Abstract
Newly isolated, not previously reported, di-(2-ethylhexyl) phthalate (DEHP)-degraders were augmented to assess their role in polyvinyl chloride (PVC) shower curtain deterioration and DEHP leaching. The biofilms that developed on the surfaces of the bioaugmented shower curtains with Gram-positive strains LHM1 and LHM2 were thicker than those of the biostimulated and Gram-negative strain LHM3-augmented shower curtains. The first derivative thermogravimetric (DTG) peaks of the bioaugmented shower curtains with the Gram-positive bacteria were observed at ~287°C, whereas the control and Gram-negative strain LHM3-augmented shower curtains were detected at ~283°C. This slight delay in the first DTG peak temperature is indicative of lower plasticizer concentrations in the shower curtains that were bioaugmented with Gram positive bacteria. Despite bioaugmentation with DEHP-degraders, aqueous solutions of the bioaugmentation reactors were not DEHP-free due probably to the presence of co-solutes that must have supported microbial growth. Generally, the bioaugmented reactors with the Gram-positive strains LHM1 and LHM2 had greater aqueous DEHP concentrations in the first-half (<3 wk) of the biodeterioration experiment than the biostimulated and strain LHM3-augmented reactors. Therefore, strains LHM1 and LHM2 may play an important role in DEHP leaching to the environment and PVC biodeterioration.
Keywords: Bioaugmentation, Biofilms, Biostimulation, Phthalates, PVC shower curtains
1. Introduction
Phthalic acid esters (phthalates) have been used as additives in plastic or polymeric materials to enhance their flexibility and integrity. These compounds are not bound chemically to the polymer matrix and they may migrate to the environment. Di-(2-ethylhexyl) phthalate (DEHP) is one of the most produced and widely used phthalates. For example, 23% (w/w) of the world’s phthalates production in 1979 was DEHP (Shelton, 1984). United States production was approximately 117,500 metric tons in 1994 (IARC, 2000). DEHP is known to have a low water solubility (0.285 mg l−1 at 24°C), long side chain, and high octanol-water partitioning coefficient (log Kow = 7.5). These characteristics make DEHP relative stable in the natural environment and, consequently, difficult to degrade (Staples et al., 1997). However, several Gram-negative bacterial strains were found capable of degrading DEHP under aerobic and anaerobic conditions (Zeng et al., 2004; Chang et al., 2005). It has been reported that suspected endocrine disrupting DEHPs could be linked to hepatocellular tumors and are developmental and reproductive toxicants (Voss et al., 2005; Heudorf et al., 2007).
Most plastic or polymeric materials containing DEHP, such as polyvinyl chloride (PVC), are typically disposed of in landfills, where they are subjected to dynamic physiochemical and biological deterioration and degradation. There are many studies conducted on thermal and chemical degradation of PVC, but only a few have evaluated PVC biodeterioration and biodegradation and subsequent DEHP leaching (Mersiowsky et al., 2001; Shah et al., 2008). This study investigated biodeterioration of shower curtains as the model DEHP-containing PVC material by bioaugmentation and biostimulation. For bioaugmentation, aerobic bacteria isolated from a local landfill leachate and capable of using DEHP as the sole carbon source were inoculated. The bacteria used for bioaugmentation in the current study have not been reported previously as DEHP-degraders. For biostimulation, ambient bacteria were provided with fresh growth medium.
2. Materials and methods
2.1. Shower curtains
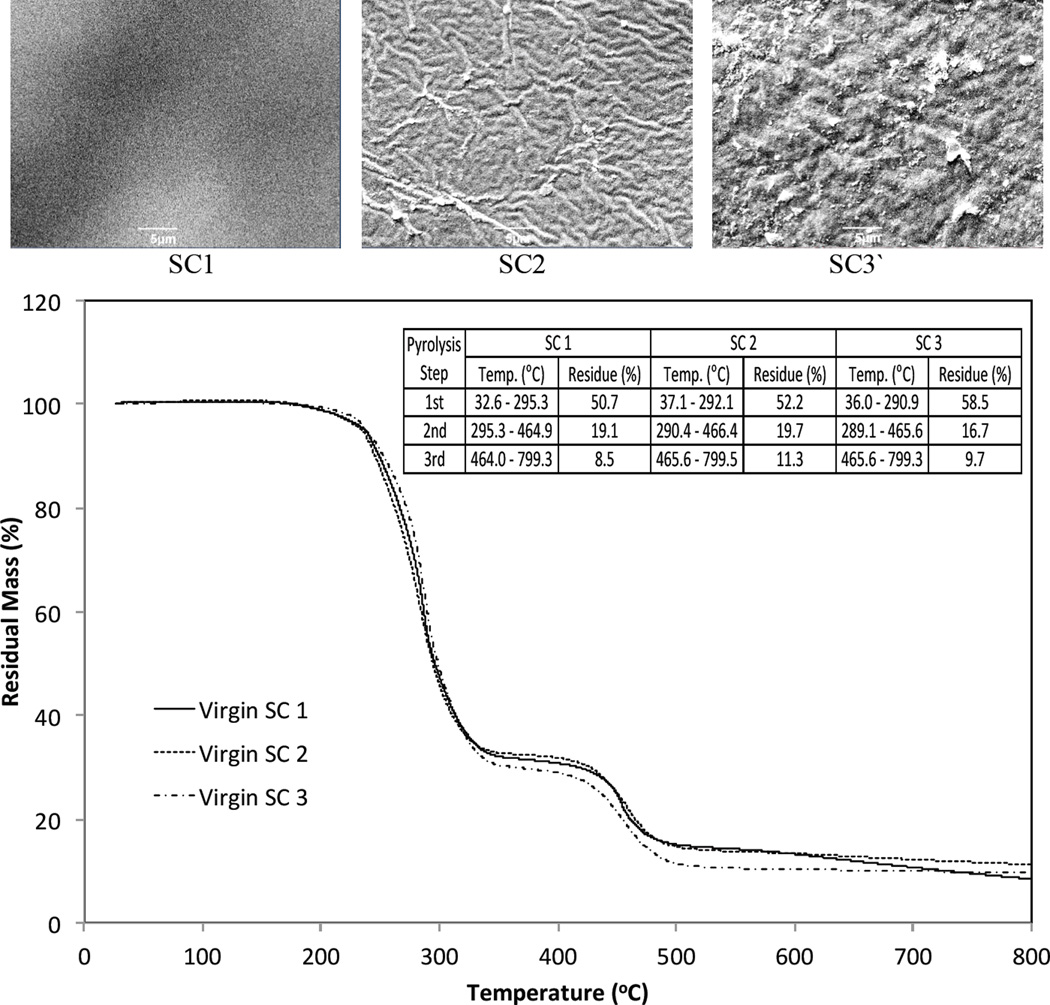
Three transparent, flexible PVC shower curtains (manufacturers: Bath Elements, Panache, and Sultan’s Linens) were purchased from a local store and cut into 2.5- × 15-cm pieces. Although their visual morphology looked different under scanning electron microscopy (SEM), the three shower curtains showed a similar thermal stability by thermogravimetric analysis (TGA; Fig. 1).
