management efforts that increase reef resistance to phase shifts and/or facilitate reef recovery.
The goals of this study were to: (1) assess the relative influence of top–down (herbivory) versus bottom–up (nutrient supply) processes on the development of benthic macroalgal communities, (2) determine how these processes differ on coral- versus macroalgal-dominated reefs, and (3) monitor the cascading impacts of these resultant algal communities on sediment accumulation and coral growth. To accomplish these goals, we conducted field experiments that assessed the individual and interactive effects of herbivore exclusion and nutrient enrichment on macroalgal proliferation, sediment accumulation, and coral growth on a coral-dominated Fijian reef protected from fishing, and on an adjacent macroalgal-dominated reef subject to local artisan fishing and riverine discharge, over 152 days.
Materials and methods
Study site and experimental design
We assessed the effects of herbivore exclusion, nutrient enrichment, and the interaction of these factors on algal community development, sediment accumulation, and coral growth at two shallow reef flat sites (~0.5 km apart) along the Coral Coast of Viti Levu, Fiji (18°13.049′S, 177°42.968′E), 20 October 2008 to 20 March 2009 (duration = 152 days). Using a fully factorial design [herbivores/no nutrient enrichment (+H−N), herbivores/nutrient enrichment (+H+N), herbivore exclusion/no nutrient enrichment (−H−N), herbivore exclusion/nutrient enrichment (−H+N)], we deployed spatially blocked sets of treatments onto shallow reef flats (~1 m depth low tide; ~2 m depth high tide) (1) within the boundaries of a no-take marine protected area on a minimally developed shoreline (herein referred to as “MPA”) and (2) within the boundaries of an adjacent area subject to impacts associated with local artisan fishing, an immediately adjacent village, and the nutrient/sediment input from a small river that runs by the village (herein referred to as “non-MPA”). Treatments were spatially blocked to control for small-scale variation in herbivory and ambient nutrient supply. The MPA is characterized by 57% coral cover, 3% upright fleshy macroalgal cover, and high rates of macrophyte removal by fishes; the non-MPA is characterized by 3% coral cover, 47% macroalgal cover, and low macroalgal removal rates (Rasher and Hay 2010). Thus, our experimental design allowed us to assess the localized effects of herbivory and nutrient enrichment under differing levels of fishing, adjacent human settlement, and riverine discharge. Treatments within blocks were separated by 1–3 m, while blocks (n = 10 per site) were separated by 20–25 m.
This design allows independence and interspersion of treatments within each larger site, but potentially confounds MPA versus non-MPA contrasts with location since there is only one larger site of each type. This limitation should be noted, but is reduced somewhat by the close proximity, similar depth, similar orientation, etc. of the two sites. Additionally, villager statements indicate that 30+ years ago, both sites supported high coral and low algal abundance, suggesting similar biotic communities were historically supported at both sites.
Each experimental unit was constructed from a concrete cinder block (~10 × 20 × 40 cm), cemented flat to the reef substrate. The upper surface of each block (800 cm2) provided a settlement site for benthic organisms, and allowed for the slow diffusion of nutrients to the upper surface of the block for treatments where fertilizers were sealed into the center spaces within each block (Miller et al. 1999; Burkepile and Hay 2009). To exclude large herbivores, we encircled mesh wire (1 cm2 grid) around each block to form a tube with a diameter of ~50 cm and closed the ends of the tube with the same wire mesh. To control for shading and hydrodynamic effects of the mesh, but allow for block access to both small and large herbivores, we enclosed “herbivore” treatment blocks within the same types of mesh tubes but left the ends open. Previous studies using this design found no significant difference in algal communities between blocks with partial cages and cage-free blocks (Miller and Hay 1998; Sotka and Hay 2009). Cages were inspected for damage and brushed clean every 30 days.
Nutrient enrichment
To produce nutrient enrichment treatments, we sealed one side of both internal chambers on a block with cement, placed 100 ± 10 g Osmocote (Scotts, USA) commercial slow-release fertilizer pellets (19:6:12, N:P:K) held inside a mesh pouch (L’eggs stockings, USA) within each block chamber, and plugged each of the opposite sides of the block opening with a section of removable closed-cell foam (Miller et al. 1999). Additional nutrients were added every 30 days as previous studies demonstrated that this frequency of addition maintained enhanced nutrient levels (Miller et al. 1999; Burkepile and Hay 2009). As with previous applications of this method (Miller et al. 1999; Burkepile and Hay 2009; Sotka and Hay 2009), our goal was to deliver a localized supply of nutrients to algal tissues growing directly on the experimental surface. Blocks without nutrient enrichment treatments were sealed in the same way, but no nutrients were placed within chambers of those blocks.
To assess the efficacy of our nutrient enrichment treatment, we measured carbon:nitrogen (C:N) ratios within tissues of Padina boryana (the most abundant macrophyte) growing on enriched versus non-enriched blocks excluded from herbivores at the end of the 152-day study. These same Padina tissues were also sampled for elemental and isotopic (15N, 13C) composition to assess the degree of nutrient limitation between sites, as well as the relative contribution of marine- versus terrestrially-derived nutrients incorporated into macrophyte tissues from ambient waters.
