Executive Summary
Objective
The purpose of this evidence based analysis was to determine the effectiveness and safety of ultraviolet phototherapy for moderate-to-severe plaque psoriasis.
Research Questions
The specific research questions for the evidence review were as follows:
What is the safety of ultraviolet phototherapy for moderate-to-severe plaque psoriasis?
What is the effectiveness of ultraviolet phototherapy for moderate-to-severe plaque psoriasis?
Clinical Need: Target Population and Condition
Psoriasis is a common chronic, systemic inflammatory disease affecting the skin, nails and occasionally the joints and has a lifelong waning and waxing course. It has a worldwide occurrence with a prevalence of at least 2% of the general population, making it one of the most common systemic inflammatory diseases. The immune-mediated disease has several clinical presentations with the most common (85% - 90%) being plaque psoriasis.
Characteristic features of psoriasis include scaling, redness, and elevation of the skin. Patients with psoriasis may also present with a range of disabling symptoms such as pruritus (itching), pain, bleeding, or burning associated with plaque lesions and up to 30% are classified as having moderate-to-severe disease. Further, some psoriasis patients can be complex medical cases in which diabetes, inflammatory bowel disease, and hypertension are more likely to be present than in control populations and 10% also suffer from arthritis (psoriatic arthritis). The etiology of psoriasis is unknown but is thought to result from complex interactions between the environment and predisposing genes.
Management of psoriasis is related to the extent of the skin involvement, although its presence on the hands, feet, face or genitalia can present challenges. Moderate-to-severe psoriasis is managed by phototherapy and a range of systemic agents including traditional immunosuppressants such as methotrexate and cyclospsorin. Treatment with modern immunosuppressant agents known as biologicals, which more specifically target the immune defects of the disease, is usually reserved for patients with contraindications and those failing or unresponsive to treatments with traditional immunosuppressants or phototherapy.
Treatment plans are based on a long-term approach to managing the disease, patient’s expectations, individual responses and risk of complications. The treatment goals are several fold but primarily to:
1) improve physical signs and secondary psychological effects,
2) reduce inflammation and control skin shedding,
3) control physical signs as long as possible, and to
4) avoid factors that can aggravate the condition.
Approaches are generally individualized because of the variable presentation, quality of life implications, co-existent medical conditions, and triggering factors (e.g. stress, infections and medications). Individual responses and commitments to therapy also present possible limitations.
Phototherapy
Ultraviolet phototherapy units have been licensed since February 1993 as a class 2 device in Canada. Units are available as hand held devices, hand and foot devices, full-body panel, and booth styles for institutional and home use. Units are also available with a range of ultraviolet A, broad and narrow band ultraviolet B (BB-UVB and NB-UVB) lamps. After establishing appropriate ultraviolet doses, three-times weekly treatment schedules for 20 to 25 treatments are generally needed to control symptoms.
Evidence-Based Analysis Methods
The literature search strategy employed keywords and subject headings to capture the concepts of 1) phototherapy and 2) psoriasis. The search involved runs in the following databases: Ovid MEDLINE (1996 to March Week 3 2009), OVID MEDLINE In-Process and Other Non-Indexed Citations, EMBASE (1980 to 2009 Week 13), the Wiley Cochrane Library, and the Centre for Reviews and Dissemination/International Agency for Health Technology Assessment. Parallel search strategies were developed for the remaining databases. Search results were limited to human and English-language published between January 1999 and March 31, 2009. Search alerts were generated and reviewed for relevant literature up until May 31, 2009.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Summary of Findings
A 2000 health technology evidence report on the overall management of psoriasis by The National Institute Health Research (NIHR) Health Technology Assessment Program of the UK was identified in the MAS evidence-based review. The report included 109 RCT studies published between 1966 and June 1999 involving four major treatment approaches – 51 on phototherapy, 32 on oral retinoids, 18 on cyclosporin and five on fumarates.. The absence of RCTs on methotrexate was noted as original studies with this agent had been performed prior to 1966.
Of the 51 RCT studies involving phototherapy, 22 involved UVA, 21 involved UVB, five involved both UVA and UVB and three involved natural light as a source of UV. The RCT studies included comparisons of treatment schedules, ultraviolet source, addition of adjuvant therapies, and comparisons between phototherapy and topical treatment schedules. Because of heterogeneity, no synthesis or meta-analysis could be performed. Overall, the reviewers concluded that the efficacy of only five therapies could be supported from the RCT-based evidence review: photochemotherapy or phototherapy, cyclosporin, systemic retinoids, combination topical vitamin D3 analogues (calcipotriol) and corticosteroids in combination with phototherapy and fumarates. Although there was no RCT evidence supporting methotrexate, it’s efficacy for psoriasis is well known and it continues to be a treatment mainstay.
The conclusion of the NIHR evidence review was that both photochemotherapy and phototherapy were effective treatments for clearing psoriasis, although their comparative effectiveness was unknown. Despite the conclusions on efficacy, a number of issues were identified in the evidence review and several areas for future research were discussed to address these limitations. Trials focusing on comparative effectiveness, either between ultraviolet sources or between classes of treatment such as methotrexate versus phototherapy, were recommended to refine treatment algorithms. The need for better assessment of cost-effectiveness of therapies to consider systemic drug costs and costs of surveillance, as well as drug efficacy, were also noted. Overall, the authors concluded that phototherapy and photochemotherapy had important roles in psoriasis management and were standard therapeutic options for psoriasis offered in dermatology practices.
The MAS evidence-based review focusing on the RCT trial evidence for ultraviolet phototherapy management of moderate-to-severe plaque psoriasis was performed as an update to the NIHR 2000 systemic review on treatments for severe psoriasis. In this review, an additional 26 RCT reports examining phototherapy or photochemotherapy for psoriasis were identified. Among the studies were two RCTs comparing ultraviolet wavelength sources, five RCTs comparing different forms of phototherapy, four RCTs combining phototherapy with prior spa saline bathing, nine RCTs combining phototherapy with topical agents, two RCTs combining phototherapy with the systemic immunosuppressive agents methotrexate or alefacept, one RCT comparing phototherapy with an additional light source (the excimer laser), and one comparing a combination therapy with phototherapy and psychological intervention involving simultaneous audiotape sessions on mindfulness and stress reduction. Two trials also examined the effect of treatment setting on effectiveness of phototherapy, one on inpatient versus outpatient therapy and one on outpatient clinic versus home-based phototherapy.
Conclusions
The conclusions of the MAS evidence-based review are outlined in Table ES1. In summary, phototherapy provides good control of clinical symptoms in the short term for patients with moderate-to-severe plaque-type psoriasis that have failed or are unresponsive to management with topical agents. However, many of the evidence gaps identified in the NIHR 2000 evidence review on psoriasis management persisted. In particular, the lack of evidence on the comparative effectiveness and/or cost-effectiveness between the major treatment options for moderate-to-severe psoriasis remained. The evidence on effectiveness and safety of longer term strategies for disease management has also not been addressed. Evidence for the safety, effectiveness, or cost-effectiveness of phototherapy delivered in various settings is emerging but is limited. In addition, because all available treatments for psoriasis – a disease with a high prevalence, chronicity, and cost – are palliative rather than curative, strategies for disease control and improvements in self-efficacy employed in other chronic disease management strategies should be investigated.
Table ES1: RCT Evidence for Ultraviolet Phototherapy Treatment of Moderate-To-Severe Plaque Psoriasis.
| Conclusion | Evidence Level |
|---|---|
|
Moderate quality and adequate study evidence |
|
High quality but limited study evidence |
|
High quality and adequate study evidence |
|
High quality and adequate study evidence |
|
High quality and adequate study evidence |
|
High quality study but limited study evidence |
|
Inadequate study evidence |
|
High quality study but limited study evidence |
Ontario Health System Considerations
A 2006 survey of ultraviolet phototherapy services in Canada identified 26 phototherapy clinics in Ontario for a population of over 12 million. At that time, there were 177 dermatologists and 50 geographic regions in which 28% (14/50) provided phototherapy services. The majority of the phototherapy services were reported to be located in densely populated areas; relatively few patients living in rural communities had access to these services. The inconvenience of multiple weekly visits for optimal phototherapy treatment effects poses additional burdens to those with travel difficulties related to health, job, or family-related responsibilities.
Physician OHIP billing for phototherapy services totaled 117,216 billings in 2007, representing approximately 1,800 patients in the province treated in private clinics. The number of patients treated in hospitals is difficult to estimate as physician costs are not billed directly to OHIP in this setting. Instead, phototherapy units and services provided in hospitals are funded by hospitals’ global budgets. Some hospitals in the province, however, have divested their phototherapy services, so the number of phototherapy clinics and their total capacity is currently unknown.
Technological advances have enabled changes in phototherapy treatment regimens from lengthy hospital inpatient stays to outpatient clinic visits and, more recently, to an at-home basis. When combined with a telemedicine follow-up, home phototherapy may provide an alternative strategy for improved access to service and follow-up care, particularly for those with geographic or mobility barriers. Safety and effectiveness have, however, so far been evaluated for only one phototherapy home-based delivery model. Alternate care models and settings could potentially increase service options and access, but the broader consequences of the varying cost structures and incentives that either increase or decrease phototherapy services are unknown.