Fig. 1.
Images of scanning electron microscope (SEM) (x3,000) and thermograms of thermogravimetric analysis (TGA) of three different shower curtains (SCs) used for the experiment. The initial masses (in mg) for TGA were 11.51 (SC1), 9.48 (SC2), and 16.87 (SC3), respectively.
2.2. Bacteria isolation and enrichment
Isolation of bacteria that grew in the presence of high DEHP concentration was performed in a fed batch reactor that had 1.5 l of leachate collected from a local landfill (Anasco, Puerto Rico). The reactor received a weekly spike of 1.5 ml pure DEHP for 5 wk. Isolated strains were further enriched in a mineral salt medium (MSM) with DEHP as the sole carbon and energy source. The MSM consisted of (in g l−1) 2 K2HPO4, 83 2 KH2PO4, 1.6 NaNO2, 1 NH4Cl, 0.2 MgSO47H2O, 0.05 CaCl2 H2O, 0.02 MnSO4, and 1 ml of trace metals that were comprised of (in g l−1) 0.162 FeCl3 6H2O, 0.014 ZnCl2 4H2O, 0.012 CoCl2 6H2O, 0.012 NaMoO4 2H2O, 0.006 CaCl2 2H2O, 1.21 CuSO4, and 0.392 H3BO3. Pure DEHP was added to the enrichments at 1 ml l−1. Enrichments were incubated in a water bath at 30°C at 100 rpm and 2 ml of the enrichments were transferred weekly to 100 ml of fresh medium. Enrichments were transferred more than ten times serially into fresh medium, and the final enrichment was streaked on the plates containing 0.3% tryptic soy, 1.5% agar, and 1 ml of trace metals consisting of (in g l−1) 8.5 KH2PO4, 33.4 Na2HPO4 7H2O, 22.5 MgSO4 7H2O, 27.5 CaCl2, 0.25 FeCl2, and 1.7 NH4Cl.
2.3. Bacterial identification
Gram staining and 16S rRNA gene sequencing were performed to identify the isolated and enriched bacteria capable of using DEHP as the sole carbon source. Extraction of genomic DNA was made with the Wizard® Genomic DNA Purification Kit (Promega) and a gel electrophoresis picture was taken with the BioDoC-it Imagining System.
The reaction conditions for PCR included an initial denaturation at 95°C for 2 min, followed by 30 cycles of denaturation (1 min at 94°C), annealing with two universal primers, 519 F µM (5’-CAGCMGCCGCGGTAATWC-3’) and 1392 R µM (5’-ACGGGCGGTGTGTRC-3’) (1 min at 55°C), and extension with dNTPs to synthesize the complimentary sequence of DNA (2 min at 72°C) with a final extension at 72°C for 5 min. PCR amplification was performed with the following reaction components: 15 ng µl−1 of DNA extracted and a master mix solution containing: 1X of buffer, 2.5 mM of MgCl2, 1 pm of 519 F, 1 pm of 1392 R, 4X of BSA, 400 dNTPs µM, and 0.026 ug µl-1 of Taq polymerase. The 16S rRNA gene related taxa were obtained from the GenBank database using the Ez Taxon algorithm.
Pairwise and multiple-sequence alignment with 16S rRNA gene sequences of closely related organisms (as identified by Ez Taxon analysis) was made by the ClustalW program using MEGA ver. 5.05. Phylogenetic analyses were conducted using the neighbor-joining method of MEGA ver. 5.05. The bootstrap test and p-distance model were used for the phylogeny analysis. Final trees were constructed with 814 and 832 bp nucleotides for strains LHM1 and LHM2, respectively.
2.4. Biodeterioration reactors
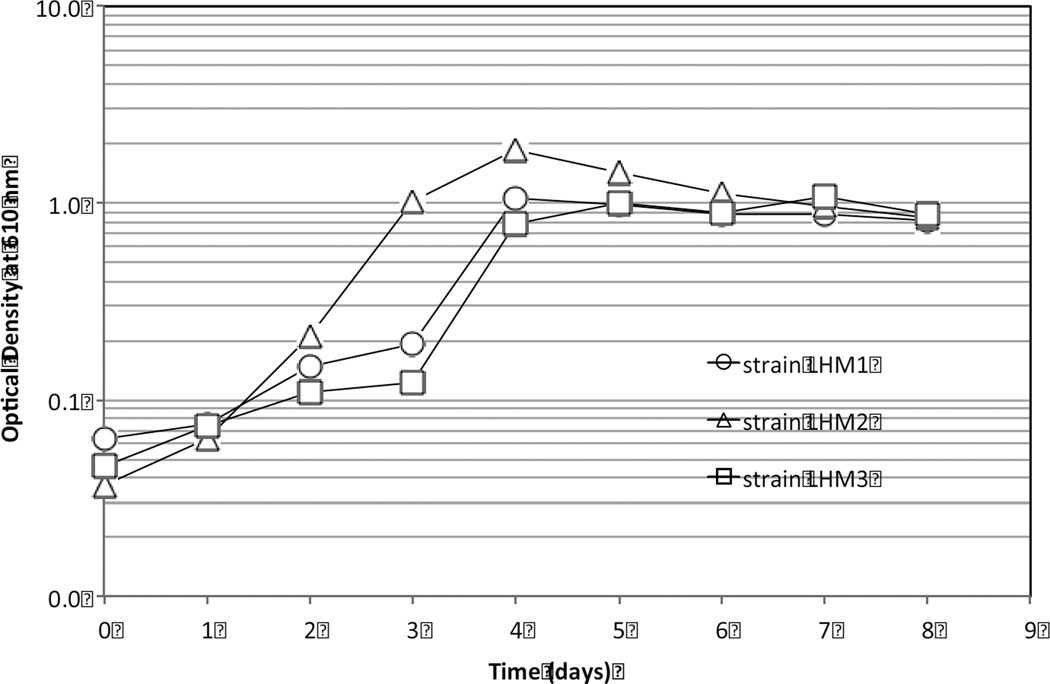
A glass cylindrical reactor (2 l) had six shower curtain strips (2.5 × 15 cm, two from each brand) that were hung vertically in a 1.2 l MSM. Three DEHP-degraders (strains LHM1, LHM2, and LHM3) were selected for the bioaugmentation. They were inoculated individually and in a consortium. For this, 1 ml of the enriched bacteria solution was added to a phosphate buffer solution (pH 7.2, 42.5 mg l−1 KH2PO4 + 405.5 mg l−1 MgCl2 6H2O) on the 4th day (stationary-growth phase, Fig. 2). They were centrifuged at 3400 rpm for 30 min and re-suspended in a phosphate buffer solution. Centrifugation and re-suspension were repeated three times prior to bacterial inoculation in the reactors. Aerobic conditions were maintained throughout the experiment by aerating the reactors to achieve a dissolved oxygen concentration of ~5 mg l−1. A total of five reactors (Table 1) were run for 6 wk. Fifty percent of the reactor volume was replenished weekly with the fresh sterilized growth medium. The biostimulation reactor was prepared in the same manner as the bioaugmentation reactors, but without bacterial inoculation.