Algal community development
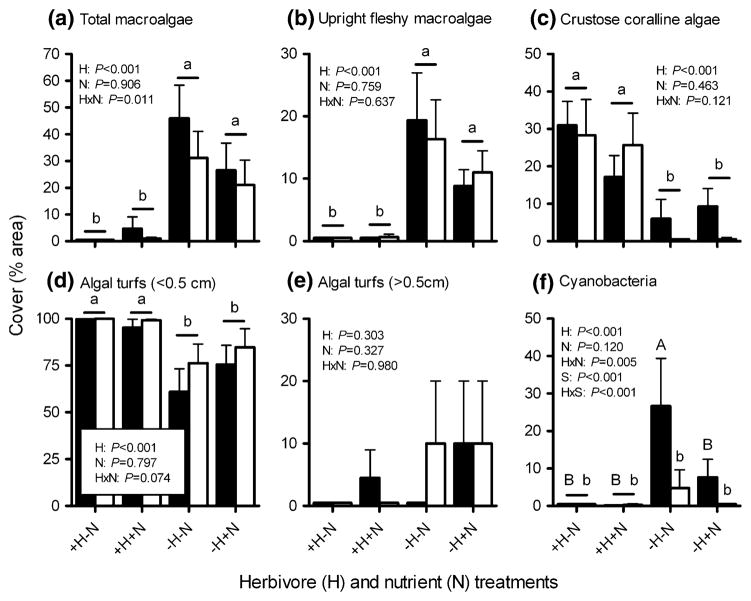
At the end of the 152-day experiment, we quantified cover of algae on the upper surface (a 20 × 40 cm rectangle = 800 cm2) of each experimental block by laying a beaded chain over the block surface and identifying algae under each of 60 randomly pre-marked points. Algae were identified to the lowest taxonomic level possible in the field, but most algae were categorized into morphological or taxonomic groups [upright fleshy macrophytes, algal turfs <0.05 cm, algal turfs >0.05 cm, cyanobacteria, crustose coralline algae (herein known as “CCA”)] because high-resolution taxonomic identification in the field was problematic. Greater than 95% of all upright fleshy macrophyte biomass was Padina spp.; thus, upright macroalgae were pooled for analyses. If more than one species was present under a single point (e.g., CCA overgrown by an upright macrophyte), both species were counted; as such, cover could exceed 100%. We also removed upright macroalgae from the top surface of each block (a 20 × 40 cm rectangle = 800 cm2), transported them to the laboratory in sealed plastic bags, removed excess water with a salad spinner (10 revolutions), and obtained total wet mass (g) of upright macroalgae. These macroalgal samples were then frozen for elemental and isotopic analysis (see below). Blocks were visually inspected for coral recruits, but none were noted on the blocks. “Total algal cover” (see Fig. 1a) was calculated as the sum of upright fleshy macroalgae, cyanobacteria, and tall algal turf (>0.05 cm) cover. We excluded algal turfs <0.5 cm and CCA from this grouping, as these groups are (1) unlikely to impact the size-class of corals we deployed on our experimental blocks, (2) are unlikely to suppress coral recruitment (Birrell et al. 2008), and (3) are characteristic of healthy reefs with high rates of herbivory (Steneck 1988; Burkepile and Hay 2006). At the end of the experiment, we also scraped sediments and filamentous algae from each block into a plastic bag, brushed and washed each block (above water), and then quantified cover of CCA in the absence of larger algae and sediments that could have obscured cover of CCA. CCA cover was quantified using 100 points set randomly within a 15 × 30 cm quadrat. However, in situ and post-scraping point counts did not differ (Wilcoxon signed rank test, P = 0.155, n = 80), so in situ counts were used for analyses to maintain consistency in scoring. Data for algal cover and biomass violated parametric assumptions, so the effects of herbivores, nutrients, and site on algal accumulation were analyzed with three-factor analyses of variance (ANOVA) on rank-transformed data.
Fig. 1.
Percent cover (area per 800 cm2, mean + SE) of a total macroalgae and b–f common algal types on settlement blocks accessible (+H) or inaccessible (−H) to herbivores, both without (−N) and with (+N) nutrient enrichment, when deployed on a reef in a no-take marine protected area (MPA; black bars) or on an adjacent fished reef (non-MPA; white bars) for 152 days (n = 10 per treatment per site). P values are from three-factor analyses of variance (ANOVA) of rank-transformed data. See Table 1 for complete ANOVA results. Letters indicate significant groupings by Tukey multiple comparisons tests. Horizontal bars indicate non-significant differences between sites (S), within a treatment. For (f), upper and lower case letters distinguish contrasts within the MPA and within the non-MPA, respectively. Note scale differences on y-axis
Sediment accumulation
Following the scoring of algal percent cover in the field, sediments, small filamentous algae, and small invertebrate infauna were scraped from blocks into plastic bags and frozen for analyses. In the laboratory, each sample was defrosted, transferred to a sieve (1 mm mesh), and water slowly passed through the sample to break up consolidated sediments. Microfauna and flora retained on the sieve were removed. Each sediment-laden water sample was then suctioned through a pre-ashed and -weighed glass fiber filter (Whatman, UK) to trap all particles. Filters holding sediments were then dried to a constant mass (80°C), and ashed (500°C for 12 h) to obtain dry, ash, and ash-free dry masses for each sediment sample.
Elemental and isotopic composition of macroalgae
Returning our frozen macroalgal samples to the laboratory, we measured the elemental (N and C) content and isotopic composition of lyophilized Padina boryana samples by continuous-flow isotope-ratio mass spectrometry (CF-IRMS) using a Micromass Optima interfaced to a CE Elantech NA2500 elemental analyzer. All nitrogen isotope abundances are reported as δ15N and δ13C values relative to atmospheric N2 and VPDB, respectively. Each analytical run included a size series of elemental (methionine) and isotopic (peptone) standards, which provided a check on the stability of the instrument and allowed us to remove the contribution of any analytical blank from our isotopic measurements (Montoya 2008).
Coral growth
We also assessed the effects of herbivore exclusion and nutrient enrichment on coral growth. To monitor growth, we stained 6- to 8-cm-height branches of the corals Porites cylindrica and Acropora millepora in a 15 mg/l solution of Alizarin red (Sotka and Hay 2009; Burkepile and Hay 2010) for 12 h (4 h day/8 h night) in large coolers filled with seawater, and then epoxied one fragment of each species into equidistant holes drilled on opposite ends of each block surface (n = 10 per species per treatment per site). At the end of the field experiment, we removed and bleached corals. To assess growth, corals were imbedded into blocks of paraffin wax, and sectioned 2–3 times vertically on a diamond saw (MK Diamond Products, USA). Growth was determined by calculating the % 2-dimensional area of new growth, relative to the stain demarking initial size, using ImageJ (National Institutes of Health, USA) photo analysis software. Growth quantified for each sectioned piece was averaged within a coral replicate. Some replicates did not incorporate the stain clearly for accurate scoring, or were missing at the termination of the experiment; these were excluded from the analyses. Data for Porites were not normally distributed and for Acropora were heteroscedastic, and so were analyzed with three-factor analyses of variance (ANOVA) on rank-transformed data.
Results
Effectiveness of nutrient enrichment
Nitrogen was significantly enriched in tissues of Padina boryana growing on nutrient enriched versus non-enriched blocks protected from herbivores, regardless of site (C:N ratios were 22.21 ± 0.64 and 24.19 ± 0.70, respectively; 2-factor ANOVA, Site: F1,26 = 0.981, P = 0.331; Enrichment: F1,26 = 4.996, P = 0.034; SxE: F1,26 = 1.189, P = 0.285; n = 6–8 per treatment per site). Thus, our nutrient enrichment was successful in that nutrients from the blocks were physiologically available to, and used by, macroalgae on enriched blocks. C:N ratios for non-enriched macroalgae did not differ between algae on blocks in the non-MPA versus MPA; thus, macroalgal access to, or use of, nutrients did not differ between sites, despite riverine input and greater human population density near the non-MPA. The δ15N of Padina growing on enriched and non-enriched blocks did not differ as a function of our fertilization treatments (n = 6–8 per treatment per site; 2-factor ANOVA, Enrichment: F1,26 = 0.434, P = 0.516), but there was a large effect of site; Padina growing on blocks in the non-MPA had a significantly lower δ15N than Padina from the MPA (0.90 ± 0.32‰; n = 14 vs. 2.09 ± 0.14‰; n = 16, respectively; 2-factor ANOVA, Site: F1,26 = 11.358, P = 0.002), suggesting the sites differed in sources of nutrients. Although Padina δ13C tended to be lower in the non-MPA (−11.44 ± 1.22‰, n = 14) than in the MPA (−10.33 ± 1.79‰, n = 16) and lower on enriched blocks (−11.19 ± 1.86‰, n = 16) than on non-enriched blocks (−10.45 ± 1.28‰, n = 14), these differences were not statistically significant (2-factor ANOVA, Site: F1,26 = 3.320, P = 0.080; Enrichment: F1,26 = 1.328, P = 0.260), but the trend for a site effect is suggestive.