Economic Analyses
The focus of the current economic analysis was to characterize the costs associated with the provision of NB-UVB phototherapy for plaque-type, moderate-to-severe psoriasis in different clinical settings, including home therapy. A literature review was conducted and no cost-effectiveness (cost-utility) economic analyses were published in this area.
Hospital, Clinic, and Home Costs of Phototherapy
Costs for NB-UVB phototherapy were based on consultations with equipment manufacturers and dermatologists. Device costs applicable to the provision of NB-UVB phototherapy in hospitals, private clinics and at a patient’s home were estimated. These costs included capital costs of purchasing NB-UVB devices (amortized over 15-20 years), maintenance costs of replacing equipment bulbs, physician costs of phototherapy treatment in private clinics ($7.85 per phototherapy treatment), and medication and laboratory costs associated with treatment of moderate-to-severe psoriasis.
NB-UVB phototherapy services provided in a hospital setting were paid for by hospitals directly. Phototherapy services in private clinic and home settings were paid for by the clinic and patient, respectively, except for physician services covered by OHIP. Indirect funding was provided to hospitals as part of global budgeting and resource allocation. Home therapy services for NB-UVB phototherapy were not covered by the MOHLTC. Coverage for home-based phototherapy however, was in some cases provided by third party insurers.
Device costs for NB-UVB phototherapy were estimated for two types of phototherapy units: a “booth unit” consisting of 48 bulbs used in hospitals and clinics, and a “panel unit” consisting of 10 bulbs for home use. The device costs of the booth and panel units were estimated at approximately $18,600 and $2,900, respectively; simple amortization over 15 and 20 years implied yearly costs of approximately $2,500 and $150, respectively. Replacement cost for individual bulbs was about $120 resulting in total annual cost of maintenance of about $8,640 and $120 for booth and panel units, respectively.
Estimated Total Costs for Ontario
Average annual cost per patient for NB-UVB phototherapy provided in the hospital, private clinic or at home was estimated to be $292, $810 and $365 respectively. For comparison purposes, treatment of moderate-to-severe psoriasis with methotrexate and cyclosporin amounted to $712 and $3,407 annually per patient respectively; yearly costs for biological drugs were estimated to be $18,700 for alefacept and $20,300 for etanercept-based treatments.
Total annual costs of NB-UVB phototherapy were estimated by applying average costs to an estimated proportion of the population (age 18 or older) eligible for phototherapy treatment. The prevalence of psoriasis was estimated to be approximately 2% of the population, of which about 85% was of plaque-type psoriasis and approximately 20% to 30% was considered moderate-to-severe in disease severity. An estimate of 25% for moderate-to-severe psoriasis cases was used in the current economic analysis resulting in a range of 29,400 to 44,200 cases. Approximately 21% of these patients were estimated to be using NB-UVB phototherapy for treatment resulting in a number of cases in the range between 6,200 and 9,300 cases. The average (7,700) number of cases was used to calculate associated costs for Ontario by treatment setting.
Total annual costs were as follows: $2.3 million in a hospital setting, $6.3 million in a private clinic setting, and $2.8 million for home phototherapy. Costs for phototherapy services provided in private clinics were greater ($810 per patient annually; total of $6.3 million annually) and differed from the same services provided in the hospital setting only in terms of additional physician costs associated with phototherapy OHIP fees.
Keywords
Psoriasis, ultraviolet radiation, phototherapy, photochemotherapy, NB-UVB, BB-UVB PUVA
Background
Objective
The purpose of this evidence based analysis was to determine the effectiveness and safety of ultraviolet phototherapy for moderate-to-severe plaque psoriasis.
Clinical Need: Target Population and Condition
Incidence and Prevalence of Psoriasis
Psoriasis is a common chronic, systemic inflammatory disease affecting the skin, nails and occasionally the joints. (1) It has a worldwide occurrence with an estimated point prevalence varying from 0% to 11.8%. (2) Variation in reported prevalence has been attributed to psoriasis’ remitting relapsing course, its wide spectrum in clinical presentation, heterogeneous disease course, varying levels of severity among patients, and a lack of standardized classification criteria. (1) With a commonly quoted prevalence of 2%, psoriasis is one of the most common systemic inflammatory diseases. (3-6) Onset of the condition can occur at any age, but the estimated mean age of onset has been reported to be 33 years of age with 75% of cases occurring before age 46. (7) Several studies have suggested that the occurrence of psoriasis may be bimodal with those having an early onset experiencing distinct characteristics from those with late onset. (8-10)
Two population-based studies in the United States reported prevalence rates of 2.2% and 2.6% based on patient reports. (11;12) In another survey examining prevalence of psoriasis based on patient reports and physician examinations, the rate of undiagnosed active psoriasis was reported to be 2.28% (95% CI; 1.47 – 3.50) and was higher for men [1.8% (95% CI; 1.15 – 2.8)] than women [0.48% (95% CI; 0.22-1.03)]. (13) In terms of the extent of the condition, the majority (83.4%) of the psoriatic population was also reported to have little or no psoriasis (51.6%) or mild psoriasis (31.7%), with limited skin disease being defined as disease affecting less than 3% of the body surface area (BSA).. A minority (16.6%) of patients were reported to have moderate to severe psoriasis with 11.4% having moderate psoriasis (defined as 3% – 10% BSA) and 5.3% with severe psoriasis (BSA > 10%).
There have been three studies reporting on incidence of psoriasis, two in the United States (1;14) and one in United Kingdom. (15) A population-based retrospective study in Minnesota examined trends in incidence, characteristics, and survival of psoriasis patients aged 18 and older in Olmsted County in Minnesota between 1970 and 2000. (1) The overall annual age and sex-adjusted incidence rate of psoriasis between 1970 and 2000 was 78.9 per 100,000 (95% CI; 75.0 – 82.9) but, when restricted to dermatologist-confirmed cases, the overall incidence was reduced to 62.3 per 100,000 (95% CI; 58.8 – 65.8). The majority (79%) of subjects in the incidence cohort had chronic plaque psoriasis, followed less commonly by guttate psoriasis (8%) and sebo psoriasis (5.3%). The age-adjusted incidence rate was significantly (p = .003) higher in males (85.5 per 100,000) than in females (73.2 per 100,000). There was also a significant (p = .001) trend of linear increasing annual incidence rates over three decades from 50.5 per 100,00 (95% CI; 41.9 – 59.6) between 1970 to 1974 to 100.5 per 100,000 (95% CI; 90.8 – 110.2) between 1995 – 1999. The trend of increasing incidence remained when corrected to dermatology-confirmed cases with an incidence rate of 38.5 per 100,000 between 1970 – 1974 to 79.1 per 100,000 between 1995 – 1999. The reasons for the increase in incidence are unknown and may be attributable to true changes in risk factors or due to art factual causes including diagnostic changes or misdiagnosis.
