Abstract
OBJECTIVE
To evaluate intravenous mannitol during minimally invasive partial nephrectomy (PN) by comparing the renal function outcomes of the patients who received it versus those who did not.
METHODS
Of 285 consecutive elective minimally invasive PN cases from February 2005 to July 2010, 164 patients (58%) were treated with mannitol. We compared the renal function recovery using a multivariate generalized estimating equation linear model of estimated glomerular filtration rate (eGFR) controlling for nephrometry complexity, preoperative eGFR, American Society of Anesthesiologists score, ischemia time, estimated blood loss, age, and sex. Sensitivity analyses were performed to adjust for cold ischemia and individual surgeon differences corrected for year of surgery.
RESULTS
Of the 285 patients who underwent minimally invasive treatment, 164 received mannitol and 121 did not. Those who received mannitol had a better preoperative eGFR (median 72 vs 69 mL/min/m2, P =.046), less complex nephrometry scores (P =0.051), and were less likely to have an American Society of Anesthesiologists score of ≥3 (42% vs 54%, P =.005). Renal function recovery was similar in both groups (estimated effect of mannitol −0.7 mL/min/m2, 95% confidence interval −3.6-2.2, P =.6). At no point in the postoperative period did mannitol make a significant difference in the eGFR according to the generalized estimating equation model after adjusting for multiple potential renal function confounders.
CONCLUSION
Mannitol use did not influence renal function recovery within 6 months of minimally invasive PN as measured by the eGFR in our analysis. An appropriately designed prospective study of mannitol is being conducted to validate its use during PN.
Traditionally, mannitol has been used to reduce the risk of perioperative renal dysfunction during surgery involving the kidney. Mannitol’s purported effects of increasing renal blood flow (ie, by way of prostaglandins and atrial natriuretic peptide), decreasing intravascular cellular swelling, free radical scavenging, decreasing renin production, and increasing intravascular volume are thought to mitigate the effects of hypoxia and ischemic renal injury that can occur during related surgical procedures.1 The clinical evidence of its effectiveness for this purpose, however, is conspicuously sparse in the field and across much of medical science.1–5 No randomized trial exists to support mannitol’s use during PN, although support has been drawn from clinical experience in renal transplantation and preclinical animal studies of prolonged warm ischemia.6 As the complex renal physiologic milieu is elucidated, evidence is mounting that mannitol might ay be detrimental to kidney function by a competitive mechanism of increasing metabolic demand.5 Although standard dosing exists, mannitol use during PN is discretionary and can be preferentially avoided by some surgeons. We evaluated the use of mannitol during minimally invasive PN and its effects on postoperative renal function recovery in a consecutive series.
MATERIAL AND METHODS
Patient Selection and Technique
An institutional review board-approved retrospective review was conducted of 285 consecutive patients who underwent elective minimally invasive partial nephrectomy (PN) performed by 1 of 4 surgeons at the Memorial Sloan-Kettering Cancer Center from February 2005 to July 2010. Cases missing estimated glomerular filtration rate (eGFR) data were excluded.
When used, intravenous mannitol was administered according to a standardized dosing regimen. A total of 12.5 g of mannitol in 200 mL sterile water was given intravenously within 15 minutes of tumor excision. Mannitol was inconsistently used across the surgeons: 1 surgeon preferentially avoided mannitol (<25% of associated procedures), and the other surgeons preferentially used mannitol (>80% of associated procedures). Mannitol was not consistently ordered or omitted by individual fellows who assisted in the procedures and no clear variables other than surgeon factors could be identified.
Robotic-assisted laparoscopic and laparoscopic PNs were completed by experienced urologic oncology surgeons at the Memorial Sloan-Kettering Cancer Center. Most minimally invasive PNs were performed under warm ischemic conditions, and 70 procedures were performed with renal hypothermia, which was achieved by either cold perfusion with iced Ringer’s lactate or retrograde renal pelvis infusion with cold saline irrigation.