Effects of herbivore exclusion and nutrient enrichment on algal community development
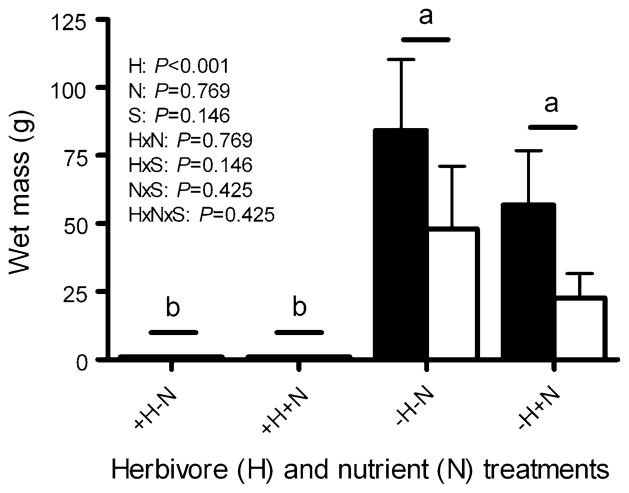
Exclusion of large herbivores increased the cover of total macroalgae and upright fleshy macroalgae by 9–46 times, increased cover of cyanobacteria by 0–27 times, and decreased cover of CCA by 46–100% and short (<0.5 cm) algal turfs by 14–39% (Fig. 1; Table 1). In contrast, nutrient enrichment did not significantly increase cover of any algal group [although suggestive for short algal turfs in the absence of herbivores (P = 0.074)], and suppressed cyanobacteria cover in the MPA by 71%, but only when large herbivores were excluded (Fig. 1; Table 1). In the absence of herbivores, nutrient enrichment also suppressed total macroalgal cover by 33–42% as indicated by a significant herbivore × nutrient interaction term (P = 0.011, ANOVA; Fig. 1a). However, post-hoc analysis did not rigorously detect this difference (P = 0.058), but the nearly significant P value is suggestive. When we assessed wet mass, rather than percent cover, of upright fleshy macroalgae per 800 cm2 (the top of each block), the patterns were similar (Fig. 2); herbivore exclusion increased upright macrophyte mass 23–84 times (P <0.001), while nutrient addition had no detectable effect (P = 0.769).
Table 1.
Results from three-factor analyses of variance (ANOVA) of algal percent cover data
| Effect | df | Total algal cover (%)
|
Upright macro-algae (%)
|
Crustose coralline algae (%)
|
Algal turf <0.5 cm (%)
|
Algal turf >0.5 cm (%)
|
Cyanobacteria (%)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | ||
| Herbivory (H) | 1 | 175.522 | <0.001 | 78.263 | <0.001 | 42.384 | <0.001 | 80.638 | <0.001 | 1.076 | 0.303 | 15.059 | <0.001 |
| Nutrients (N) | 1 | 0.014 | 0.906 | 0.095 | 0.759 | 0.544 | 0.463 | 0.067 | 0.797 | 0.975 | 0.327 | 2.473 | 0.120 |
| Site (S) | 1 | 1.372 | 0.245 | 0.204 | 0.653 | 2.315 | 0.133 | 2.007 | 0.161 | 0.001 | 0.980 | 13.649 | <0.001 |
| H × N | 1 | 6.800 | 0.011 | 0.225 | 0.637 | 2.456 | 0.121 | 3.295 | 0.074 | 0.001 | 0.980 | 8.430 | 0.005 |
| H × S | 1 | 1.205 | 0.276 | 0.110 | 0.742 | 0.554 | 0.459 | 0.695 | 0.407 | 0.975 | 0.327 | 13.995 | <0.001 |
| N × S | 1 | 0.141 | 0.709 | 0.371 | 0.544 | 0.377 | 0.541 | 0.000 | 0.982 | 0.975 | 0.327 | 2.054 | 0.156 |
| H × N × S | 1 | 0.201 | 0.655 | 0.030 | 0.863 | 0.666 | 0.417 | 0.005 | 0.941 | 0.001 | 0.980 | 1.923 | 0.170 |
| Error | 72 | ||||||||||||
Data were rank-transformed. Significant results are highlighted in bold
Fig. 2.
Wet mass (grams per 800 cm2, mean + SE) of larger upright fleshy macroalgae on settlement blocks accessible (+H) or inaccessible (−H) to herbivores, both without (−N) and with (+N) nutrient enrichment, when deployed on a protected reef (MPA; black bars) or on an adjacent fished reef (non-MPA; white bars) for 152 days (n = 10 per treatment per site). P values are from a three-factor analysis of variance (ANOVA) on rank-transformed data. Letters indicate significant groupings from a Tukey multiple comparisons test. Horizontal bars indicate non-significant differences between sites (S), within a treatment
With the exception of cyanobacteria, the placement of experimental blocks in the MPA versus the non-MPA had no significant effect on algal community development after 152 days (Fig. 1; Table 1). Cyanobacteria were unusual in that exclusion of herbivores increased cyanobacteria cover for blocks within the MPA, but nutrient addition suppressed this effect to levels similar to treatments including herbivores. In the non-MPA, herbivore exclusion and nutrient enrichment had no effect on cyanobacteria growth (Fig. 1f).
Effects of herbivore exclusion and nutrient enrichment on sediment accumulation
Excluding large herbivores significantly increased sediment accumulation on experimental blocks; dry mass of inorganic sediments was 66–89% higher and ash-free dry mass of organic sediments was 49–60% higher on herbivore exclusion blocks than blocks subject to herbivory (Fig. 3; Table 2). Nutrient enrichment had no effect on sediment accumulation, but blocks of all treatments accumulated significantly more sediments when deployed within the non-MPA versus the MPA (Fig. 3a, b).
Fig. 3.