Associations with other Conditions
It has been long recognized that psoriasis is more than a skin deep disease condition. (16;17) The condition has been reported to be associated with a range of systemic disorders including Chrohn’s disease, diabetes (particularly Type 2) and metabolic syndrome. (18-22) Psoriatic arthritis, for example, is an inflammatory seronegative arthropathy associated with psoriasis and has a wide range of joint involvement occurring with a variable and unpredictable clinical course. (23) Although it has features in common with psoriasis, it is considered a distinct entity. Approximately 10% of patients with psoriasis also have arthritis complicating management of the disease. (24) The prevalence of this condition has been estimated to be between 0.1% and 0.25% of the population. (25)
The association of psoriasis with cardiovascular disease is increasingly of interest. Cardiovascular risk factors have been found to be associated with psoriasis. A United Kingdom (UK) population-based study compared the prevalence of cardiovascular risk factors (i.e. diabetes, hypertension, hyperlipidemia, obesity or smoking) in patients with mild versus severe psoriasis (defined as having systemic therapy), and the general population. (26) Risk factors were significantly higher among both mild and severe psoriasis patients compared to the general population. Cardiovascular risk factors that are key components of the metabolic syndrome, i.e. hyperlipidemia, obesity were more strongly associated with severe psoriasis. Cardiovascular disease as measured by atherosclerosis has also been found to be higher among psoriasis patients. The prevalence and severity of coronary artery calcification (CAC), assessed by computed tomography was found to be more common [59.4% vs. 28.1%, (p =0.015)] and more severe [3.7 vs. 0.0 (p = 0.019)] by Agatston CAC score in psoriasis patients than in age and sex matched control patients. (27)
Cardiovascular outcomes in psoriasis patients have been evaluated in several studies. A UK population-based cohort study evaluated the occurrence of myocardial infarction (MI) in psoriasis patients compared to control patients. (28) The incidence of MI per 1000 person-years of follow-up for control patients and patients with mild and severe psoriasis (receiving systemic therapy) were: 3.58 (95% CI; 3.52 – 3.65), 4.04 (95% CI; 3.88 – 4.21), and 5.13 (95% CI; 4.22 – 6.17). The risk for MI was also found to be independently influenced by age and was greatest in younger patients with severe psoriasis. The relative risk for MI in a 30 year-old patient with severe psoriasis compared to control patients of same age was 3.10 (95% CI; 1.98 - 4.86). In a Swedish historical cohort study comparing psoriasis inpatients, outpatients, and the general population, an increased risk of cardiovascular death was found among outpatients but a 50% increase in mortality [SMR 1.52 (95% CI; 1.44 – 1.60)] was found in those with severe psoriasis and admitted to hospital at least once. Those admitted to hospital at a younger age (i.e. 20 to 39 years) were found to have the greater risk [SMR 2.62 (95% CI; 1.91 -3.49)]. (29)
Aetiology
Psoriasis is characterized by three main features: increased proliferation and incomplete differentiation of the epidermis, increased cutaneous blood flow, and leucocytic infiltration of the papillary dermis and epidermis. (2) The condition has a multifactorial disease aetiology resulting from complex interactions between predisposing genes and the environment. (2;17;30) Known environmental triggers for psoriasis are: infection (by streptococcus or human immunodeficiency virus), stress, drugs (including β – adrenergic receptor blockers, anti-malarial drugs, non-steroidal anti-inflammatory drugs, and lithium), withdrawal of glucosteroids, and alcohol. (24)
Genetic factors have a well known basis for psoriasis. Based on population studies, the risk of having the disease if both parents are affected is 41%, 14% if one parent is affected and 6% if one sibling is affected. (2) The mode of inheritance is complex with genome-wide linkage analysis identifying at least nine chromosomal loci with statistical significant linkages with psoriasis. (17)
Although it is clear that genes are important it is less clear how they influence the disease. Molecular studies indicate psoriasis is polygenic, although the molecular events that trigger the events are unknown. Genome analysis has also provided insights into disease-relevant cells and pathways. Genome signatures in psoriatic lesions suggest that dendritic cells and T cells are key cell types and type 1 interferons, interferon-γ, and TNF-α as key cytokines.
Several lines of evidence support that psoriasis results from an immune dysfunction including: the presence of increased number of immune cells (dendritic and T cells) in psoriatic lesions, the appearance of clonal T cells in psoriatic lesions over time, therapeutic activity of drugs targeting the immune system, possible curing of the disease in those undergoing bone marrow transplantation, transferral of disease from transplant donor to recipient, and the fact that the top hits in the whole-genome scans of genes and messenger RNA are immune-related. (17)
Disease Measurement
Three main measurement tools are used to evaluate the extent and severity of psoriasis and to monitor treatment progress. They include the Body Surface Involvement (BSI), the Physicians Global Assessment (PGA) and the Psoriasis Area and Severity Index (PASI). (31) The BSI rates the degree of psoriasis by the degree of body surface involvement. The ranges for this index have been classified as mild (< 5%), moderate (5-10%), or severe (>10%) degrees of psoriasis. (32) The proportion of body surface area involvement is approximated by assuming that the surface of the palm is approximately 1% of the body area. The PGA is a rating of the physicians overall subjective impression of disease severity. (32) The assessor rates the disease on a seven-point ordinal scale used to assess the global severity of disease over the body as a whole. The categories were defined as: clear (C), almost clear (AC), mild, mild to moderate, moderate, moderate to severe, and severe and for analysis were assigned scores of 0 to 6.
The PASI, first developed in 1978, is the most commonly used clinical measurement tool for psoriasis. (33) The index has been used by both physician raters (PASI) and by the patient or self raters (S-PASI). The score incorporates both the proportion of skin involved and the severity of signs such as erythema (redness), induration (thickness) and induration (scaliness). It divides the body into four quadrants (head, trunk, upper and lower extremities) and takes a weighted average representing the body surface area multiplied by a plaque severity score. The proportion of skin affected in each area (0-9%, 10-29%, 30-49%, 50-69%, 70-89%, 90–100%) is rated with a numerical score from 1 to 6. In addition, within each area the severity of each of 3 signs (erythema, induration/thickness, desquamation/scaling) is assessed on a 5-point scale: 0 (none), 1 (mild), 2 (moderate), 3 (severe), 4 (very severe). The total PASI score ranges from 0 to 72, although most psoriasis cases score under 15. Those with the most severe forms of psoriasis have PASI’s of 40 or less. The PASI is highly reproducible when administered by trained raters [Jacobson, Berth-Jones] and has a good correlation with the self-administered PASI (S-PASI). (32) The psoriasis disease state of moderate-to-severe has been defined by varying PASI levels (≥ 8-12), although the majority of reports classify moderate-to-severe disease by a PASI of ≥ 12. (33) A BSI score ≥ 10 is also commonly used as criteria for mild-to-moderate disease but usually used in conjunction with PASI scores.
Clinical improvement is measured by the percent change in PASI score. A PASI-75 represents the number of patients who experience a 75% improvement or reduction in the PASI score from their baseline after treatment. The FDA uses PASI-75 as a primary efficacy endpoint for new psoriasis drugs. (33) Some authors feel that PASI-75 under-represents clinical improvement and is too high a standard and that a PASI-50 should also represent significant clinical change in disease. (4;34) The argument for the lower threshold was also based on the observations that effective therapy can be differentiated from placebo at this point and that disability scores are also usually improved. (34)
Disease Impact and Quality of Life
Psoriasis disease severity can be variable and depend on the perspectives and interests of the assessor. A poor correlation between PASI and quality of life (QoL) in psoriasis patients suggests that both should be measured. (4) Clinicians managing these patients have reported a wide range of patient reactions to their condition. For example, those with small areas of involvement (5%) may experience large impacts on their quality of life if the plaques are visible, whereas some with large surface area involvement (50% or more) in less visible areas may not be as greatly affected. (4) Patients may well be unsatisfied with the aggressiveness of their treatment if their physician fails to consider the impact of psoriasis on their lives or those of their families, or the broader context of their social and work relationships.
The severity of psoriasis can also be evaluated from different perspectives. Some authors and societies have suggested that the impact on quality of life is a more adequate definition of disease severity than by measures of physical disease severity. (35) Perspectives “from the patient’s point of view” are that psoriasis should be considered ‘severe’ if it causes embarrassment or anxiety, pruritis or soreness, if it affects relationships, everyday activity, or if there is joint involvement. The Medical Advisory Board of the National Psoriasis Foundation formally recommended the following QOLbased classification scheme for psoriasis severity (36):
Mild: disease that does not alter quality of life
Moderate: disease that alters quality of life; therapies would be expected to improve QOL with minimal risk of side effects
Severe: disease that alters QOL; response to treatments that have minimal side effects have been ineffective and patients are willing to accept life altering side effects to achieve a better QOL
From the employer’s point of view, psoriasis is severe if it impacts on an employees’ ability to work, causes time off, or has an adverse impact on other employees or customers. From the dermatologists point of view, the disease is considered severe if it is widespread, if the patient is concerned, or if the patient is erythrodermic or widely pustular. Severity is also suggested by poor response to therapy, especially over the long term or if it requires impatient or intensive treatment.
Disease Management
Management of psoriasis is dependent on the extent and location of skin involvement, with disease presence on the hands, feet, face or genitalia presenting particular challenges. (37;37) Treatment plans are based on a long-term approach to disease management, patient’s expectations, individual responses, and risk or complication events. The treatment goals are several fold but primarly to :
1) improve physical signs and secondary psychological effects,
2) reduce inflammation and control skin shedding
3) control physical signs as long as possible, and to
4) avoid factors that can aggravate the condition.
Approaches are also generally individualized because of variability in presentation, quality of life implications, coexistent psoriatic arthritis, comorbid medical conditions, and triggering factors such as stress, infections and medications.
There are a range of therapeutic options available for psoriasis, which are usually considered in a staged manner with increasing disease severity matched to treatments with increasing invasiveness and risk. A treatment pathway for patients presenting with psoriasis is outlined in Figure 1.
Figure 1: Treatment Pathways For Psoriasis.