Measurements
The clinical parameters, including demographics and patient comorbidities, pathologic data, intraoperative details, and follow-up data were reviewed. Renal function was assessed by estimating the eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation.7 All renal tumors were categorized according to the nephrometry score determined from preoperative imaging as low, moderate, and high complexity.8 Baseline laboratory studies were completed within 7 days before the patient’s procedure. All available postoperative laboratory studies were included. Only creatinine measurements completed at our institution were included in the analysis.
Statistical Analysis
To evaluate whether mannitol affected the eGFR after surgery, a multivariate generalized estimating equation (GEE) linear model was created to predict eGFR according to the interval from surgery, preoperative eGFR, American Society of Anesthesiologists score (1–2 vs 3–4), estimated blood loss, age, sex, total nephrometry score, and ischemia time. A GEE model with an identity link function was used because patients were followed up longitudinally and most had >1 eGFR measurement. In brief, standard regression models assume that all observations are independent. Because multiple eGFR measurements from the same patient are not independent, adjustment is required for correlation within patients. We hypothesized that the eGFR would initially decline and subsequently improve after surgery. Because the association between eGFR and the interval after surgery was hypothesized to be nonlinear, we included restricted cubic splines in our model, which allowed the curve to bend at prespecified knots (15 days and 2 months after surgery). To illustrate the relationship between the interval from surgery and the eGFR in our cohort, we plotted the adjusted eGFR by the interval after surgery from the GEE models that were created separately for the mannitol and nonmannitol groups. Sensitivity analyses were performed to adjust for cold ischemia and individual surgeon differences.
RESULTS
Of the 287 consecutive minimally invasive patients included in the final analyses, 2 patients were excluded because of insufficient laboratory data. Of the remaining 285 patients, 164 received mannitol and 121 did not. The patient characteristics are listed in Table 1. The patients who received mannitol tended to have a better preoperative eGFR (median 72 vs 69 mL/min/m2, P =.046), were more likely to undergo ischemia (98% vs 88%, P <.001), and tended to be healthier (42% vs 54% had an American Society of Anesthesiologists score of 3 or 4, P =.005) than patients who did not. The tumors, as classified by the nephrometry score, tended to be more complex in the nonmannitol group (P =.05).
Table 1.
Patient characteristics stratified by mannitol treatment (n =285)*
| Characteristic | No Mannitol (n =121) | Mannitol (n =164) | P Value |
|---|---|---|---|
| Age at surgery (y) | 60 (51–68) | 60 (51–67) | .9 |
| Preoperative eGFR | 69 (57–81) | 72 (62–84) | 0.046 |
| Male gender | 78 (64) | 109 (66) | .8 |
| Estimated blood loss (mL) (n =284) | 200 (50–300) | 200 (100–350) | .002 |
| Tumor size (cm) (n =284) | 2.9 (1.8–4.3) | 2.7 (2.0–4.2) | .7 |
| Ischemia | 107 (88) | 161 (98) | <.001 |
| Cold perfusion/irrigation (n =285) | 49 (46) | 21 (13) | |
| Clamp time (min) (n =241) | 32 (24–39) | 32 (25–40) | |
| Pathologic high grade (n =188) | 34 (42) | 38 (36) | .4 |
| Pathologic stage (n =259) | .8 | ||
| T1 | 91 (83) | 130 (87) | |
| T2a | 4 (4) | 5 (3) | |
| T2b+ | 14 (13) | 15 (10) | |
| ASA score (n =284) | .005 | ||
| 1 | 12 (10) | 6 (4) | |
| 2 | 44 (36) | 89 (55) | |
| 3 | 63 (52) | 67 (41) | |
| 4 | 2 (2) | 1 (1) | |
| Year of surgery | <.001 | ||
| 2005 | 3 (2) | 27 (16) | |
| 2006 | 5 (4) | 18 (11) | |
| 2007 | 5 (4) | 48 (29) | |
| 2008 | 31 (26) | 25 (15) | |
| 2009 | 50 (41) | 24 (15) | |
| 2010 | 27 (22) | 22 (13) | |
| Total nephrometry score | .051 | ||
| 4–6 (low complexity) | 45 (37) | 68 (41) | |
| 7–9 (moderate complexity) | 59 (49) | 87 (53) | |
| 10–11 (high complexity) | 17 (14) | 9 (5) |
eGFR, estimated glomerular filtration rate; ASA, American of Anesthesiologists.