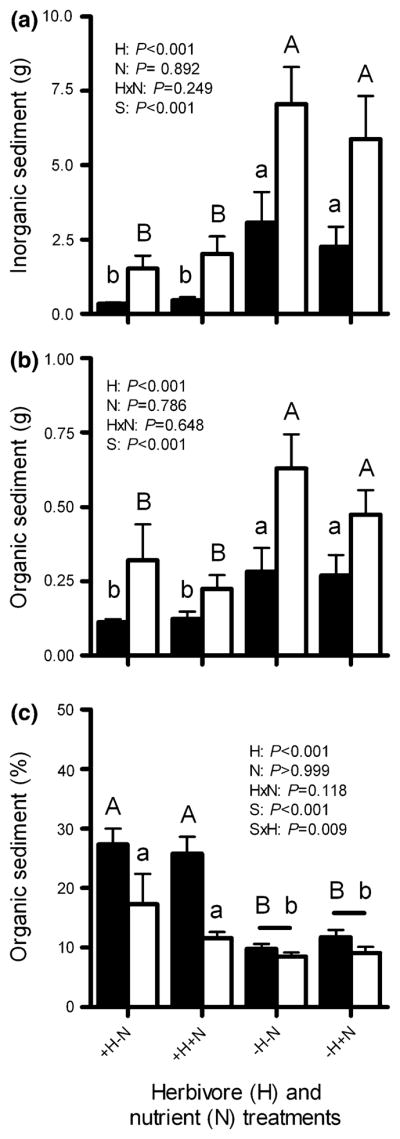
a Inorganic and b organic sediments (grams per 800 cm2, mean + SE), or c percent (mean + SE) of total sediments that are organic on settlement blocks accessible (+H) or inaccessible (−H) to herbivores, both without (−N) and with (+N) nutrient enrichment, when deployed on a protected reef (MPA; black bars) or on an adjacent fished reef (non-MPA; white bars) for 152 days (n = 10 per treatment per site). P values are from three-factor analyses of variance (ANOVA) of rank-transformed data. See Table 2 for complete ANOVA results. Letters indicate significant groupings by Tukey multiple comparisons tests. Horizontal bars indicate non-significant differences between sites (S), within a treatment. Upper and lower case letters distinguish within-site contrasts among treatments. Note scale differences on y-axis
Table 2.
Results from three-factor analyses of variance (ANOVA) of sediment accumulation data
| Effect | df | Inorganic sediment (g)
|
Organic sediment (g)
|
Organic sediment (%)
|
|||
|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | ||
| Herbivory (H) | 1 | 53.595 | <0.001 | 24.281 | <0.001 | 64.439 | <0.001 |
| Nutrients (N) | 1 | 0.019 | 0.892 | 0.075 | 0.786 | 0.000 | 0.999 |
| Site (S) | 1 | 32.249 | <0.001 | 17.310 | <0.001 | 29.571 | <0.001 |
| H × N | 1 | 1.353 | 0.249 | 0.211 | 0.648 | 2.504 | 0.118 |
| H × S | 1 | 0.243 | 0.624 | 0.662 | 0.419 | 7.160 | 0.009 |
| N × S | 1 | 0.032 | 0.859 | 0.528 | 0.470 | 0.902 | 0.346 |
| H × N × S | 1 | 0.270 | 0.605 | 0.161 | 0.690 | 0.001 | 0.977 |
| Error | 72 | ||||||
Data were rank-transformed. Significant results are highlighted in bold
Organic contributions to total sediment loads were 22–64% greater on blocks subject to herbivory versus blocks excluded from herbivores; nutrient enrichment had no effect on the proportion of organic sediments accumulated. Moreover, organic contributions to sediments were significantly greater within the MPA versus non-MPA, but only for blocks accessible to herbivores (Fig. 3c; Table 2).
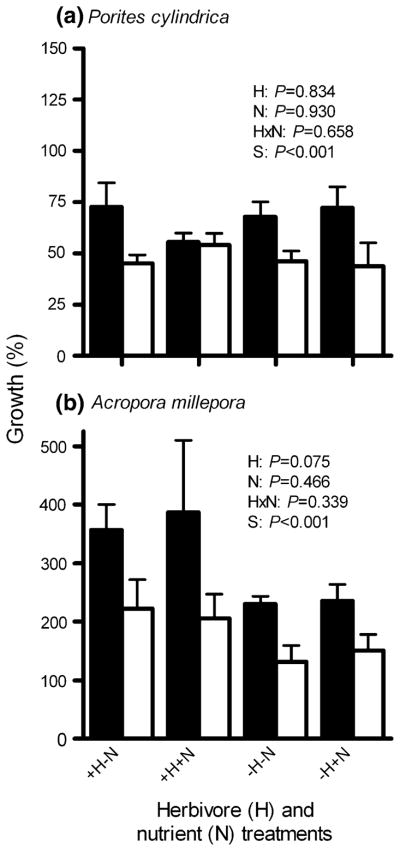
Effects of herbivore exclusion and nutrient enrichment on coral growth
Neither exclusion of large herbivores, addition of nutrients, nor their interaction affected the growth of the mounding coral Porites cylindrica over the 152-day experimental period. However, P. cylindrica growth averaged a significant 30% greater in the MPA than in the non-MPA (Fig. 4a; Table 3). Although the faster-growing, tabular coral Acropora millepora grew 27–41% more on blocks subject to grazing by large herbivores (with or without nutrient enrichment), this effect was suggestive but not statistically significant (P = 0.075; Fig. 4b; Table 3). Our power to detect among-treatment differences for Acropora was compromised due to unexplained deaths of 9 of 40 outplants in the MPA and 2 of 40 in the non-MPA within the first month of our experiment; after this initial death, survivorship of Acropora was high (>98%). Like Porites, A. millepora growth averaged a significant 41% greater when deployed in the MPA versus the non-MPA (Fig. 4b; Table 3).
Fig. 4.
Percent growth (two-dimensional, cross-sectional area, mean + SE) of the corals (a) Porites cylindrica and (b) Acropora millepora transplanted onto settlement blocks accessible (+H) or inaccessible (−H) to herbivores, both without (−N) and with (+N) nutrient enrichment, when deployed on a protected reef (MPA; black bars) or on an adjacent fished reef (non-MPA; white bars) for 152 days (n = 5–10 per treatment per site). P values are from three-factor analyses of variance (ANOVA) of rank-transformed data. See Table 3 for complete ANOVA results. Horizontal bars indicate non-significant differences between sites (S), within a treatment. Note scale differences on y-axis
Table 3.