Fig. 2.
Growth of Gram-positive strains LHM1 and LHM2 and Gram-negative strain LHM3 in MSM with DEHP as the sole carbon source. Vertical bars in the data symbols are standard deviation of two measurements.
Table 1.
The types of biodeterioration reactors and the initial bacteria populations.
| Reactor ID | Reaction type | Inoculated bacteria type |
Initial bacteria population* (CFU/100 mL) |
|---|---|---|---|
| R1 | Biostimulation | - | - |
| R2 | Bioaugmentation | Strain LHM1 | 1.6 × 106 |
| R3 | Strain LHM2 | 1.1 × 105 | |
| R4 | Strain LHM3 | 2.4 × 105 | |
| R5 | Consortium | 1.3 × 106 |
Measured via heterotrophic plate count (HPC), colony forming unit (CFU)
2.5. Analysis
Biodeterioration on the surface of the shower curtains was monitored after air-drying overnight by bright field microscopy (BFM) (OMANO Opima OM159T) equipped with an OPTIXCAM video camera and by SEM (JEOL JSM-6390 scanning electron microscope) operated at 5 keV. The SEM samples were coated with gold using a Denton Vacuum Desk IV sputter-coater to improve the conductivity of the samples and thus the quality of the images.
Thermal degradability of the control and biodeteriorated shower curtains was assessed by TGA (Mettler Toledo, TGA DSC 1). Biofilms on the biodeteriorated shower curtains were eliminated for TGA by sonication in distilled deionized water for 1 h at 42 kHz followed by rinsing with distilled deionized water and air-drying overnight. The TGA results indicated no DEHP losses after the sonication process. TGA was carried out using sample masses between 10 and 20 mg in a standard 150-µl alumina sample holder. The sample was analyzed in a nitrogen atmosphere at a flow rate of 20 ml min−1. The temperature range was from 25 to 800°C at a heating rate of 10°C min−1.
To quantify the concentrations of water-soluble total organic carbon (TOC) and DEHP in an abiotic condition, the shower curtain strips were placed in the reactor containing the MSM amended with 200 mg l−1 sodium azide (NaN3) to inhibit microbial growth. The reactors were incubated in a water bath at 30°C at 100 rpm for > 5 wk. Aliquot samples were taken at regular intervals for the TOC and DEHP analyses. Total organic carbon was analyzed with a TOC Reagent Set (Hach Method 10129).
For DEHP analysis, the aqueous samples were extracted with dichloromethane at a volumetric ratio of 10:1 (sample:dichloromethane) and put on a shaker for 15 min at 100 rpm. Dichloromethane on the bottom of the extraction vial was transferred to a 2-ml gas chromatography vial and allowed to air dry (at least 10 h). One milliliter of hexane was then added to the GC vial that was vortexed prior to analysis with a gas chromatograph (GC, Varian CP3800). The recovery percentage was 98% for DEHP. The GC was equipped with an electron capture detector and DB-5 capillary column (15 m; 0.25 um film thickness; 0.25 mm ID) (Phenomenex, USA). One microliter was injected from an automatic sample tray to the GC. The initial column temperature was set at 100°C for 1 min, increased by 20°C min−1 to 280°C, and held for 2 min. Injector and detector temperatures were set at 250 and 300°C, respectively. Helium was used as a carrier at a flow rate of 2 ml min−1 and nitrogen was a make-up gas at a flow rate of 25 ml min−1.
The pH levels were measured by submerging an Orion 9157BNMD pH probe into the liquid samples. Bacterial growth in the liquid phase was monitored by measuring OD at a wavelength of 610 nm with a Perkin Elmer UV/VIS Spectrophotometer Lambda 20. Heterotrophic plate count (HPC) was made using membrane filtration (0.45 µm) followed by incubation on an HPC plate (HACH) for 48 h at 35°C.