Data presented as medians, with interquartile ranges in parentheses, or numbers, with percentages in parentheses.
Fisher’s exact test or Wilcoxon rank-sum test.
The median number of eGFR measurements per patient was 5 (interquartile range 3–8), and the median interval to the last eGFR assessment for those treated before 2010 was 13 months (interquartile range 6–29). We did not find any evidence that patients with worse preoperative eGFR were followed up longer (P =0.11) or more intensely (P =0.13). Similarly, after adjusting for the year of surgery, we did not find evidence of significant differences in intensity (P =.11) of follow-up according to mannitol use but did find a significant difference in length of follow-up (P =0.016).
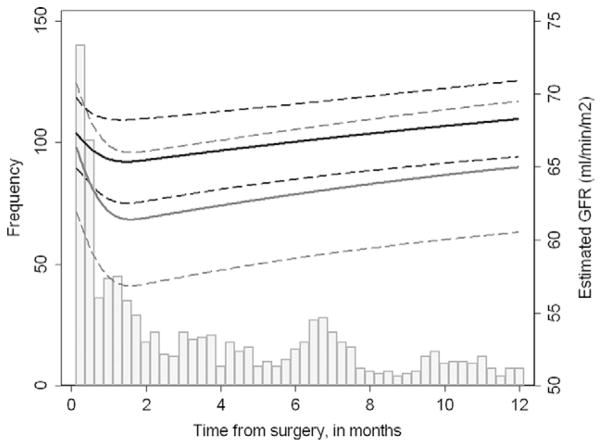
Overall, we did not find any evidence that mannitol was significantly associated with improved renal function recovery over time. Mannitol use did not lead to a significantly greater eGFR (estimated effect of mannitol on eGFR from the GEE model was −0.7 mL/min/m2, 95% confidence interval −3.6-2.2; P =.6). Figure 1 illustrates the eGFR over time after minimally invasive PN when modeled separately for the mannitol and nonmannitol groups. The predicted eGFR for a typical patient 2 months after surgery was 70 mL/min/1.73 m2 for patients receiving mannitol (difference of −3 mL/min/m2 compared with the baseline eGFR) and 68 mL/min/1.73 m2 for those who did not (difference of 1 ml/min/m2 compared with the baseline eGFR). At 6 months postoperatively, the predicted eGFR measurement was 72 mL/min/1.73 m2 (baseline difference of −1 ml/min/m2) and 70 mL/min/1.73 m2 (baseline difference of 3 ml/min/m2) for the mannitol and nonmannitol groups, respectively. These predicted eGFR measurements can be estimated at any point in the postoperative period using the data in Figure 1, and the baseline differences were not statistically significant.
Figure 1.
Population-averaged changes in eGFR after minimally invasive PN for patients given (gray line) and not given (black line) mannitol, adjusting for interval from surgery (modeled with splined terms), preoperative eGFR, American Society of Anesthesiologists score (1–2 vs 3–4), ischemia time, estimated blood loss, age, sex, year of surgery, and total nephrometry score (4–6, 7–9, 10–11). Dashed lines indicate 95% confidence intervals. Histogram shows number of eGFR measurements over time, excluding postoperative measurements taken within 2 days of surgery, which were recorded for all patients.