Results from three-factor analyses of variance (ANOVA) of coral growth data
| Effect | df |
Porites cylindrica growth (%)
|
df |
Acropora millepora growth (%)
|
||
|---|---|---|---|---|---|---|
| F | P | F | P | |||
| Herbivory (H) | 1 | 0.044 | 0.834 | 1 | 3.287 | 0.075 |
| Nutrients (N) | 1 | 0.008 | 0.930 | 1 | 0.540 | 0.466 |
| Site (S) | 1 | 13.512 | <0.001 | 1 | 14.896 | <0.001 |
| H × N | 1 | 0.198 | 0.658 | 1 | 0.931 | 0.339 |
| H × S | 1 | 2.101 | 0.152 | 1 | 0.061 | 0.806 |
| N × S | 1 | 0.205 | 0.652 | 1 | 0.653 | 0.422 |
| H × N × S | 1 | 1.534 | 0.220 | 1 | 0.205 | 0.653 |
| Error | 67 | 54 | ||||
Data were rank-transformed. Significant results are highlighted in bold
Discussion
The processes mediating large-scale shifts in coral reef community structure are debated (McCook 1996; Lapointe 1997; Hughes et al. 1999; Littler et al. 2006a, b; Burkepile and Hay 2006; Heck and Valentine 2007; Houk et al. 2010; Smith et al. 2010), in part due to a reasonable assumption that nutrients may commonly be limiting in tropical waters and due to a few conflicting results from field experiments manipulating nutrients and herbivory. It can also be argued that several previous studies documenting strong effects of herbivory and weak effects of nutrient enrichment may have underestimated nutrient effects because studies did not run for the 3–4 months it may take for nutrient effects to appear, and/or were conducted on reefs dominated by algae instead of corals (Smith et al. 2010). However, a preponderance of rigorous field experiments suggest that herbivory plays a critical role in controlling algal community development, while nutrients play a more minor role (Burkepile and Hay 2006; Heck and Valentine 2007). Our study supports that emerging consensus; we found strong effects of herbivory and minimal effects of nutrients on algal proliferation. These effects were documented on both a coral-dominated and an algal-dominated reef, and over a duration sufficient to allow slower-acting nutrient effects to emerge. On coral-dominated (MPA) and macroalgal-dominated (non-MPA) reefs, the exclusion of large herbivores significantly increased total macroalgae, upright fleshy macroalgae, and cyanobacteria cover, but nutrient addition did not stimulate cover or mass of these algae (Figs. 1 and 2) and, in fact, inhibited accumulation for some algal types under reduced herbivory. Moreover, herbivory significantly enhanced the cover of CCA (some of which cue coral recruitment) and short algal turfs—both characteristic components of healthy reefs. Nutrients had no significant effect on these algal types (Fig. 1). Thus, between-site and between-experiment differences in nutrient effects cannot be explained consistently by benthic community composition or experiment duration alone.
Debates over the importance of top–down versus bottom–up regulation of algal communities on coral reefs may stem, in part, from discrepancies between empirical findings and theoretical predictions. The relative dominance model (RDM) (Littler and Littler 1984; Littler et al. 2006b) predicts algal and coral community structure as a function of interactions between grazing intensity and nutrient enrichment, and suggests that turf algal communities will develop with reduced herbivory, but that elevated nutrients are required for the proliferation of upright macroalgae. While a limited number of studies suggest that nutrients can drive macroalgal production in some locations (Smith et al. 2001; Lapointe et al. 2004; Littler et al. 2006b), especially when herbivores are excluded (Smith et al. 2010), our study and the majority of other field tests (e.g., McCook 1996; Miller et al. 1999; Thacker et al. 2001; Belliveau and Paul 2002; Diaz-Pulido and McCook 2003; McClanahan et al. 2003; Burkepile and Hay 2009; Sotka and Hay 2009) find limited support for the RDM. Although the RDM has been a poor predictor of most experimental outcomes, herbivory and nutrient enrichment can interact in complex ways that may vary with ecosystem productivity, latitude, algal functional group, intensity of herbivory, and duration of study—making variance between locations or times likely (Burkepile and Hay 2006; Houk et al. 2010; Smith et al. 2010).
Exclusion of large herbivores, but not nutrient enrichment, increased sediment accumulation on our experimental blocks by 49–89% (Fig. 3). Interestingly, mean total algal cover was significantly correlated with mean total sediment load across our treatments and sites (Spearman rank correlation; r = 0.79, P = 0.015, n = 8), suggesting a strong link between herbivory and sediment accumulation, likely by algal entrapment of sediments. Indeed, other field studies have also found a relationship between algal biomass and sediment load (Smith et al. 2001; Belliveau and Paul 2002; Stamski and Field 2006), and that sediments can strongly suppress herbivory (Bellwood and Fulton 2008)—suggesting positive feedbacks among herbivore loss, macroalgal proliferation, and sediment accumulation could reinforce phase-shifts to macroalgae. How feedbacks might vary with domination by different algal types (e.g., small turfs versus intermediate sized species like Padina versus large macrophytes like Sargassum) has not been directly addressed, but net sediment accumulation and the strength of feedbacks might vary with stage of algal development, wave exposure, and depth (Steneck 1997). While the exclusion of herbivores increased sediments on blocks at both our MPA and non-MPA sites, net sediment loads were significantly higher in the non-MPA (regardless of treatment), indicating that attributes unique to our non-MPA site (e.g., decreased grazing due to fishing, riverine discharge of sediments, domination by large macroalgae) contributed to net sediment accumulation at this location.
In contrast with previous field experiments documenting that macroalgae can suppress coral growth and survivor-ship (Lewis 1986; Hughes et al. 2007; Burkepile and Hay 2008, 2009), the manipulation of herbivores and nutrients in our experiment had no statistically detectable effect on the growth of the corals Porites cylindrica or Acropora millepora, but the nearly significant (P = 0.075) effect of herbivores on A. millepora is suggestive (Fig. 4). It should be noted that greater than 95% of upright macrophyte biomass found on our herbivore exclusion blocks was Padina boryana, a macrophyte that has little effect on P. cylindrica or A. millepora relative to several other algal species on this reef (Rasher and Hay 2010; Rasher et al. 2011). In addition, our studies started with corals transplanted to unoccupied experimental blocks; effects of macroalgae on corals would have been delayed until macrophytes had time to colonize and grow to appreciable size. Because macroalgae generally take about 3–5 months to recruit and grow to cover ≥20% of substrate in such experiments (Miller et al. 1999; Burkepile and Hay 2009; Smith et al. 2010), it is possible that we would have detected an effect of herbivores on corals (via increased competition from macroalgae) if our experiment had run longer (see Fig. 4b).