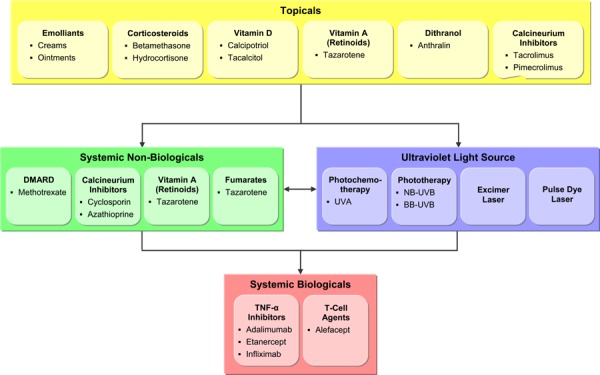
Initially patients can be treated using a range of topical agents. For those not responding or becoming refractory to topical agents, phototherapy or a range of oral systemic agents are considered. Traditional systemic immunosuppressive agents such as methotrexate and cyclosporin, however have a wide range of known side effects including hair loss, liver toxicity, hypertension, renal impairment, and bone marrow suppression and require extensive monitoring and surveillance. (5;37;38) Phototherapy, particularly chemophototherapy (PUVA), also has risks, in particular, photo aging of the skin and an increased risk of skin cancer associated with long term cumulative exposure. (39-41)
An increased understanding of the pathogenesis of psoriasis has led to the development and approval of more systemic agents by the US Food and Drug Administration (FDA) in the last 5 years than in the last 50. (42) Modern immunosuppressive injectable agents known as biologicals have been designed to selectively target the immune deficiencies of psoriasis, particularly the activation of T cells and inflammatory cytokines. (43-45) There’s also a range of current FDA and Health Canada approved biological agents (monoclonal antibodies and fusion proteins) developed to selectively target key cytokines and receptor molecules on T-cells and antigen-presenting cells involved in the immune process. (6) Aalefacept was the first biological approved for psoriasis and is one of two biological agents (i.e. efalizumab and alefacept) designed to modulate T-cell function. In addition to their short term effectiveness in controlling psoriasis (43;45) these agents have risks involving potential cancers and fatal infections.
Because of the risks associated with cumulative exposures to systemic agents or phototherapy, a variety of treatment approaches have evolved designed to control symptoms or limit the life-long disease process, while minimizing varied treatment risks. The strategies involve combination, sequential or rotational treatment approaches. (38) Combination therapy involves more than one agent indented to enhance each others’ effects and referred to as synergistic effect. Rotational therapies involve administering one therapy for a limited time and then switching to another therapy with the intent to minimize the extended or prolonged period of exposure to any one agent. Sequential treatment approaches are more complex and intended to manage flare ups that may occur with any sudden cessation of therapy. An example of this strategy is the initial quick clearance of psoriasis with cyclosporin followed by a transitional phase consisting of the gradual addition of acitretin and cyclosporin. The final phase involves tapering off cyclosporin and continuing with a maintenance dose of acitretin.
Patient Satisfaction
Despite the various therapeutic options for psoriasis, surveys of patient satisfaction in several countries have indicated a high level of patient dissatisfaction with their treatments. (35;46;47) Two US surveys (11;35) conducted by the National Psoriasis Foundation (NSF) in 1998 and 2002 reported high levels of patient dissatisfaction. In the 1998 mail and telephone survey (35), nearly 18,000 respondents from the membership (40,350) were surveyed with 500 interviewed in depth by telephone. Among those interviewed by telephone:
49% reported that that they were only ‘somewhat’ or ‘not at all’ satisfied with their therapy.
Among those with severe psoriasis, 78% reported feeling frustrated that their treatment did not work well enough and did not make their disease more manageable.
More than half of the patients felt that their physicians could be more helpful in assisting them to live with psoriasis.
Half of the patients with severe psoriasis felt that their treatment wasn’t aggressive enough.
Patients did report being told of effective therapies by their physicians. Phototherapy with UVB (65%) and methotrexate (50%) were the most commonly mentioned. Among those with severe psoriasis, only 27% had ever tried methotrexate and only 23% had tried PUVA. The majority of these patients reported good to excellent treatment response - 70% for methotrexate and 64% for PUVA.
A second benchmark population-based survey on psoriasis of 27,220 patients, aged 18 or older, was conducted by the NPF in 2002 to estimate disease prevalence, impact, and treatment satisfaction. (11) The study found that:
Overall, 25% of the respondents reported being ‘dissatisfied’ and 27% reported being ‘only somewhat satisfied’ with their treatment.
Those with severe psoriasis (>10% BSI) were more likely than those with less severe cases to report that their condition was a large problem [OR, 14.50 (95% CI; 5.00, 42.04)] and more likely to indicate dissatisfaction [(OR, 3.14 (95% CI; 1.20,8.23)] with therapy.
In another study, a combined group of 1,197 psoriasis patients from the general population and from among NPF members were surveyed to assess their satisfaction with four systemic treatments widely used in 2002 for psoriasis. (48) The results on treatment utilization were as follows:
A minority of the respondents (26%; 311) reported using at least one of the four systemic therapies - methotrexate, cyclosporin, PUVA and/or acitretin.
Those with severe psoriasis (≥10%BSI), however, were more likely [OR, 2.90 (95% CI; 1.87, 4.49)] to report use of systemic treatments than patients with less severe (< 3% BSI) disease.
73% (227/311) indicated that they had used only one of the treatments and 4.5% (14/311) indicated that they had used all four treatments.
For those with severe psoriasis, the most common treatments used were methotrexate (46%), PUVA (44%,), cyclosporin (31%;) and acitretin (21%).
Cyclosporin and acitretin users were more likely to have used the other systemic therapies PUVA and methotrexate.
The results on patient satisfaction with systemic therapies were:
Of the patients using systemic therapies, 37.6% were dissatisfied and 36.3% were very satisfied with their therapy.
The dissatisfied with individual treatments were: cyclosporin [46% (95% CI; 36%, 56%)], methotrexate [36%, (95% CI; 28%, 44%)], and acitretin [36% (95% CI; 25%, 49%)], and PUVA [14% (95% CI; 9%, 20%)].
Except for PUVA, 35% of patients who used a therapy were dissatisfied with it.
Patients who used PUVA were significantly (p < .05) less likely to be dissatisfied with it compared to those using other treatments.
Only a few patients reported using the new biological agents and the majority in both groups were dissatisfied with these therapies – 4/7 using inflixamab and 8/14 using etanercept. Although the sample was small, dissatisfaction was an issue for some of these patients.
A postal survey conducted by the European Federation of Psoriasis Patient Organizations (EUROPSO) with 18,386 respondents reported that 40% of psoriasis patients were currently using a therapy. (5;46) Of the treatments, 4% reported using a topical agent, 20% a systemic therapy, and 13% used phototherapy. Only 59% of patients with severe psoriasis were currently using prescription medication. Overall, 72% reported low or only moderate satisfaction with their psoriasis treatment. The disadvantages cited by patients with their treatments were that it was time consuming (50%), ineffective (32%), costly (30%), or associated with unpleasant adverse effects (23%).
The findings of a Canadian survey examining awareness and satisfaction of treatment among 514 patients with moderate-to-severe psoriasis were similar to those found in other countries. (47) Overall, only 24% of the respondents indicated that they were ‘very satisfied’ with their current medication. Dissatisfaction was high for both current and past treatment efficacies, 68% reported that no medication worked well for their psoriasis. Respondents most commonly cited a lack of efficacy (60%) and inconvenience (23%) as reasons for discontinuation of their anti-psoriatic treatments. Other reasons cited for discontinuing treatments included symptoms improved (22%), concern over side effects (20%), cost (14%), and doctor’s advice (14%). Although patients had lived with psoriasis on average over 21 years, 64% reported desiring more information about psoriasis.
Ultraviolet Phototherapy
Regulatory Status
Ultraviolet phototherapy units have been licensed since February 1993 for use as a class 2 device in Canada. Currently, units are licensed and available from at least four different manufacturers as hand held devices, hand and foot devices, full body panels, and in booth styles. The full body units use 6-foot long bulbs and are rated at 100 watts. The booth type units are for institutional use and commonly employ 44 or 48 bulbs, whereas the smaller panel units are designed for home use and employ between 4 and 10 bulbs. Units are available with ultraviolet A (UVA), broad band ultraviolet B (BB-UVB), and narrow band ultraviolet B (NB-UVB) lamps.
Ultraviolet Radiation
Exposure to UV irradiation occurs naturally in sunlight and spans the wavelengths from 200 to 400 nm and within that range are UVA (320 – 400 nm), UVB (280 – 320 nm) and UVC (200 – 280 nm). (49) Of these UVA and UVB reach the earth’s surface and have biological consequences to the skin and eyes. UVA is the main component of solar radiation and penetrates more deeply into the skin than UVB but is less biologically active. UVB wavelengths in the solar spectrum are more biologically active than UVA and, when absorbed into the skin, produces erythema, burns, and potentially skin cancer. (49) Both sources of UV can have phototoxic effects on the skin including aging and wrinkling.
Mode of Action
UVA and UVB are the therapeutic classes of ultraviolet therapy. (49). UVB phototherapy uses bulbs that emit ultraviolet in several action spectra, notably UVB broad band in the 290 – 315 nm spectra, UVB selective in the 300 – 312 nm spectra, and UVB narrow band in the 311 – 312 nm spectra. Broad band UVA is in the 320 – 400 nm spectral range and can be further divided into UVA1 (340 – 400 nm) and UVA2 (315 – 340 nm).