3. Results and discussion
3.1. Bacterial identification
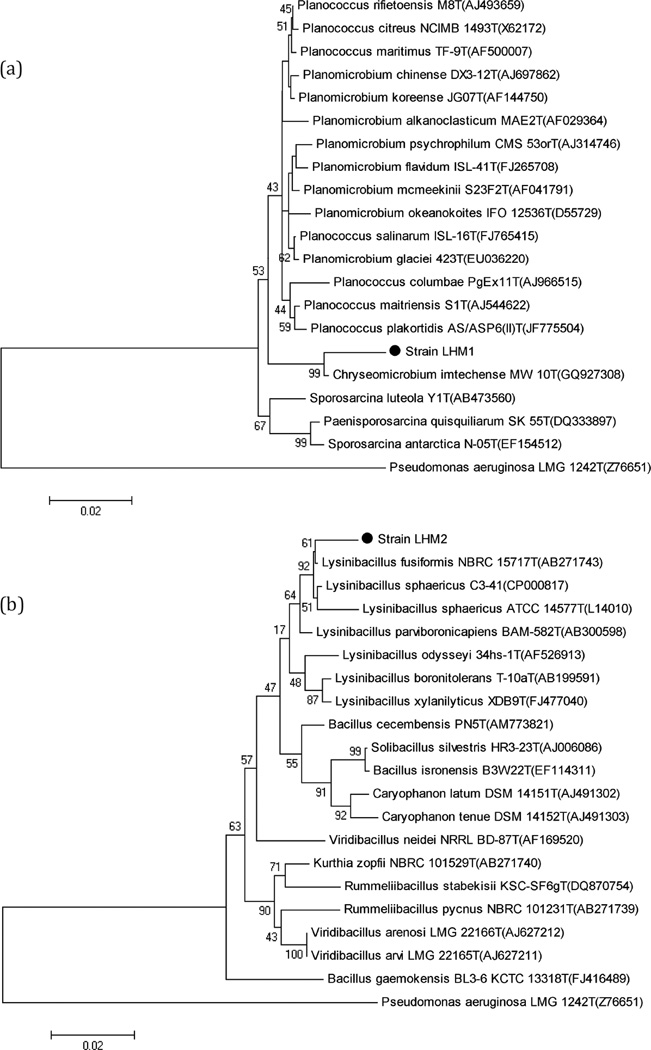
Four bacteria strains, LHM1, LHM2, LHM3, and LHM4, not previously reported as DEHP-degraders, were identified by their 16S rRNA gene sequences. Gram-positive strain LHM1 and strain LHM2 had a greater than 97% similarity to Chryseomicrobium imtechense MW 10(T) and Lysinibacillus fusiformis NBRC 15717(T), respectively. Gram-negative strain LHM3 and strain LHM4 were related to Acinetobacter calcoaceticus DSM 30006(T) (90.7% similarity) and Stenotrophomonas pavanii ICB 89(T) (96.0% similarity), respectively. For the bioaugmentation, two Gram-positive strains (LHM1 and LHM2) and one Gram-negative strain (LHM3) were selected for their healthy growth during the enrichment. It is, however, noted that strain LHM3 did not have as close a level of similarity as strains LHM1 and LHM2 had.
Phylogenetic analysis was conducted to corroborate the results of the molecular identification of the DEHP-degraders (strains LHM1 and LHM2). Figure 3 illustrates that strains LHM1 and LHM2 are closely related to Chryseomicrobium imtechense MW 10T, with a bootstrap value of 99%, and Lysinibacillus fusiformis NBRC 15717T, with a bootstrap value of 61%, respectively.
Fig. 3.
Neighbour-joining distance tree using the 16S rRNA gene sequences of strain LHM1 (a) and strain LHM2 (b) and closely related species. Bootstrap values higher than 35% are shown. Accession numbers are shown in the parenthesis. Pseudomonas aeruginosa LMG 1242T (Z76651) was used as the outgroup. Bar, 1 substitution per 200 nt.
3.2. Growth of the isolates in MSM
As enriched with DEHP as the sole carbon source for at least wk in MSM, three isolates did not have a lag phase (Fig. 2). However, strain LHM2 entered the stationary phase faster than strains LHM1and LHM3 and reached a maximum OD value of 1.8 AU after four days of incubation. Then, their growth declined gradually to 0.9 AU. The growth patterns of strains LHM1 and LHM3 were very similar. They reached the stationary growth phase at ~1.0 AU after 4 days of incubation. It was construed that strain LHM2 metabolized more DEHP at a faster rate than the other two bacteria, resulting in the growth decline at a later period of incubation due probably to an unbalanced carbon-to-nutrients ratio and/or production of toxic metabolites.
3.3. TOC and DEHP leaching in abiotic MSM
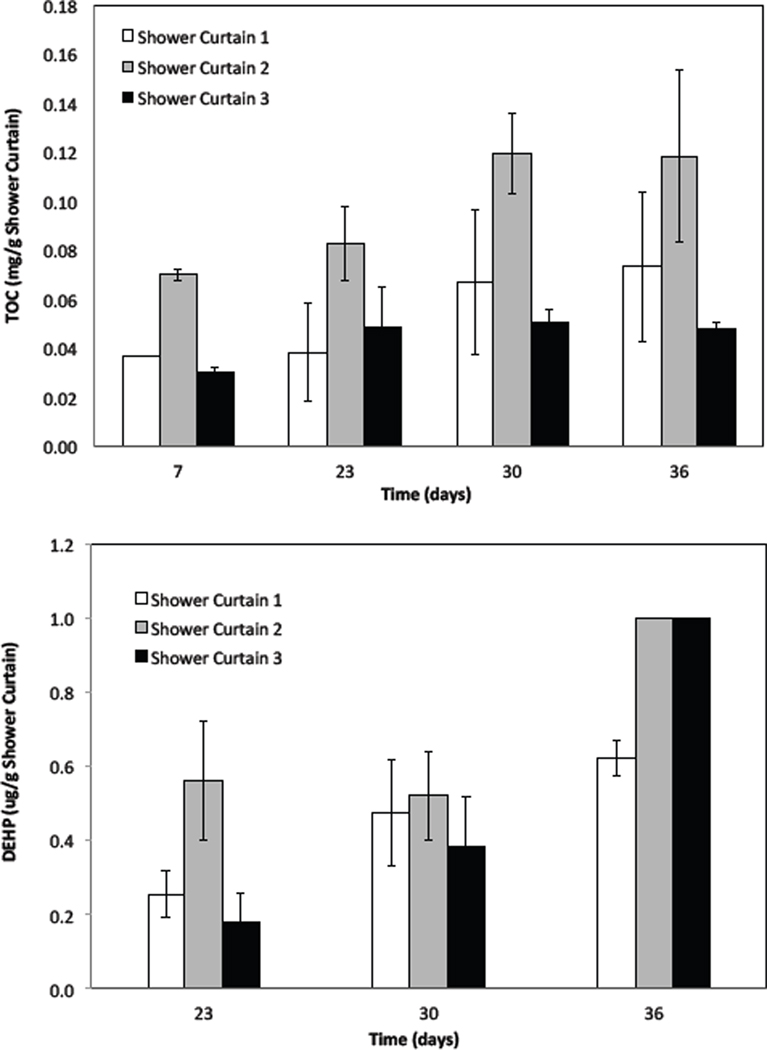
Just as their surface morphology differed (SEM, Fig. 1), the three shower curtains produced dissimilar water-soluble TOC concentrations in the abiotic MSM. The TOC values were generally increased and reached a plateau after 30 days (Fig. 4). As shown, shower curtain 2 produced the greatest TOC concentration of 0.12 mg g−1 followed by shower curtain 1 (0.07 mg g−1) and shower curtain 3 (0.05 mg g−1) at 36 days.