Porites cylindrica and Acropora millepora grew significantly less on blocks deployed on a reef subject to fishing and riverine discharge versus a protected reef. Hypotheses to explain this site difference could include effects of sediments, salinity, or abundant nearby macro-algae on coral growth. Because sediment accumulation can suppress coral growth and survivorship (Nugues and Roberts 2003; Birrell et al. 2005), and net sediment accumulation was significantly greater within the non-MPA versus MPA, it is possible that between-site differences in net sediment accumulation contributed to differences in coral growth between MPA and non-MPA reefs (Fig. 4). Alternatively, algal canopies and mats can produce a physio-chemical environment that is detrimental to corals, and have been reported to release water-soluble compounds that indirectly harm corals by stimulating harmful, coral-associated microbes (Smith et al. 2006; Hauri et al. 2010); thus, the preponderance of macroalgae surrounding our blocks within the non-MPA (47% cover) could have negatively impacted coral growth relative to blocks deployed within the MPA (3% macroalgal cover) (Rasher and Hay 2010).
We conducted our manipulative study on geographically similar, adjacent reefs subject to either (1) fishing and riverine input or (2) protection from harvest to assess whether herbivory, eutrophication, or the interaction of these processes differ based on human fishing practice or riverine influence. A limitation of the MPA versus non-MPA contrast is that there is only one of each, thus potentially confounding MPA effect with location. This limitation is reduced to some extent by the sites being adjacent and by statements of villagers that the algal-dominated non-MPA site supported a coral community like that in the MPA some 30+ years ago. One might expect greater macroalgal cover on blocks accessible to herbivores within the non-MPA versus the MPA, given (1) the potential for increased propagule supply due to surrounding high macroalgal cover (47 vs. 3% cover of macroalgae; Rasher and Hay 2010), (2) the low macrophyte removal rates at this site (Rasher and Hay 2010), (3) the potential for terrestrially-derived nutrients to increase algal growth via riverine discharge onto this reef, and/or (4) the dilution of herbivore grazing effort over increasing substrate as corals decline and are replaced by macroalgae (Mumby et al. 2007a). Yet, herbivores strongly impacted algal communities even on a heavily fished reef dominated by macroalgae (Figs. 1 and 2), highlighting the primacy of top–down effects on algae and their cascading impacts on reef community state (Birrell et al. 2008; Hughes et al. 2010). However, high grazing rates on open blocks within the non-MPA could have resulted from exploited herbivore species concentrating their grazing on these blocks (in preference to the surrounding natural substrate) because these herbivores prefer algae found on new substrates undergoing primary succession (such as small turfs) over large macroalgae common on older substrates in the non-MPA (Burkepile and Hay 2010). Herbivore effects can differ dramatically on substrates supporting communities of different ages (Burkepile and Hay 2008, 2010).
Patterns of algal abundance documented here (Figs. 1 and 2) suggest that the 15 times greater cover of macroalgae on natural substrates in the non-MPA compared to the MPA (Rasher and Hay 2010) is not due to nutrient stimulation of macroalgal growth in the non-MPA. When large herbivores were excluded in the presence of ambient nutrients (−H−N), macroalgae grew as well or better in the coral-dominated MPA as in the non-MPA (Figs. 1 and 2), where one might expect nutrient input from the river and nearby village. Additionally, nutrient concentrations (C:N ratio) of Padina boryana growing on non-enriched blocks excluded from herbivores (−H−N) did not differ between reefs, suggesting similar baseline nutrient levels between sites. Although algal nutrient analyses showed that macroalgae utilized our enriched nutrient supply (see C:N ratios of enriched vs. non-enriched blocks), this did not result in increased algal cover at either site, indicating that macroalgae were not nutrient limited on either reef. Thus, the 47% macrophyte cover in the non-MPA versus 3% cover in the MPA (Rasher and Hay 2010) appears to be from differential rates of algal removal by herbivory, not differential rates of algal growth based on nutrient supply or other differing physical regimes.
Our elemental and isotopic measurements are consistent with this top–down interpretation. The C:N ratio of P. boryana varied between 18.4 and 28.9, which matches the upper portion of the range reported for samples of Padina australis collected across a set of reefs with differing degrees of exposure to terrigenous nutrients (11.8–30.1; Umezawa et al. 2002). Umezawa et al. (2007) explored the controls on Padina C:N ratio by incubating field-collected algae (C:N = 22) under varying conditions of light and nutrient limitation, yielding a range of about 16.5 (low light, high nutrients) to >45 (high light, low N). In our study, C:N ratios averaged ~22–23, suggesting that ample nutrients were available for growth at both sites, and were significantly elevated within our fertilization treatment, but did not result in increased macroalgal production. Moreover, our elemental composition data imply that the P. boryana grew under conditions of neither severe nutrient limitation (i.e., C:N ratio >30) nor very high nutrient availability (C:N ratio <15).
Our N and C isotopic data provide additional insights into the growth conditions experienced by P. boryana across the study area. The δ13C of Padina tissues increases linearly with growth rate (Umezawa et al. 2007). Our data show intriguing but not significant contrasts with higher δ13C values, implying higher growth rates, in the MPA than in the non-MPA, and higher δ13C values for Padina growing on non-enriched versus enriched blocks. The site (MPA vs. non-MPA) difference may reflect reduced competition for light, or some other non-nutrient resource, on the MPA experimental blocks because of reduced macroalgal biomass on the surrounding reef.
The above interpretation is supported by our nitrogen isotopic measurements, which provide an integrative record of the nutrient sources supporting growth (Umezawa et al. 2002, 2007). We found significantly higher δ15N values for P. boryana collected on MPA blocks than on non-MPA blocks, but no significant δ15N contrast between non-enriched and enriched blocks within study sites. The higher δ15N in the non-MPA contrasts with previous reports of a simple relationship between terrigenous input (high δ15N) and algal δ15N (Umezawa et al. 2007), but is consistent with a relative lack of nutrient limitation and an isotopically uniform supply of N throughout the study area. In this scenario, variation in the δ15N of macroalgae is driven by isotopic fractionation and reflects a greater fractional consumption of nutrients in the MPA than in the non-MPA, perhaps because of the higher terrigenous inputs to the non-MPA.
Emerging research suggests the human harvest of marine herbivores plays a pivotal role in reef decline (Lewis 1986; Jackson et al. 2001; Bellwood et al. 2004; Mumby and Steneck 2008; Hughes et al. 2010) by compromising processes such as herbivory and coral recruitment that facilitate coral recovery from, and resistance to, a range of disturbances (Hughes et al. 2007; Mumby et al. 2007a, b). Indeed, our study and numerous other recent field experiments (e.g., Belliveau and Paul 2002; Diaz-Pulido and McCook 2003; Burkepile and Hay 2009; Sotka and Hay 2009) indicate that herbivores limit the establishment of algae (Fig. 1), limit sediment accumulation (Fig. 3), and promote the establishment of CCA (Fig. 1), all of which are critical to successful coral recruitment and/or growth following disturbance (Birrell et al. 2008). These critical ecological processes are reduced or lost with the removal of functionally important herbivores, and the impacts of their loss may be magnified by nutrient enrichment (Burkepile and Hay 2006; Smith et al. 2010). Prioritization of management approaches that protect critical processes, such as herbivory, that bolster coral reefs against phase-shifts to macroalgae should slow reef decline and facilitate coral recovery from the numerous stresses impacting present-day reefs (Knowlton and Jackson 2008; Carilli et al. 2009; Mumby and Harborne 2010; Selig and Bruno 2010).