The objective of the treatment is to maximize the effects of phototherapy on diseased tissue and to minimize burning effects to normal tissue. When initiating treatment, it’s necessary to evaluate skin types as the required dose and dosing increments are related to skin type with higher doses being less tolerated by fair skin. (50) The standard procedure therefore is to initially obtain the minimal erythema dose (MED) to remain effective and limit the side effects of erythema and burning (the procedure takes about 15 to 20 minutes). Treatment is then started with an initial dose at 50% to 70% of the MED limit. Once the appropriate dose is determined, treatment regimens can range from 3- to 5-times weekly, during which doses of successive treatments are increased by at least 10% of the MED.
The therapeutic effects of UV light on the skin occur in several stages. (50) Initially, there is a phase of rapid change involving cell membrane and DNA damage, induction of cytoplasmic transcription factors, and isomerization of uric acid. The next phase involves subacute changes including alteration of the antigen presenting cell populations and the modification of intra- and intercellular signaling mechanisms.
The overall skin effects of UV are to create a change in the environment in the cytokine patterns of dermis and epidermis which is more favourable to the development of T-cell (Th-2) helper response. The action of UVA differs from UVB. UVA by itself is not biologically active, so a photosensitizing compound (usually psoralen) is taken, either orally or topically, prior to UVA exposure, and is referred to as PUVA treatment. This process of adding a photosentizing agent prior to UV exposure is referred to as photochemotherapy. UVA can then produce both oxygen-dependent and oxygen-independent photochemical reactions. The oxygen independent reactions occur as the formation of DNA crosslinks and development of cyclobutane rings. The DNA cross linking is a strong bond (covalent bond) that remains as a defect in the DNA. Increased numbers of DNA cross links in the epidermis are an important consideration as they are a predisposition to squamous cell carcinoma with long term treatment. The oxygen dependent reactions generate reactive oxygen species that result in membrane damage at the cell and mitochondrial level. Lymphocytes appear to be more susceptible than keratinocyytes to the effects of PUVA and the depletion of CD3 lymphocytes in the epidermis has been found to correlate well with the clinical response to PUVA. (51)
Ultraviolet Treatment History
The application of ultraviolet light for medical treatments first received recognition in 1903 when Nies Finsen received a Nobel prize for the medical application of phototherapy. (50) The first major use of ultraviolet phototherapy for the treatment of psoriasis was introduced by William Goeckerman in 1925 at the Mayo clinic. (52;53) The treatment referred to as the Goeckerrman therapy consisted of hospital admission for several weeks during which crude coal tar was applied to the entire body for several hours and then exposed to hot quartz mercury vapour lamps. In the 1950’s, Ingram introduced anthralin paste as a substitute to the crude coal tar. (54) In the 1970’s, Levine et al. discovered that lubricating base was as effective as coal tar and that outpatient treatments 3-times weekly were as effective as inpatient treatments. (54) In 1976, Fischer et al. (55) reported on the therapeutic action spectra of ultraviolet radiation for treatment of plaque psoriasis. In 1981, Parrish et al. (56) expanded the investigations on treatment with ultraviolet wavelengths and included broader ranges of UVA, UVB and UVC. A notable finding from this study was that UV doses below 300 nm produced significant clearing but also produced the most erythema and burning.
UVA was first developed therapeutically for psoriasis treatment in 1970. (24) Exposure to UVA after the ingestion of a photosensitizing chemical (psoralens) known as PUVA was found to result in therapeutic action. Psoralen were successfully isolated in 1948 and first used prior to UVA topically in 1973 and orally in 1974 for psoriasis treatment. (24) The most common psoralen used in North America is 8-methoxypsoralen (8-MOP) and in Europe it is 5-MOP. (50)
In 1984 the Phillips TL-01 lamp which emitted UV light in a very narrow range (311 ± 2 nm) was sent to dermatology centers in Europe for clinical testing. (57) This spectral range had been found to provide the optimal balance between therapeutic response and limiting erythermogenic or skin burning. These NB-UVB lamps only became available in the US in 1998. (50) The more focused TL-01 bulb also allowed for the development of home phototherapy units; self treatment with phototherapy has since been on the rise across Europe and North America. (58)
Potential Advantages, Risks, and Limitations
Generally, phototherapy or photochemotherapy is indicated for patients with moderate-to-severe psoriasis who have failed or are unresponsive to topical therapy. (6) A major disadvantage for both therapies are that treatments are time consuming as many successive treatments are required and treatment may necessitate extensive travel to a phototherapy clinic.
There are a range of contraindications for UVB phototherapy and for PUVA. (5;6;59) Both treatments have contraindications including any history of light sensitivity disorders (i.e. lupus erythematosus, porphyria, cutanea tarda, xeroderma pigmentosum etc.), melanoma, squamous cell carcinoma, aphakia, and/or basal cell carcinoma. (6) The safety for PUVA has also not been established in pregnancy, nursing mothers, or children. There are also contraindications for patients with significant hepatic impairment and for those taking warfarin or phenytoin.
Short Term Adverse Effects
Adverse short term side effects are possible with both UVB and UVB including: erythema, swelling, blisters, generalized pruritus, tingling, fever, general malaise and skin pain. (60) In addition, there are risks and disadvantages unique to PUVA photochemotherapy. The need for prior ingestion of psoralen adds complexity to the treatment schedule. The results of photochemotherapy can also be variable due to the variable serum levels of the psoralen molecule necessitating a consistent approach to ingestion methods including accompanying foods and liquids. The use of eye protection for 24 hours after the procedure is also mandatory. Significant gastrointestinal side effects (nausea and vomiting) are also often associated with oral psoralens and may result in discontinuation of the therapy. These complications, however, can be avoided by using bath or cream based PUVA therapies.
A study by Pearce et al. (61) evaluating treatment history and adverse events occurring in a cohort of psoriasis patients managed at a large academic practice compared the risks of phototherapy with other topical and systemic antipsoriatic treatments. The retrospective review was conducted through electronic records and all clinic visits for 753 patients over an 8 year study period (mean minimum follow-up was 3 years). The average age of the patients was 55 years, 42% were male, 88% were white and 75% had a comorbidity. The mean duration of psoriasis was 6 years and 51% of the patients had plaque psoriasis and 12% had psoriatic arthritis.
Approximately 60% of patients receiving a systemic therapy experienced an adverse event, 40% of which were classified as significant. For the individual therapies, the rates of significant adverse events were: 63% for cyclosporin (10 patients), 34% for methotrexate (61 patients), 33% for oral retinoids (43 patients), 13% for hydroxyurea (1 patient), and 5% for PUVA (2 patients). UVB was used as the reference group and exhibited a significant adverse event rate of 4%, including five cases of skin cancer and one ulcer in 168 treated patients. The odds ratio for significant adverse events occurring in different treatment groups compared to UVB were: 20.3 (95% CI; 4.3, 96.6) for cyclosporin, 11.4 for methotrexate (95% CI; 2.9, 45.0), 6.6 for acitretin (95% CI; 1.6, 26.9), 6.4 for etretinate (95% CI; 1.3, 32.3), and 1.5 for PUVA (95% CI; 0.28,7.5).
Martin et al. (62) conducted a three-center review of acute adverse events occurring over a 1-year period in a cohort of 8,784 patients, largely with psoriasis treated with NB-UVB or oral, bath, or hand/foot PUVA. A total of 8,784 treatments involving 70 treatment related acute adverse events (four rated as severe) were recorded in the 1-year audit survey period for an overall adverse event rate of 0.8%. Adverse events had been categorized as: non-treatment related (n=3), equipment related, operator error (n=2), patient non-compliance with standard operating procedures (n=15), or treatment related (i.e., photo-toxic/allergic, pruritis, light eruption, reactivation of viral infections, disease flares, and symptoms related to systemic psoralen). Episodes of marked painful erythema with edema and/or blistering were classed as severe adverse events. NB-UVB had the lowest adverse event rate of 0.6% (37/5974) with 0.05% (3/5974) rated as severe. Adverse rates were 1.3% (22/1675) for bath-PUVA with none rated as severe and 1.3% (4/299) for oral-PUVA with 0.3% (1/299) rated as severe.
Cancer Risk with Ultraviolet Irradiation
Many of the systemic therapies for moderate-to-severe psoriasis, including phototherapy, traditional immunosuppressants such as methotrexare and cysclosporin, as well as the new biological agents may increase the risk of malignancy. (63) The main risk associated with ultraviolet therapy is the risk of skin cancer with long term use. Concerns for increased skin cancer risk with cumulative ultraviolet exposure are for both UVA and UVB irradiation although they have been better evaluated for PUVA.
The increased risks of skin cancer with exposure to PUVA was first documented in a prospective cohort study of 1,380 psoriasis patients treated at 16 centers in the United States. (64-66) The study, known as the PUVA Follow-Up study was conducted to determine the long term risks and benefits of PUVA treatment. The majority (69%) were followed for at least 5 years and the overall incidence rate of squamous cell carcinoma (SCC) in the prospective cohort was 20 per 1,000 person-years of follow-up and a strong dose response effect was noted. The dose response was compared as low dose defined as <100 treatments or 1,000 J/cm2 compared to high dose defined as >200 treatments or 2,000 J/cm2. Based on those dose levels, the risk that SCC would develop at least 22 months after the first exposure to PUVA was 12.8 times greater (95% CI; 5.8 – 28.5) in patients exposed to a high dose than those exposed to a low dose. (64) Increased risks were not seen for basal cell carcinoma (BCC).