We performed several sensitivity analyses. We excluded 70 patients who received cold perfusion/irrigation during surgery (21 from the mannitol group and 49 from the nonmannitol group). We again found no evidence that mannitol was significantly associated with improved renal function (−1.5 mL/min/m2, 95% confidence interval −5.0-2.0; P =.4), and there did not appear to be important differences in renal function between the mannitol and nonmannitol groups at either 2 months (71 vs 69 mL/min/m2) or 6 months (72 vs 73 mL/min/m2). Finally, we were concerned about differences in the rates of mannitol use by surgeon and their corresponding PN technique. Of the 4 surgeons performing minimally invasive PN, 3 used mannitol for most of their patients (≥80%), and 1 surgeon gave mannitol to fewer than one quarter of his patients (21%). One surgeon who frequently prescribed mannitol had significantly greater eGFR outcomes (16.5 mL/min/m2, 95% confidence interval 6.5–26.5; P <.001). However, that surgeon treated only 5 patients included in the present study. Our results were not importantly changed, however, when we adjusted for the surgeon as a fixed effect among the remaining 3 surgeons who performed most procedures. Owing to the lower number of patients included in these analyses, the confidence intervals were wider.
COMMENT
We did not find evidence of clinically meaningful improvements in intermediate eGFR outcomes associated with mannitol use in minimally invasive PN; thus, the results of our study do not support the use of intravenous mannitol infusion as a renal protective agent in this setting. Additional research of mannitol use in this setting is warranted. This is the first clinical study in the modern elective PN era to investigate mannitol’s role in renal protection.
Mannitol is an osmotic diuretic and a renal vasodilator that promotes tubular flow, prevents intratubular cast formation, decreases postischemic cellular swelling, and might serve as a free radical scavenger.1 However, no randomized trials have assessed its effect. Therefore, a clinician is left with data on limited short-term physiologic gains in animal studies not specific to PN techniques. A recent 4-center, 660-patient retrospective solitary kidney PN series by Lane et al9 attempted to assess the effect of modifiable and nonmodifiable factors in determining long-term renal function. Their results suggested the nonmodifiable factors, the quantity and quality of the remaining renal parenchyma, were the main determinant of follow-up renal function. However, the modifiable factor of intraoperative mannitol use was not consistent (using 12.5–37.5 g) and was not factored into their analysis.
The seminal review by Novick6 drew on a number of well-performed animal studies to support mannitol use in early PN series. However, that study stressed the importance of future investigation into pharmacologic prevention strategies that would translate into the clinical setting.
Other medical specialties have conducted trials evaluating mannitol’s renal protective effects. In vascular surgery, 3 randomized controlled trials reported no reduction in the incidence of renal failure in patients undergoing abdominal aortic aneurysm repair.10 All these patients underwent elective, infrarenal abdominal aortic aneurysm repair, with no cross-clamping of the renal arteries. No significant differences were seen between the groups in terms of eGFR from 2 hours to 7 days (with the exception of postoperative day 2 in the trial by Wijnen et al,4 P =.047).3,11 In interventional cardiology, initial uncontrolled clinical studies suggested renal protective effects of mannitol against radiocontrast nephropathy12,13; however, the use of mannitol has since ceased in coronary angiography after the publication of randomized clinical trials that demonstrated not only mannitol’s in effectiveness for renal protection, but also its potential to cause renal failure.14,15
Perhaps the strongest arguments for the use of mannitol as a renal protective agent come from the published transplantation data, although these studies are not completely parallel to the physiologic alterations of PN patients. Green et al,16 motivated by the indiscriminate use of multiple agents in renal transplantation without “comparative experimental data,” completed an initial study in a 1-hour unilateral normothermic ischemia rabbit model. The right kidney served as the internal control for the clamped left kidney. The investigators summarized their results by categorizing renal protective agents into acute (measurements of creatinine excretion [mg/kg/h] from each kidney every 5 minutes for 1 hour after renal artery clamping) and chronic (measurements of creatinine [mg/dL] for 7 days) studies. Only mannitol conferred a significant benefit in both the acute and the chronic models. The investigators went further to characterize the timing and dose of mannitol in a subsequent study, and they found that 0.25 mg/kg of intravenous mannitol given 15 minutes before the onset of renal ischemia provided the best outcomes on post-treatment day 3.17 Shilliday and Allison1 provided a summary of the animal models supporting mannitol’s beneficial role but concluded their discussion with the disclaimer that the “case for mannitol must remain, in the words of the Scottish legal verdict, ‘Not proven.’” Weimar et al18 reported on a prospective trial of 50 patients undergoing cadaveric donor transplant patients randomly assigned to receiving 50 g intravenous mannitol versus saline infusion just before graft revascularization. They found a significant decrease in the incidence of acute tubular necrosis in the early postoperative period in the mannitol group, but this effect was not significant at 3 months when the eGFR was compared.