Acknowledgments
We thank the Fijian government and Korolevu-iwai district elders for research permissions. T. Andras and C. Dell provided valuable laboratory assistance. Support was provided by research grants from the National Institutes of Health (U01-TW007401) and the National Science Foundation (OCE 0929119), a National Science Foundation Integrative Graduate Education and Research Traineeship grant (DGE-0114400), and the Teasley Endowment to the Georgia Institute of Technology. The experiments reported here comply with the current laws of the country in which the experiments were performed.
Abbreviations
- MPA
Marine protected area
- Non-MPA
Non-marine protected area
- CCA
Crustose coralline algae
- RDM
Relative dominance model
Footnotes
Conflict of interest The authors declare no conflict of interest with the organizations that funded this research.
Contributor Information
Douglas B. Rasher, School of Biology, Georgia Institute of Technology, Atlanta, GA 30332, USA
Sebastian Engel, School of Biology, Georgia Institute of Technology, Atlanta, GA 30332, USA.
Victor Bonito, Reef Explorer Fiji, PO Box 183, Korolevu, Fiji.
Gareth J. Fraser, Department of Animal and Plant Sciences, University of Sheffield, Sheffield S10 2TN, UK
Joseph P. Montoya, School of Biology, Georgia Institute of Technology, Atlanta, GA 30332, USA
Mark E. Hay, Email: mark.hay@biology.gatech.edu, School of Biology, Georgia Institute of Technology, Atlanta, GA 30332, USA
References
- Baker AC, Glynn PW, Riegl B. Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci. 2008;80:435–471. [Google Scholar]
- Belliveau SA, Paul VJ. Effects of herbivory and nutrients on the early colonization of crustose coralline and fleshy algae. Mar Ecol Prog Ser. 2002;232:105–114. [Google Scholar]
- Bellwood DR, Fulton CJ. Sediment-mediated suppression of herbivory on coral reefs: decreasing resilience to rising sea levels and climate change? Limnol Oceanogr. 2008;53:2695–2701. [Google Scholar]
- Bellwood DR, Hughes TP, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- Birrell CL, McCook LJ, Willis BL. Effects of algal turfs and sediment on coral settlement. Mar Poll Bull. 2005;51:408–414. doi: 10.1016/j.marpolbul.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Birrell CL, McCook LJ, Willis BL, Diaz-Pulido GA. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr Mar Biol Annu Rev. 2008;46:25–63. [Google Scholar]
- Bruno JF, Petes LE, Harvell CD, Hettinger A. Nutrient enrichment can increase the severity of coral diseases. Ecol Lett. 2003;6:1056–1061. [Google Scholar]
- Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, Harvell CD, Sweatman H, Melendy AM. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biology. 2007;5:e124. doi: 10.1371/journal.pbio.0050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkepile DE, Hay ME. Herbivore versus nutrient control of marine primary producers: context-dependent effects. Ecology. 2006;87:3128–3139. doi: 10.1890/0012-9658(2006)87[3128:hvncom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Burkepile DE, Hay ME. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc Natl Acad Sci USA. 2008;105:16201–16206. doi: 10.1073/pnas.0801946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkepile DE, Hay ME. Nutrient versus herbivore control of macroalgal community development and coral growth on a Caribbean reef. Mar Ecol Prog Ser. 2009;389:71–84. [Google Scholar]
- Burkepile DE, Hay ME. Impact of herbivore identity on algal succession and coral growth on a Caribbean reef. PLoS One. 2010;5:e8963. doi: 10.1371/journal.pone.0008963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carilli JE, Norris RD, Black BA, Walsh SM, McField M. Local stressors reduce coral resilience to bleaching. PLoS One. 2009;4:e6324. doi: 10.1371/journal.pone.0006324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheal A, MacNeil M, Cripps E, Emslie M, Jonker M, Schaffelke B, Sweatman H. Coral–macroalgal phase shifts or reef resilience: links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs. 2010;29:1005–1015. [Google Scholar]
- Diaz-Pulido G, McCook LJ. Relative roles of herbivory and nutrients in the recruitment of coral-reef seaweeds. Ecology. 2003;84:2026–2033. [Google Scholar]
- Elmhirst T, Connolly SR, Hughes TP. Connectivity, regime shifts and the resilience of coral reefs. Coral Reefs. 2009;28:949–957. [Google Scholar]
- Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, Holling CS. Regime shifts, resilience, and biodiversity in ecosystem management. Annu Rev Ecol Evol Syst. 2004;35:557–581. [Google Scholar]
- Harvell D, Jordan-Dahlgren E, Merkel S, Rosenberg E, Raymundo L, Smith G, Weil E, Willis B. Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography. 2007;20:172–195. [Google Scholar]
- Hauri C, Fabricius KE, Schaffelke B, Humphrey C. Chemical and physical environmental conditions underneath mat- and canopy-forming macroalgae, and their effects on understorey corals. PLoS One. 2010;5:e12685. doi: 10.1371/journal.pone.0012685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck KL, Valentine JF. The primacy of top-down effects in shallow benthic ecosystems. Estuar Coasts. 2007;30:371–381. [Google Scholar]
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- Hoey AS, Bellwood DR. Suppression of herbivory by macroalgal density: a critical feedback on coral reefs? Ecol Lett. 2011;14:267–273. doi: 10.1111/j.1461-0248.2010.01581.x. [DOI] [PubMed] [Google Scholar]
- Houk P, Musburger C, Wiles P. Water quality and herbivory interactively drive coral-reef recovery patterns in American Samoa. PLoS One. 2010;5:e13913. doi: 10.1371/journal.pone.0013913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TP. Catastrophes, phase-shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Szmant AM, Steneck RS, Carpenter R, Miller S. Algal blooms on coral reefs: what are the causes? Limnol Oceanogr. 1999;44:1583–1586. [Google Scholar]
- Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol. 2007;17:360–365. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS. Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol. 2010;25:633–642. doi: 10.1016/j.tree.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- Jompa J, McCook LJ. The effects of nutrients and herbivory on competition between a hard coral (Porites cylindrica) and a brown alga (Lobophora variegata) Limnol Oceanogr. 2002;47:527–534. [Google Scholar]