A dose response relationship was also found in a review analyses of eight published studies examining skin cancer risk with PUVA exposure. All studies reported a higher incidence of SCC in high versus low PUVA exposures. (39) The pooled SCC incidence rate ratio (high versus low dose) for the studies was 14.0 (95% CI; 8.3 – 24.1) and was higher than the 5.9 (95% CI; 4.0 – 8.7) incidence rate ratio for the PUVA follow-up study.
In contrast to the American PUVA prospective trial, an increased risk of SCC with exposure to PUVA was not found in a European follow-up study of 1,643 patients treated with PUVA at 18 European centers from 11 countries. (67) In this study however, almost half of the cohort was unavailable at 10-year follow-up. Although the study did report an occurrence of skin cancers in PUVA treated patients with prior exposure to carcinogens, a dose relationship between PUVA exposure and risk of skin cancer was not shown.
It was found that all patients with cancers had been exposed to other carcinogens prior to treatment with PUVA including methotrexate, arsenic, ionizing radiation (x-rays), UVB, solar irradiation and coal tar. However, only patients with a history of treatment with arsenic prior to PUVA exhibited a significantly higher risk of skin cancer than those without prior arsenic treatment: 11.9% (25/211) versus 0.7% (6/917). Also, all patients having SCC in the low dose PUVA exposure category had a history of exposure to x-rays, methotrexate, or arsenic although increased risk was not shown with patients having prior history of these exposures. In addition, patients with a history of risk factors developed SCC at the same rate irrespective of total PUVA dose. Incidence rates in those receiving >3,000 J/cm2 over 105.5 months was approximately equal to the rate in those exposed to <1,000 J/cm2 over 75 months. The finding that basal cell carcinomas mainly occurred in the low PUVA dose exposure group was consistent with observations made in the American PUVA longitudinal cohort study.
Skin Cancer Risk with UVB Exposure
The risk of skin cancer with UVB treatment for psoriasis was evaluated in two separate reviews. (40;41). The Pasker et al. (41) report was a review of published studies evaluating skin cancers among psoriasis patients attributable to UVB exposure. Skin cancer risk was assessed as a function of the total dose specific for time since exposure, age at first treatment and other antipsoriatic treatments. Although four studies (65;68-70) were identified that allowed calculation of excess incidence, the variables of treatment dose and time since first exposure could not be assessed as they were insufficiently reported in all studies.
Only one of the studies (68) evaluated the incidence of skin cancers in psoriasis patients treated with UVB alone and compared them to expected rates from the general population. In that study, 85 Swedish patients treated with UVB alone were followed for up to 25 years (average 16.2 years). The prevalence of premalignant/malignant skin lesions was found to be lower among the psoriasis patients than their age, sex, and address-matched population controls.
The Pittelkow et al. study (69) compared SCC incidence rates in 260 psoriasis patients treated with UVB and tar at the Mayo Clinic and followed them for 25 years. Patients with UVB and tar exposure did not exhibit more SCC cancers (19 cases) than that expected (26.6 cases) number of cases based on general population rates for the same geographic region.
Stern et al. (65) reported a nested case control design of patients in the PUVA follow-up study. Among 1,373 psoriasis patients, 75 cases of skin cancers occurred. A history of UVB exposure was compared in patients with skin cancer compared to those who did not develop skin cancer. High exposure to coal tar or UVB was defined as exposures to over 300 treatments with UVB and/or >90 months treatment with coal tar compared to low exposure or <300 treatments with UVB and <90 months treatment with coal tar. The crude (unmatched) odds ratio for skin cancer risk with UVB exposure was 2.4 (95% CI 1.4 – 4.2). After a matched analyses, controlling for age, sex, skin type, address, ionizing radiation and PUVA, the OR of SCC increased to 4.7 (95% CI 2.2-10.0).
Stern et al. (70) also examined the risk for invasive squamous cell carcinomas of the genitals in men in the PUVA follow-up study. The PUVA cohort was grouped according to men with (>300 treatments with UVB and/or >90 months coal tar) and without UVB and coal tar exposure estimated by patients. Men with psoriasis and UVB exposure and a similar PUVA exposure, were 4.6 times more likely than men without UVB exposure to have invasive SCC of the genitals.
The Lee et al. review (40) examining skin cancer risk with UVB exposure identified 11 studies examining UVB exposure in which six involved UVB as the primary treatment modality for psoriasis. Only the Finnish cohort study (71) was an additional study to the earlier review by Pasker et al. (41) on UVB exposure and risk of skin cancer. Treatment and cancer was examined in 5,687 Finnish patients. A nested case control analysis was performed with 30 SCC cases compared to 137 age and sex matched controls for UVB exposure. A history of UVB exposure was found in 21 (70%) of the cases and 63 controls (46%), yielding an OR of 1.6 (95% CI; 0.4, 6.4) for SCC with UVB treatment. A history of Goeckerman therapy (UVB and coal tar) was found in 12 (43%) cases and 33 (24%) controls, yielding an OR of 1.5 (95% CI; 0.3-7.3). Neither risk estimate for SCC was statistically significant.
In general, the conclusions in the Lee review on skin cancer risk with UVB exposure were similar to those in the earlier review by Pasker. The major difficulty in assessing the risk of skin cancer from UVB exposure is that UVB was rarely the only treatment for psoriasis and the other treatments involved exposures to other potential carcinogens. Also, the reporting of exposures was often inadequate to evaluate dose-response relationships with exposure. Despite these limitations, the reviewers concluded that there was adequate evidence to support an increased risk of skin cancer with UVB exposure. The PUVA follow-up study was the only one showing an increased risk of genital cancer. For that reason, and to avoid unnecessary tissue exposure, the practice of genital shielding during phototherapy continues to be recommended.
The main limitation in the previous studies is the lack of information on cancer risk with exposure to NB-UVB, which is being increasingly used as the ultraviolet source of choice for phototherapeutic treatment of psoriasis. (58) Recently, a few studies (72-74) have reported on the risk of skin cancer with exposure to NB-UVB. Although these studies have not identified significant risk with exposure to NB-UVB, the studies are small and/or present preliminary findings in the cohort follow-up. The adequate evaluation of the carcinogenic potential of UVB phototherapy is likely to require large, multi-center, registry-based cohort studies with a follow-up of 10 ten years or more. (75)
Evidence-Based Analysis of Safety and Effectiveness
Objective of Evidence Based Analysis
The purpose of this evidence review was to determine the effectiveness and safety of ultraviolet phototherapy for moderate-to-severe plaque psoriasis. The specific research questions for the evidence review were:
What is the safety profile of phototherapy for use in the treatment of moderate-to-severe plaque psoriasis?
How effective is phototherapy for the treatment of moderate-to-severe plaque psoriasis?
Methods
Literature Search
The literature search strategy employed keywords and subject headings to capture the concepts of 1) phototherapy and 2) psoriasis. The search was run on March 31, 2009 in the following databases: Ovid MEDLINE (1996 to March Week 3 2009), OVID MEDLINE In-Process and Other Non-Indexed Citations, EMBASE (1980 to 2009 Week 13), the Wiley Cochrane Library, and the Centre for Reviews and Dissemination/International Agency for Health Technology Assessment. Search alerts were generated and reviewed for relevant literature up until May 31, 2009. The literature search strategies for MEDLINE and EMBASE are reproduced in Appendix 1. Parallel search strategies were developed for the remaining databases. Search results were limited to human and English-language published between January 1999 and March 31, 2009. The resulting citations were downloaded into Reference Manager, v.11 ((ISI Researchsoft, Thomson Scientific, U.S.A)), and duplicates were removed. The bibliographies of all relevant articles were scanned. The literature search strategies for MEDLINE and EMBASE are reproduced in Appendix 1.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
The citations from different databases were merged into one database using Reference Manager software and duplicates were subsequently removed. In total, 146 citations were identified. The citation lists were reviewed, and articles were excluded based on title and abstract. Excluded articles included those discovered to be review articles or commentaries, dosing studies, halfbody comparisons, other skin conditions (egg acne vulgaris, palmoplantar psoriasis, guttae or psoriatic arthritis, atopic dermatitis). Copies of original articles of eligible articles were obtained and reference lists were further hand searched.
Additional Information Sources
Consultations held with several clinical experts and industry representatives.