Fig. 4.
Total organic carbon (TOC) and Di-2-(ethylhexyl) phthalate (DEHP) concentrations in the same mineral growth medium that the biodeterioration used, except for the addition of biocide (sodium azide, NaN3) at 200 mg L−1. Vertical bars are standard deviations of duplicate reactors.
The shower curtains produced approximately one order of magnitude lower DEHP concentrations than their solubility (0.285 mg l−1 at 25°C) in the abiotic MSM at 36 days; 1.0 µg DEHP g−1 was found in both shower curtain 2 and 3 systems, whereas 0.6 µg DEHP g−1 was for the shower curtain 1 system (Fig. 4). Based on the results obtained after a 36-day abiotic leaching, it can be calculated that the leached DEHP concentrations were equivalent to ~0.8% (w/w) of the water-soluble TOC concentrations for shower curtains 1 and 2, whereas they were ~2.1% (w/w) for shower curtain 3. In this regard, further analyses of SEM and TGA were conducted for the shower curtain 3 samples.
3.4. Biofilms and surface deterioration
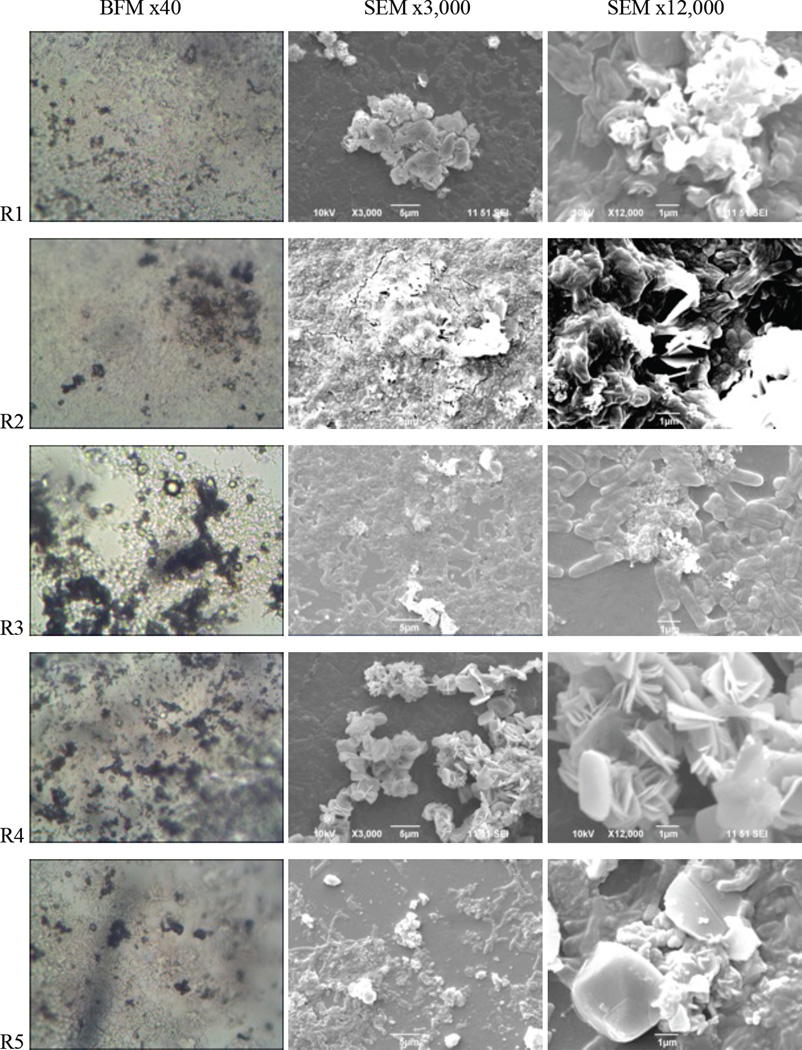
The surfaces of shower curtain 3 were monitored via SEM before and after the biodeterioration experiments (Fig. 5). In general, microorganisms tend to develop biofilm (i.e., attached growth) in low-nutrient environments and this causes biodeterioration of the surfaces of polymeric materials (Flemming, 1998). In the current study, biofilms were observed on the surfaces of the shower curtains in both biostimulated and bioaugmented reactors. However, the reactors bioaugmented with the Gram-positive strains LHM1 and LHM2 (R2 and R4, respectively) showed thicker biofilm development on the surfaces of shower curtains than the reactor bioaugmented with the Gram-negative strain LHM3 (R3). Pan et al. (2009) also reported that Gram-positive Nocardia corynebacterioides S3 colonized more thickly on the surfaces of bath mat than did the Gram-negative bacteria Alcaligenes xylosoxidans T2 and Pseudomonas aeruginosa GP10. The reactor bioaugmented with the consortium of three isolates (R5) also showed thick biofilm development on the surfaces of shower curtains.
Fig. 5.
Comparison of the biofilm images on the shower curtain type 3 (SC3) after 6-week biodeterioration. BFM: bright field microscopy, SEM: scanning electron microscopy.
In contrast, the biostimulated reactor (R1) had relatively weaker colonization than the bioaugmented reactors. The biofilms developed on the biostimulated shower curtains were isolated and subjected to 16S rRNA analysis. The results indicated that they were Pseudomonas sp. with a 97.7% similarity to Pseudomonas aeruginosa LMG 1242(T). A sterile control reactor run at a biocide (sodium azide, NaN3) concentration of 200 mg l−1 did not show any observable biofilm developments on the surfaces of the shower curtains.
Pigmentation with dark brown pigment was also observed in both the biostimulated and bioaugmented reactors (BFM images in Fig. 5). indicating the ability of the bacteria to produce extracellular enzymes for metabolic degradation (Pan et al., 2009) of the shower curtains. Morton and Surman (1994) commented that such biodeterioration could be attributed to either assimilatory degradation for its nutritive value or dissimilatory deterioration by corrosion, pigmentation, or toxic metabolites, or both. Scanning electron microscope images of the shower curtains after removal of biofilms revealed structural deterioration, as described in the following section on thermogravimetric analysis.