- Knowlton N, Jackson JBC. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol. 2008;6:e54. doi: 10.1371/journal.pbio.0060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe BE. Nutrient thresholds for bottom-up control of macroalgal blooms on coral reefs in Jamaica and southeast Florida. Limnol Oceanogr. 1997;42:1119–1131. [Google Scholar]
- Lapointe BE, Barile PJ, Yentsch CS, Littler MM, Littler DS, Kakuk B. The relative importance of nutrient enrichment and herbivory on macroalgal communities near Norman’s Pond Cay, Exumas Cays, Bahamas: a “natural” enrichment experiment. J Exp Mar Biol Ecol. 2004;298:275–301. [Google Scholar]
- Lewis SM. The role of herbivorous fishes in the organization of a Caribbean reef community. Ecol Monogr. 1986;56:183–200. [Google Scholar]
- Littler MM, Littler DS. Models of tropical reef biogenesis: the contribution of algae. In: Round FE, Chapman DJ, editors. Progress in phycological research. Biopress; Bristol: 1984. pp. 323–364. [Google Scholar]
- Littler MM, Littler DS, Brooks BL, Lapointe BE. Nutrient manipulation methods for coral reef studies: a critical review and experimental field data. J Exp Mar Biol Ecol. 2006a;336:242–253. [Google Scholar]
- Littler MM, Littler DS, Brooks BL. Harmful algae on tropical coral reefs: bottom-up eutrophication and top-down herbivory. Harmful Algae. 2006b;5:565–585. [Google Scholar]
- McClanahan TR, Sala E, Stickels PA, Cokos BA, Baker AC, Starger CJ, Jones SH. Interaction between nutrients and herbivory in controlling algal communities and coral condition on Glover’s Reef, Belize. Mar Ecol Prog Ser. 2003;261:135–147. [Google Scholar]
- McCook LJ. Effects of herbivores and water quality on Sargassum distribution on the central great barrier reef: cross-shelf transplants. Mar Ecol Prog Ser. 1996;139:179–192. [Google Scholar]
- Miller MW, Hay ME. Effects of fish predation and seaweed competition on the survival and growth of corals. Oecologia. 1998;113:231–238. doi: 10.1007/s004420050373. [DOI] [PubMed] [Google Scholar]
- Miller MW, Hay ME, Miller SL, Malone D, Sotka EE, Szmant AM. Effects of nutrients versus herbivores on reef algae: a new method for manipulating nutrients on coral reefs. Limnol Oceanogr. 1999;44:1847–1861. [Google Scholar]
- Montoya JP. Nitrogen stable isotopes in marine environments. In: Capone DG, Carpenter EJ, Mulholland MR, Bronk DA, editors. Nitrogen in the marine environment. Academic; London: 2008. pp. 1277–1302. [Google Scholar]
- Mumby PJ, Harborne AR. Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS One. 2010;5:e8657. doi: 10.1371/journal.pone.0008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby PJ, Steneck RS. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol. 2008;23:555–563. doi: 10.1016/j.tree.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007a;450:98–101. doi: 10.1038/nature06252. [DOI] [PubMed] [Google Scholar]
- Mumby PJ, Harborne AR, Williams J, Kappel CV, Brumbaugh DR, Micheli F, Holmes KE, Dahlgren CP, Paris CB, Blackwell PG. Trophic cascade facilitates coral recruitment in a marine reserve. Proc Natl Acad Sci USA. 2007b;104:8362–8367. doi: 10.1073/pnas.0702602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugues MM, Roberts CM. Coral mortality and interaction with algae in relation to sedimentation. Coral Reefs. 2003;22:507–516. [Google Scholar]
- Rasher DB, Hay ME. Chemically rich seaweeds poison corals when not controlled by herbivores. Proc Natl Acad Sci USA. 2010;107:9683–9688. doi: 10.1073/pnas.0912095107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasher DB, Stout EP, Engel S, Kubanek J, Hay ME. Macroalgal terpenes function as allelopathic agents against reef corals. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1108628108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymundo LJ, Halford AR, Maypa AP, Kerr AM. Functionally diverse reef-fish communities ameliorate coral disease. Proc Natl Acad Sci USA. 2009;106:17067–17070. doi: 10.1073/pnas.0900365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig ER, Bruno JF. A global analysis of the effectiveness of marine protected areas in preventing coral loss. PLoS One. 2010;5:e9278. doi: 10.1371/journal.pone.0009278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JE, Smith CM, Hunter CL. An experimental analysis of the effects of herbivory and nutrient enrichment on benthic community dynamics on a Hawaiian reef. Coral Reefs. 2001;19:332–342. [Google Scholar]
- Smith JE, Morrigan S, Edwards RA, Obura D, Pantos O, Sala E, Sandin SA, Smriga S, Hatay M, Rohwer FL. Indirect effects of algae on coral: algae-mediated, microbe-induced mortality. Ecol Lett. 2006;9:835–845. doi: 10.1111/j.1461-0248.2006.00937.x. [DOI] [PubMed] [Google Scholar]
- Smith JE, Hunter CL, Smith CM. The effects of top-down versus bottom-up control on benthic coral reef community structure. Oecologia. 2010;163:497–507. doi: 10.1007/s00442-009-1546-z. [DOI] [PubMed] [Google Scholar]
- Sotka EE, Hay ME. Effects of herbivores, nutrient enrichment, and their interactions on macroalgal proliferation and coral growth. Coral Reefs. 2009;28:555–568. [Google Scholar]
- Stamski RE, Field ME. Characterization of sediment trapped by macroalgae on a Hawaiian reef flat. Estuar Coast Shelf Sci. 2006;66:211–216. [Google Scholar]
- Steneck RS. Herbivory on coral reefs: a synthesis. Proceedings of 6th International Coral Reef Symposium. 1988;1:37–49. [Google Scholar]
- Steneck RS. Crustose corallines, other algal functional groups, herbivores and sediment: complex interactions along reef productivity gradients. Proceedings of 8th International Coral Reef Symposium. 1997;1:695–700. [Google Scholar]
- Thacker RW, Ginsburg DW, Paul VJ. Effects of herbivore exclusion and nutrient enrichment on coral reef macroalgae and cyanobacteria. Coral Reefs. 2001;19:318–329. [Google Scholar]
- Umezawa Y, Miyajima T, Yamamuro M, Kayanne H, Koike I. Fine-scale mapping of land-derived nitrogen in coral reefs by delta N-15 in macroalgae. Limnol Oceanogr. 2002;47:1405–1416. [Google Scholar]
- Umezawa Y, Miyajima T, Tanaka Y, Koike I. Variation in internal delta N-15 and delta C-13 distributions and their bulk values in the brown macroalga Padina australis growing in subtropical oligotrophic waters. J Phycol. 2007;43:437–448. [Google Scholar]