Assessment of Quality of Evidence
An evaluation of the quality of evidence was based on the grading of recommendations assessment, development, and evaluation (GRADE) system. The recommendations of the GRADE working group can be viewed at http://www.gradeworkinggroup.org. Accordingly, the quality of the evidence was assessed as either high, moderate, low, or very low according to the GRADE method. The potential level of impact of further evidence on decision making was also rated according to the following GRADE definitions:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain |
Results of Evidence-Based Analysis
Previous Systematic Reviews
The MAS evidence-based review identified one previous health technology assessment report by The National Institute Health Research (NIHR) Health Technology Assessment Program in the UK, which examined the overall management of psoriasis. (24) That review identified 195 RCT studies on psoriasis management published between 1966 and June 1999. Of these, 109 fit the eligibility criteria and were included in the review. The trials involved four major treatment approaches: 51 RCTs on phototherapy, 32 RCTs on oral retinoids, 18 RCTs on cyclosporin and five RCTs on fumarates. The authors raised the issue of an absence of RCTs on methotrexate and noted that the original studies with this agent had been performed prior to 1966.
Of the 51 RCT studies involving phototherapy, 22 involved UVA, 21 involved UVB, five involved both UVA and UVB, and three involved natural light as a source of UV. The RCT studies were also divided into six different groups based on the intent of the study’s research objective, which included comparisons of treatment schedules, ultraviolet sources, addition of adjuvant therapies, and comparisons between phototherapy and topical treatment schedules. Because of the heterogeneity of the studies, no synthesis or meta-analysis of the study groups were performed.
The reviewers concluded that the efficacy of only five therapies could be supported from the RCT-based evidence review: photochemotherapy or phototherapy, cyclosporin, systemic retinoids, combination topical vitamin D3 analogues (calcipotriol), and corticosteroids in combination with phototherapy and fumarates. Although there was no RCT evidence to support methotrexate, it’s efficacy for psoriasis was reported to be well known and continues to be a mainstay in the treatment of psoriasis.
The overall conclusion of the evidence review was that photochemotherapy and phototherapy were both effective treatments for clearing psoriasis, although their comparative effectiveness was unknown. Trade offs between the therapies were noted in that although phototherapy involving UVB may have offered a treatment that was less efficacious than photochemotherapy, it appeared to involve a lower radiation dose and lower cumulative lifelong exposure thought to represent a lower skin cancer risk. The combination of topical agents such as retinoids or vitamin D3 analogues appeared to lower the cumulative dose of ultraviolet (both UVA and UVB) needed to achieve treatment success. The use of topical agents in combination with phototherapy was also thought to more closely represent “real-life” clinical practice than the restrictive monotherapy approach used to evaluate efficacy in clinical trials. In summary the authors concluded that both phototherapy and photochemotherapy had important roles in psoriasis management and were standard therapeutic options offered in dermatology practices.
Despite the conclusions on efficacy of phototherapy for psoriasis, a number of issues were identified in the evidence review. The outcome measures reported in the trials were highly variable and often presented in forms that would not allow cross-study comparisons. The definition and measurement of “severe” psoriasis was not uniform and the clinical measures of severity commonly employed were generally considered to be inadequate, frequently represent an underestimate of disease severity. Many of the trials also appeared to be under-powered and lacking relevant treatment or comparator arms. In addition, given that interventions are palliative for a lifelong chronic disease condition, there were concerns over phototherapy and other systemic treatments relating to side effects or complications attributable to long term use. A particular short coming in these studies, was thus the short, if not absent, follow-up surveillance. The reporting of side-effects, even in the short term periods of the studies, was also noted to be generally poor.
Several major limitations and areas for further investigation in psoriasis management were discussed by the reviewers including:
A better understanding of patients’ views was needed, particularly their tolerability of higher treatment risks to gain improved quality of life, an important consideration in treatment selection.
An increase in pragmatic trials involing combination therapies that better relate to usual clinical practice and that are conducted with sufficient follow up to enable improved estimates of remission, side effects, and complications was particularly recommended.
An increase in trials that focus on comparative effectiveness, either between ultraviolet sources or between classes of treatment, such as methotrexate versus phototherapy was recommended.
A better assessment of the cost-effectiveness of therapies that also take into account systemic drug costs and costs of surveillance, as well as drug efficacy.
Medical Advisory Secretariat Systematic Evidence Review
The MAS evidence review of RCT trial evidence for phototherapy or photochemotherapy for moderate-to-severe plaque psoriasis was performed as an update of the 2000 health technology review on treatments for severe psoriasis by Griffeths et al. (24) The MAS review identified an additional 26 reports involving phototherapy or photochemotherapy for psoriasis management in randomized control studies (the level of evidence for these studies is summarized in Table 1). Seven of the RCT studies were categorized as small (n < 50 patients), eight RCT were moderate in size (n = 50-100), and 11 studies were considered large (n >100). The majority (65%; 17/26) of the reports involved single site studies. The studies were conducted mainly in European countries, specifically in: Germany (n = 5), United Kingdom (n = 5), Netherlands (n = 3), Spain (n = 2), France (n = 1), and Greece (n = 2). Three reports were from North America with two in the US and one in Canada. The remainder were from Bulgaria (n = 1), China (n = 1), Thailand (n = 1), Pakistan (n = 1) and South Korea (n = 1).
Table 1. Level of Evidence Summary for Studies Included in MAS Review.
| Study Design | Level of Evidence* |
Number of Eligible Studies |
|---|---|---|
| Large RCT, systematic review of RCTs | 1 | 19 |
| Large RCT unpublished but reported to an international scientific meeting | 1(g) | 0 |
| Small RCT | 2 | 7 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | 0 |
| Non-RCT with contemporaneous controls | 3a | 0 |
| Non-RCT with historical controls | 3b | 0 |
| Non-RCT presented at international conference | 3(g) | 0 |
| Surveillance (database or register) | 4a | 0 |
| Case series (multisite) | 4b | 0 |
| Case series (single site) | 4c | 0 |
| Retrospective review, modelling | 4d | 0 |
| Case series presented at international conference | 4(g) | 0 |
| Total | 26 |
RCT refers to randomized controlled trial; g, grey literature designation given to preliminary reports presented at international scientific meetings.
For each included study, levels of evidence were assigned according to a ranking system based on a hierarchy proposed by Goodman. (87)
The reports are summarized in Table 2 and grouped according to varying treatment objectives of the trials. Overall, there were six different general treatment comparisons. Two RCTs compared ultraviolet wavelength sources, five RCT compared different forms of phototherapy, four RCTs compared phototherapy monotherapy with phototherapy and balneotherapy (i.e. prior spa saline bathing), nine RCTs combined phototherapy with topical agents, and two RCTs combined phototherapy with systemic immunosuppressive agents (methotrexate or alefacept), one RCT compared phototherapy with an excimer laser as additional light source, and one RCT compared phototherapy monotherapy with a combination of phototherapy and audiotape intervention involving mindfulness and stress reduction. Two RCT trials examined the effect of treatment setting on the effectiveness of phototherapy, one involved inpatient versus outpatient treatment; the other compared outpatient clinic treatment to home-based phototherapy.
Table 2. Ultraviolet Phototherapy Management of Psoriasis: RCT Evidence (1999 to May 2009).
| Subject of Comparison | Number of RCTs |
||||
|---|---|---|---|---|---|
| 1. Comparison of effectiveness of ultraviolet wavelength source | |||||
| A. Within Ultraviolet B | |||||
| i. NB-UVB vs. BB-UVB | 2 | ||||
| B. Between Ultraviolet Source | |||||
| i. NB-UVB vs. PUVA | 4 | ||||
| ii. P-UVB vs. PUVA | 1 | ||||
| 2. Adjuvant effects with topical agents as additive interventions to phototherapy | |||||
| A. Vitamin D3 analogues with | |||||
| i. NB-UVB | 3 | ||||
| ii. BB-UVB | 2 | ||||
| iii. PUVA | 1 | ||||
| iv. NB-UVB or UVA | 1 | ||||
| B. Oleic Acid Cream | |||||
| i. NB-UVB | 1 | ||||
| C. Bergamol oil | |||||
| i. NB-UVB | 1 | ||||
| D. Balneotherapy (Spa saline baths) | |||||
| i. NB-UVB or BB-UVB | 4 | ||||
| 3. Adjuvant effects with systemic agents as additive interventions to phototherapy | |||||
| A. Methotrexate | |||||
| i. NB-UVB | 1 | ||||
| B. Alefacept | |||||
| i. NB-UVB or BB-UVB | 1 | ||||
| 4. Additional light source to improve effectiveness of phototherapy | |||||
| A. Excimer laser | |||||
| i. PUVA | 1 | ||||
| 5. Psychological intervention as additive intervention to phototherapy | |||||
| A. Audio tape on mindfulness therapy | |||||
| i. NB-UVB or BB-UVB | 1 | ||||
| 6. Comparison of phototherapy treatment setting | |||||
| A. Inpatient versus outpatient dithranol | |||||
| i. NB-UVB or BB-UVB | |||||
| B. Home vs. outpatient phototherapy | |||||
| i. NB-UVB | 1 | ||||
NB-UVB, Narrow band ultraviolet B radiation; BB-UVB, Broad band ultraviolet B radiation; PUVA, Psoralen ultraviolet A radiation; RCT Randomized controlled trial
Although the majority (n = 20) were 2-arm RCT study designs, five were 3-arm trials (76-80) and one was a 4-arm trial (81). All the studies except one (80) involved randomization without pre-selection of eligible subjects. The trial by Sminkels et al. (80) involved parallel randomization groups where contraindications, patient preferences and tolerances were used to sort and select patients for randomization. Trials involving comparisons between ultraviolet wavelength source involved study arms of essentially two treatments. The trials involving the adjuvant effects of adding topical agents to phototherapy, involved comparisons with vehicle or placebo agents in four (77;82-85) of the nine trials. Of the two trials involving phototherapy combination therapy with systemic agents, one involved a placebo comparison. (86) Among the four trials involving balneotherapy (i.e. the addition of salt baths prior to irradiation) only one trial (78) used a control arm comparison that did not also involve ultraviolet irradiation with the intervention.