3.5. Thermogravimetric analysis
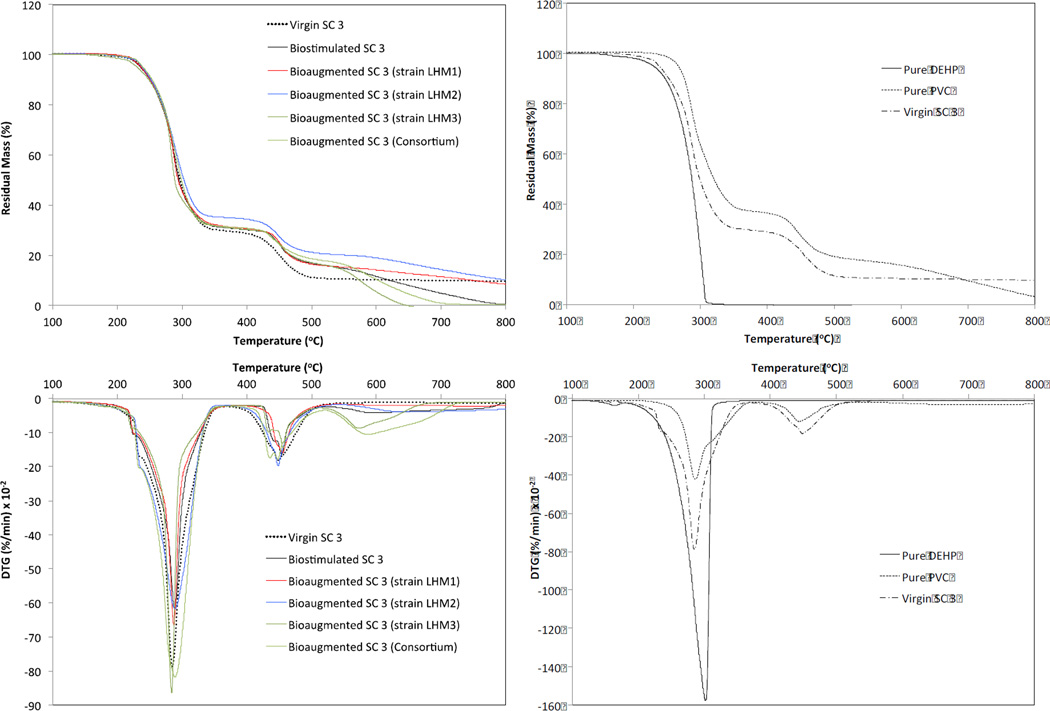
Figure 6 shows the residual mass (as a percentage) and derivative thermogravimetry (DTG, in % min−1) obtained from TGA for the shower curtain 3 samples. The thermal degradation of the control and biodeteriorated PVC shower curtains was generally in three steps at a temperature range of 200–300, 300–500, and 500–800°C. Both the control and biostimulated shower curtains had two DTG peaks (i.e., greatest mass loss rate) at ~280 and ~450°C. However, the biodeteriorated shower curtains showed additional DTG valleys at ~600°C. It is known that the thermal degradation of pure PVC is generally in two or three steps (Slapak et al., 2000; Kim, 2001; Soudais et al., 2007). A three-step thermal degradation was found for the pure PVC in the current study (Fig. 6).
Fig. 6.
Thermal gravimetric analysis (TGA) and derivative thermogravimetry (DTG) results of virgin and biodeteriorated shower curtains (left column) (SC 3) and those of pure DEHP and pure PVC (right column).
The thermograms showed the onset of mass loss at ~200°C due to evaporation of the phthalates. For example, pure DEHP evaporation would be observed in a TGA at a temperature close to its boiling point (230°C). However, as a consequence of the gelation and fusion of plasticizers during the heating process, the onset of plasticizer mass loss can be delayed. Dehydrochlorination in PVC resin to a polyene-like structure by a free radical mechanism is generally considered responsible for the first step occurring in the lower temperature region (200–300°C) (Soudais et al., 2007). The first peak temperatures of the bioaugmented shower curtains with the Gram-positive strains LHM1 and LHM2 were observed at ~287°C, whereas the control and Gram-negative strain LHM3-augmented shower curtains had the first peak temperature at ~283°C. This slight delay in the first peak temperature is indicative of lower plasticizer concentrations in the shower curtains that were bioaugmented with Gram-positive bacteria.
It is believed that a high plasticizer concentration accelerates the PVC resin decomposition due possibly to an autocatalytic effect exerted by hindered diffusion of the evolved HCl (Marcilla and Beltran, 1997; Slapak et al., 2000). Therefore, it is assumed that the shower curtains would have lower DEHP after a 6-wk biodeterioration due probably to more DEHP degradation by the biofilms and/or more DEHP leaching to the solution phase. This assumption was supported by greater aqueous DEHP concentrations in the bioaugmented shower curtain reactors with the Gram-positive bacteria and consortium as shown in the following section (3.6).
The thermal degradation of PVC in the higher temperature range of 400–500°C is attributed to decomposition of the polyene structure (Slapak et al., 2000; Kim, 2001). In the current study, the second peaks occurred at ~450°C. Generally, the onset of polyene decomposition was delayed and the temperature ranges were narrower for the biodeteriorated shower curtains in comparison to the control shower curtain. Additional DTG valleys at ~600°C were produced for the shower curtains from the biodeterioration reactors, especially from the reactor bioaugmented with strain LHM3 and the consortium. It is unclear at this moment why the biodeteriorated shower curtains produced such delayed polyene decomposition at ~450°C with narrower temperature ranges and unique additional DTG valleys at ~600°C. Brems et al. (2011) also reported an additional DTG valley at ~650°C due to a slow decomposition of carbonaceous residue in thermogravimetric pyrolysis of waste polyethylene-terephthalate. Further studies on potential chemical and structurally changes due to biodeterioration are required to better understand these phenomena.
3.6. DEHP and OD concentrations in biodeterioration reactors
Despite bioaugmentation with highly enriched DEHP-degraders, the solutions of bioaugmentation reactors were not DEHP-free, although the DEHP concentrations were one or two orders of magnitude lower than their aqueous solubility (0.285 mg l−1) (Fig. 7). As shown in the results from water-soluble TOC analysis (Fig. 4), DEHP was only ~0.8–2.1% (w/w) of TOC, implying that there were other organic carbons readily available to the augmented bacteria from the shower curtains. Perhaps some of those other organic carbons in the shower curtains and/or biofilms as extracellular carbohydrate on the shower curtains were utilized preferentially by DEHP-degraders supporting their growth in the biodeterioration reactors.