It is well established that the pneumoperitoneum compresses the renal parenchyma and renal hilum, resulting in decreased blood flow and transient ischemia.19 The clinical significance of this phenomenon is not well established, particularly at the abdominal pressure of 12–15 mm Hg.20 Adamy et al21 compared the renal function outcomes of 987 patients who underwent open versus laparoscopic PN from 2002 to 2009. They found that the surgical approach had only a small effect on the eGFR outcomes and, in fact, slightly favored laparoscopic PN. The statistical analysis was limited to minimally invasive procedures owing to the highly discrepant differences in mannitol use between the surgical approaches. Moreover, <2% of the open procedures were performed with renal ischemia without mannitol. Thus, it is unclear whether these findings are applicable in this setting.
The results of our study provide evidence to support additional critical evaluation of mannitol as a renal protective agent. The limitations of our study included the retrospective, nonrandomized design, lack of uniformity in laboratory collection intervals, and complete eGFR follow-up for all patients. Significant heterogeneity of our comparison groups was also evident. Although the Chronic Kidney Disease Epidemiology Collaboration eGFR as an outcome measure is more precise than serum creatinine, it is not a perfect gauge of renal function and the effects of iatrogenic renal injury resulting from surgical interventions. Additional studies involving more accurate and precise testing of renal function are necessary to explore these important questions. Also, different doses of mannitol infusion at different intervals might prove to be renal protective. Our analysis used a multivariate GEE linear model to perform a comparison between the mannitol and nonmannitol groups, attempting to control for multiple potential renal function outcome confounders, such as preoperative eGFR, American Society of Anesthesiologists score, ischemia time, estimated blood loss, age, sex, year of surgery, surgeon difference, and nephrometry complexity. Although we believe that attempts to control for such confounders are superior to standard reporting of eGFR outcomes using univariate statistics, our model has not been validated in larger independent series. The GEE model might not be able to adequately control for factors influencing renal function outcomes as measured by eGFR; therefore, before discouraging mannitol use uniformly, a prospective randomized controlled trial should be performed.
CONCLUSIONS
Multiple measurements of eGFR within 6 months after minimally invasive PN procedures showed that intravenous mannitol use did not influence renal function recovery in our retrospective consecutive series analysis. An appropriately designed prospective study of mannitol is needed to validate its use as a renal protective agent during both open and minimally invasive PN procedures.
Acknowledgments
Funding Support: Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and funds provided by the Renal Carcinoma Program Fund.
To Joyce Tsoi and Clarisse Mazzola, with thanks, for the editorial assistance provided in preparing our report.