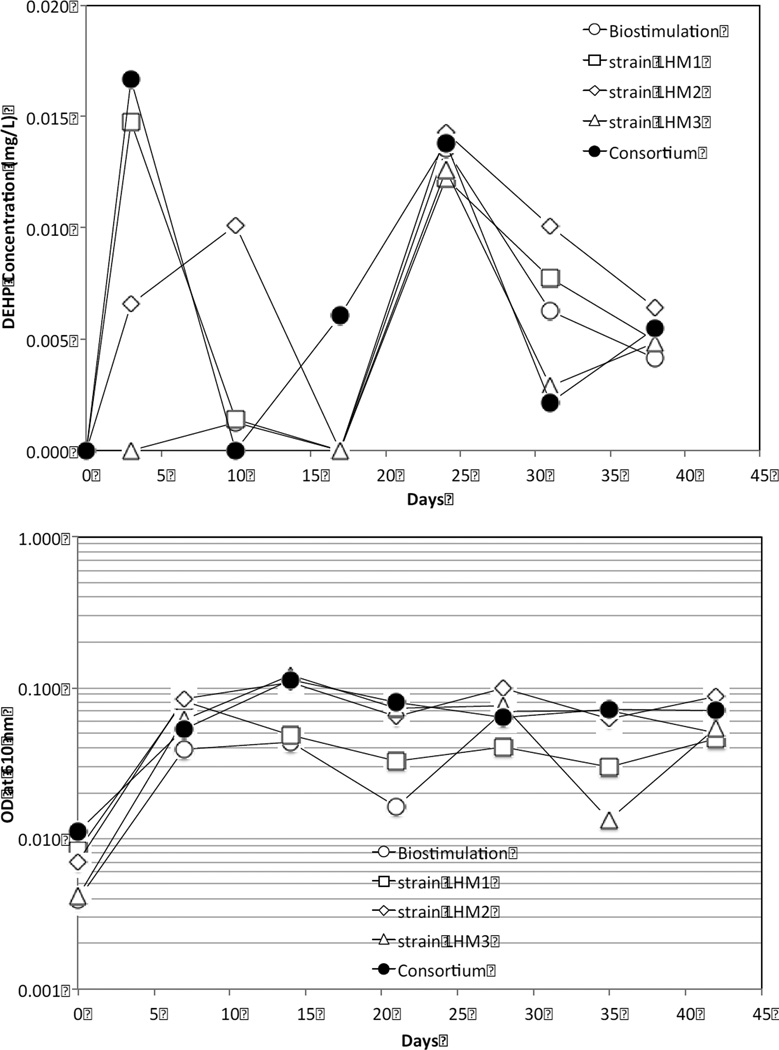
In general, the bioaugmented reactors with the Gram-positive strains LHM1 and LHM2 and consortia had greater aqueous DEHP concentrations in the first half (<3 wk) of the biodeterioration than the biostimulated and Gram-negative strain LHM3-augmented reactors. To give more support to these findings, kinetic studies on DEHP leaching and metabolism by these bacteria are warranted.
The reactors bioaugmented with strain LHM2, strain LHM3, and consortia showed a similar OD profile (Fig. 7), achieving high OD from the beginning and maintaining this at 0.08~0.1 AU in the end of the experiment. However, the OD values in the bioaugmented reactor with strain LHM1 diminished and stayed at ~0.04 AU. Generally, the trend of OD development by these bacteria in the biodeterioration experiment was similar to that observed in the enrichment flasks (Fig. 2). The biostimulated reactor generally showed the lowest OD value among the reactors up to the first half of the experiment (<3 wk) but increased to a similar OD value (~0.07 AU) to those of the bioaugmented reactors.
Fig. 7.
Comparisons of aqueous DEHP concentration (top) and optical density (OD) at 610 nm (bottom) among the biodeterioration reactors. DEHP and OD measurements were done at the 3rd and 7th day, respectively, after the weekly replenishment of 50% liquid volume with the fresh MSM.
Bioaugmentation of the consortium did not produce an additive effect on the aqueous DEHP and OD concentrations or on biofilm development and TGA in the current study. This was probably due to the competition for nutrients among the microorganisms, resulting in inhibition of their growth (Pino and Penuela, 2011).
3.7. Environmental health implications
As the water-soluble TOC concentration results indicated, the shower curtains (and other plastic materials) contained various organic ingredients, including DEHP. Due to its weak physical bonding to the plastic matrix, DEHP can easily leach to the environment (Fang et al., 2010). Higher aqueous DEHP concentrations can result from a greater extent of biodeterioration of plastic or polymeric materials by specific bacteria, as was shown in the current study. It can be metabolized as the primary carbon source by DEHP-degraders (e.g., strains LHM1, LHM2, and LHM3) or it can be degraded by cometabolism. In some cases, it can fortuitously be leached out of the plastic or polymeric materials to the solution phase, while the microorganisms utilize other carbonaceous compounds in the materials. The latter case is especially true in natural environments (e.g., landfill leachate or wastewater) where other readily available carbon sources are abundant to microorganisms despite their capability of metabolizing DEHP.
Because DEHP is a suspected endocrine disruptor and human carcinogen, enhanced DEHP leaching from plastic or polymeric materials due to biodeterioration may impose significant environmental health problems on the adjacent environmental receptors including humans. Indeed, DEHP has been found in air, sediments, water, soils, and landfill leachate (Shelton et al., 1984; Chauret et al., 1996; Turner and Rawling, 2000; Marttinen et al., 2003; ) and in at least 737 of the 1613 current or former National Priorities List sites (ATSDR, 2011).
4. Conclusions
Newly isolated, not previously reported DEHP-degraders (strains LHM1 and LHM2) were able to enhance biodeterioration of PVC shower curtains by more biofilm development. This resulted in lower DEHP concentrations in the shower curtains after 6 wk of biodeterioration with these bacteria, as shown in TGA. The onset of polyene decomposition was delayed and the temperature ranges were narrower for the biodeteriorated shower curtains in comparison to the control shower curtain. Despite their metabolic capability of degrading DEHP as the sole source of carbon, these bacteria did not make the aqueous solution DEHP-free, although aqueous DEHP was found to be one order of magnitude lower than its solubility. This must have been caused by the presence of other readily available organic carbons from the shower curtains. In fact, mass fractions of aqueous DEHP were only 2% or less of water-soluble TOC. Therefore, these bacteria may play an important role in DEHP leaching to the environment and PVC biodeterioration.
> Polyvinyl chloride (PVC) shower curtains were biodeteriorated by DEHP-degraders. > Newly isolated, not previously reported, DEHP-degraders were used. > Less DEHP was found left on the shower curtains with Gram-positive DEHP-degraders. > Greater aqueous DEHP concentrations were observed with Gram-positive DEHP-degraders. > New DEHP-degraders can signify PVC deterioration and DEHP leaching.
Acknowledgements
The authors are grateful to students Lymari Montijo in the Department of Civil Engineering, and Carolina Guerrero and Madelyn Caban in the Department of Biology, at the University of Puerto Rico at Mayaguez (UPRM) for their sincere help in conducting the experiments. Our appreciation is extended to Drs. Nelson Cardona and Carlos Rinaldi in the Department of Chemical Engineering at UPRM for their kind provision of SEM and TGA, respectively.
This project was supported by Award Number P42ES017198 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR. Public Health Statement, Di-(2-ethylhexyl) phthalate (DEHP) [accessed July 14, 2011];2002 http://www.atsdr.cdc.gov.
- Brems A, Baeyens J, Beerlandt J, Dewil R. Thermogravimetric pyrolysis of waste polyethylene-terephthalate and polystyrene: A critical assessment of kinetics modeling. Resources, Conservation and Recycling. 2011;55:772–781. [Google Scholar]
- Chang B, Liao C, Yuan S. Anaerobic degradation of diethyl phthalate, di-n-butyl phthalate, and di-(2-ethylhexyl) phthalate from river sediment in Taiwan. Chemosphere. 2005;58:1601–1607. doi: 10.1016/j.chemosphere.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Chauret C, Inniss W, Mayfield C. Biotransformation at 10 °C of Di-n-butyl phthalate in subsurface microcosms. Ground Water. 1996;34:791–794. [Google Scholar]
- Fang CR, Yao J, Zheng YG, Jiang CJ, Hu LF, Wu YY. Dibutyl phthalate degradation by Enterobacter sp. T5 isolated from municipal solid waste in landfill bioreactor. International Biodeterioration and Biodegradation. 2010;64:442–446. [Google Scholar]
- Flemming H-C. Relevance of biofilms for the biodeterioration of surfaces of polymeric materials. Polymer Degradation and Stability. 1998;59:309–315. [Google Scholar]
- Heudorf U, Merch-Sundermann V, Angerer J. Phthalates: Toxicology and exposure. International Journal of Hygiene and Environmental Health. 2007;210:623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. vol. 77. Lyon, France: International Agency for Research on Cancer; 2000. Some Industrial Chemicals. ( http://monographs.iarc.fr/ENG/Monographs/vol77/volume77.pdf) [Google Scholar]
- Kim S. Pyrolysis kinetics of waste PVC pipe. Waste Management. 2001;21:606–616. doi: 10.1016/s0956-053x(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Marcilla A, Beltran M. Effect of DMP concentration on the thermal decomposition behaviour of plastisols from a commercial PVC resin. Polymer Degradation and Stability. 1997;57:101–107. [Google Scholar]
- Marttinen SK, Kettunen RH, Rintala JA. Occurrence and removal of organic pollutants in sewages and landfill leachates. The Science of the Total Environment. 2003;301:1–12. doi: 10.1016/s0048-9697(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Mersiowsky I, Weller M, Ejlertsson J. Fate of plasticized PVC products under landfill conditions: A laboratory-scale landfill simulation reactor study. Water Research. 2001;35:3063–3070. doi: 10.1016/s0043-1354(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Morton LHG, Surman SB. Biofilms in biodeterioration – a review. International Biodeterioration and Biodegradation. 1994;34:203–221. [Google Scholar]
- Pan L, Gu J-G, Yin B, Cheng S-P. Contribution to deterioration of polymeric materials by a slow-growing bacterium Nocardia corynebacterioides. International Biodeterioration and Biodegradation. 2009;63:24–29. [Google Scholar]
- Pino N, Penuela G. Simultaneous degradation of the pesticides methyl parathion and chlorpyrifos by an isolated bacterial consortium from a contaminated site. International Biodeterioration and Biodegradation. 2011;65:827–831. [Google Scholar]
- Shah AA, Hasan F, Hameed A, Ahmed S. Biological degradation of plastics: A comprehensive review. Biotechnology Advances. 2008;26:246–265. doi: 10.1016/j.biotechadv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Shelton DR, Boyd SA, Tiedje AM. Anaerobic biodegradation of phthalic acid esters in sludge. Environmental Science and Technology. 1984;18:93–97. doi: 10.1021/es00120a008. [DOI] [PubMed] [Google Scholar]
- Slapak MJP, van Kasteren JMN, Drinkenburg AAH. Determination of the pyrolytic degradation kinetics of virgin-PVC and PVC-waste by analytical and computational methods. Computational and Theoretical Polymer Science. 2000;10:481–489. [Google Scholar]
- Soudais Y, Moga L, Blazek J, Lemort F. Coupled DTA-TGA-FT-IR investigation of pyrolytic decomposition of EVA, PVC and cellulose. Journal of Analytical and Applied Pyrolysis. 2007;78:46–57. [Google Scholar]
- Staples CA, Adams WJ, Parkerton TF, Gorsuch JW, Biggingers GR, Reiner KH. Aquatic toxicity of eighteen phthalate esters. Environmental Toxicology and Chemistry. 1997;16:875–891. [Google Scholar]
- Turner V, Rawling M. The behavior of di-2-ethylhexyl phthalate in estuaries. Marine Chemistry. 2000;68:203–217. [Google Scholar]
- Zeng F, Cui K, Li X, Fu J, Sheng G. Biodegradation kinetics of phthalate esters by Pseudomonas fluoresences FS1. Process Biochemistry. 2004;39:1125–1129. [Google Scholar]
- Voss C, Zerban H, Bannasch P, Berger MR. Lifelong exposure to di-(2-ethylhexyl)-phthalate induces tumors in liver and testes of Sprague-Dawley rats. Toxicology. 2005;206:359–371. doi: 10.1016/j.tox.2004.07.016. [DOI] [PubMed] [Google Scholar]