References
- 1.Shilliday I, Allison ME. Diuretics in acute renal failure. Ren Fail. 1994;16:3–17. doi: 10.3109/08860229409044843. [DOI] [PubMed] [Google Scholar]
- 2.Zacharias M, Conlon NP, Herbison GP, et al. Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev CD. 2008:003590. doi: 10.1002/14651858.CD003590.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson ML, Baker DM, Hopkinson BR, et al. Randomized controlled trial of the effect of mannitol on renal reperfusion injury during aortic aneurysm surgery. Br J Surg. 1996;83:1230–1233. [PubMed] [Google Scholar]
- 4.Wijnen MH, Vader HL, Van Den Wall Bake AW, et al. Can renal dysfunction after infra-renal aortic aneurysm repair be modified by multi-antioxidant supplementation? J Cardiovasc Surg. 2002;43:483–488. [PubMed] [Google Scholar]
- 5.Gelman S. Does mannitol save the kidney? Anesth Analg. 1996;82:899–901. doi: 10.1097/00000539-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Novick AC. Renal hypothermia: in vivo and ex vivo. Urol Clin North Am. 1983;10:637–644. [PubMed] [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Lane BR, Russo P, Uzzo RG, et al. Comparison of cold and warm ischemia during partial nephrectomy in 660 solitary kidneys reveals predominant role of nonmodifiable factors in determining ultimate renal function. J Urol. 2011;185:421–427. doi: 10.1016/j.juro.2010.09.131. [DOI] [PubMed] [Google Scholar]
- 10.Hersey P, Poullis M. Does the administration of mannitol prevent renal failure in open abdominal aortic aneurysm surgery? Interact Cardiovasc Thorac Surg. 2008;7:906–909. doi: 10.1510/icvts.2008.184010. [DOI] [PubMed] [Google Scholar]
- 11.Paul MD, Mazer CD, Byrick RJ, et al. Influence of mannitol and dopamine on renal function during elective infrarenal aortic clamping in man. Am J Nephrol. 1986;6:427–434. doi: 10.1159/000167248. [DOI] [PubMed] [Google Scholar]
- 12.Anto HR, Chou SY, Porush JG, et al. Infusion intravenous pyelography and renal function: effect of hypertonic mannitol in patients with chronic renal insufficiency. Arch Intern Med. 1981;141:1652–1656. [PubMed] [Google Scholar]
- 13.Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int. 1994;45:259–265. doi: 10.1038/ki.1994.32. [DOI] [PubMed] [Google Scholar]
- 14.Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–1420. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 15.Majumdar SR, Kjellstrand CM, Tymchak WJ, et al. Forced euvolemic diuresis with mannitol and furosemide for prevention of contrast-induced nephropathy in patients with CKD undergoing coronary angiography: a randomized controlled trial. Am J Kidney Dis. 2009;54:602–609. doi: 10.1053/j.ajkd.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Green RD, Boyer D, Halasz NA, et al. Pharmacological protection of rabbit kidneys from normothermic ischemia. Transplantation. 1979;28:131–134. doi: 10.1097/00007890-197908000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Collins GM, Green RD, Boyer D, et al. Protection of kidneys from warm ischemic injury: dosage and timing of mannitol administration. Transplantation. 1980;29:83–84. doi: 10.1097/00007890-198001000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Weimar W, Geerlings W, Bijnen AB, et al. A controlled study on the effect of mannitol on immediate renal function after cadaver donor kidney transplantation. Transplantation. 1983;35:99–101. [PubMed] [Google Scholar]
- 19.Demyttenaere S, Feldman LS, Fried GM. Effect of pneumoperitoneum on renal perfusion and function: a systematic review. Surg Endosc. 2007;21:152–160. doi: 10.1007/s00464-006-0250-x. [DOI] [PubMed] [Google Scholar]
- 20.Simmons MN, Schreiber MJ, Gill IS. Surgical renal ischemia: a contemporary overview. J Urol. 2008;180:19–30. doi: 10.1016/j.juro.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Adamy A, Favaretto RL, Nogueira L, et al. Recovery of renal function after open and laparoscopic partial nephrectomy. Eur Urol. 2010;58:596–601. doi: 10.1016/j.eururo.2010.05.044. [DOI] [PubMed] [Google Scholar]