Phototherapy Management of Moderate-to-Severe Plaque Psoriasis
The results of the RCT trials are detailed in Table 3 and are discussed below by their research grouping.
Table 3. Effectiveness of Different UVB Wavelengths for Moderate-To-Severe Plaque Psoriasis.
| Study | Comparison of Clinical Outcomes by Ultraviolet Source | |||
|---|---|---|---|---|
| Kirke et al. (89) 2007; N=100 | NB-UVB vs. Selective UVB | |||
| Treat Till Clear | Complete clearance |
Number of Treatments to Clearance |
Mean Cumulative UVB Dose until Clearance, J/cm2 (95% CI) |
Remission at 6 months |
| NB-UVB (3x wkly) Treat to Clear | 28/50 (56%) | 28.4 | 40.9 (28.1, 55.2) | 1/19 |
| Selective UVB (3x wkly) | 20/50 (40%) | 30.4 | 39.9 (27.4, 53.6) | 0/13 |
|
|
OR = 2.00 (0.87, 4.62) |
|
|
|
|
NSD |
NSD |
NSD |
||
| Yuehua et al. (88) 2008; N=73 | NB-UVB vs. BB-UVB | |||
| 6 wk Treatment (18 sessions) |
PASI-60 | PASI ± SD Pre / Post |
Mean Cumulative UVB Dose, J/cm2 ± SD |
Durability |
| NB-UVB (3x wkly) | 36/43 (84%) | 11.2 ± 3.6/ 2.9 ± 1.3 |
16.10 ± 4.13 | NR |
| BB-UVB (3x wkly) | 11/29 (38%) | 11.6 ± 4.1/ 2.2 ± 0.9 |
16.10 ± 4.13 | NR |
| p < .01 | NSD | NSD | ||
NR, Not reported; NSD, Not statistically different
1A. Effectiveness of Different Ultraviolet B Wavelengths
Two studies (88;89) evaluated the effectiveness of different UVB irradiation sources, broad band (BB), selective band (SEL) and narrow band (NB) for phototherapy treatment (see Table 3). NB-UVB was compared to selective UVB in a ‘treat until clearance’ study and to BB-UVB in a 6-week treatment study; each involved optimal treatment frequencies of 3-times weekly. The clinical response was found to be significantly higher in the NB-UVB group than in the BB-UVB group (p < .01) and higher (56% versus 40% clearance) but not significantly higher in the NB-UVB compared to the SEL UVB group. The mean number of treatments and cumulative UVB doses to achieve the clinical response was not significantly different between groups in either study. Remission or duration of the clinical response was not reported in one study and inadequately evaluated in the other.
1B. Effectiveness of UVB and UVA (PUVA) Ultraviolet Irradiation
Four studies (76;90-92) compared NB-UVB with PUVA, three involving oral-PUVA and one cream-PUVA (see Table 4). In two of the studies (90;92) oral-PUVA resulted in significantly greater clinical response than NB-UVB. One study did not report clinical response and no significant difference was found between cream PUVA and NB-UVB. Oral-PUVA also resulted in significantly shorter mean treatment times and longer remission periods. The mean cumulative UV dose however, was significantly higher in the oral-PUVA than the NB-UVB group in both studies reporting cumulative dose.
Table 4: Treatment Comparisons of UVB Phototherapy and UVA Photochemotherapy for Moderate-To-Severe Plaque Psoriasis.
| Study | Comparison of Clinical Outcomes by Type of Ultraviolet Irradiation | |||
|---|---|---|---|---|
| Gordon et al., (90) 1999; N = 100 | NB-UVB vs. Oral-PUVA | |||
| Treat Till Clear | Clearance (no psoriasis) |
Mean Number of Treatments to Clearance |
Mean Cumulative UV Dose (J/cm2) to Clearance |
Remission At 6 Months Randomized/Cleared |
| NB-UVB (2x wkly) | 32/51 (63%) | 25.3 | 35 | 12% / 22% |
| Oral-PUVA (2x wkly) | 41/49 (84%) | 16.7 | 70.1 | 35% / 41% |
| OR = 3.04 (1.18,7.84); p = .018 | p < .001 | p < .001 | p = .002 | |
| Markham et al., (91) 2003; N = 54 | NB-UVB vs. Oral-PUVA | |||
| Treat Till Clear | Complete clearance | Mean Number of Treatments to Clearance (95% CI) | Mean Cumulative UV Dose (J/cm2) to Clearance | Mean Number Days in Remission (95% CI) |
| NB-UVB (3x wkly) | NR | 25.5 (18.0, 32.5) | NR | 288.5 (170.6,365) |
|
Oral-PUVA (2x wkly) |
NR |
19 (14.6, 25.8) |
NR |
231 (162.7,365) |
| p = .03 | NSD | |||
| Yones et al., (92) 2006; N = 88 | NB-UVB + Placebo vs. Oral-PUVA | |||
| 15 weeks treatment (30 Treatments) | Clearance (PGA C/AC) [Median PASI, pre→post at 8 sessions] | Mean Number of Treatments to Clearance | Mean Cumulative UV Dose (J/cm2) | Remission at 6 months (Median time to relapse) |
| NB-UVB + Oral placebo (2x wkly) | 31/37 (84%) [9.6 → 5.7] | 28.5 | 41.3 | 23/34 (68%) (8 months) |
|
Oral-PUVA (2x wkly) |
22/34 (65%) [11.0 → 4.2] |
17.0 |
126 |
8/23 (35%) (4 months) |
|
p = .02, p = 001 |
p < .001 |
p < .001 |
p = .03 |
|
| Grundman-Kollman et al. (76) 2004; N = 30 | NB-UVB + Cream-PUVA vs. Cream-PUVA vs. NB-UVB | |||
| Treat Till Clear | Clearance Time (range) to Complete Clearance |
Mean Number of Treatments to Clearance ± SD (range) | Mean Cumulative UV (J/cm2) A and UVB (J/cm2) Dose to Clearance ± SD (range) | Remission |
| NB-UVB + Cream-PUVA (4x wkly) | 10/10 (3– 4 wks) | 14 ± 2 (12 – 16) | UVA: 18.7 ± 4.7 (13.2 – 28) UVB: 8.2 ± 3.3 (4.0 – 15.4) |
NR |
|
Cream-PUVA (4x wkly) |
10/10 (5–7 wks) |
24 ± 5 (18 – 33) |
45.0 ± 16.3 (26.2 – 80.5) |
NR |
|
NB-UVB (4x wkly) |
10/10 (5–7 wks) |
21 ± 3 (15 – 25) |
17.1 ± 4.1 (12.7 – 24) |
NR |
| NSD | p < .001 | p < .001 | ||
| Khurshid et al., (93) 2000; N= 44 |
Oral-PUVB vs. Oral-PUVA |
|||
| 7 week treatment (21 sessions) + 10 wk maintenance | Clearance (90% reduction) | Mean Number of exposures to clearance | Mean Cumulative UV Dose (J/cm2) to Clearance | Remission |
| Oral-PUVB (3x wkly) | 17/22 (77.3%) | 18 | 25.2 | NR |
|
Oral-PUVA (3x wkly) |
19/22 (86.4%) |
16 |
72.5 |
NR |
| NSD | NSD | p < .001 | ||
NR Not reported NSD Not statistically different
The Grundman-Kollman et al. study (76) involved a 3-arm trial design in which cream-PUVA was used with NB-UVB as a combination therapy and compared separately to NB-UVB and cream-PUVA as monotherapies. The clinical response did not differ in the three groups but the mean number of treatments was significantly lower in the NB-UVB and cream-PUVA combination therapy. The mean cumulative UV dose, however was lowest in the NB-UVB monotherapy group. The Khurshid et al. study (93) involved a comparison between oral-PUVB and oral-PUVA in which clinical response was similar in the two groups, but the mean cumulative UV dose was significantly lower in the oral-PUVB group.
2. Effectiveness of Topical Agents as Additive Interventions to Phototherapy
The clinical trials evaluating the effects of adding various topical agents to phototherapy are outlined in Table 5. Combination therapies were expected to increase clinical responses, decrease number of irradiation treatments, and decrease cumulative dose or increase the duration of remission. Calcipotriol, a vitamin D3 analogue was the most common agent employed and was evaluated as a combination therapy with NB-UVB, BB-UVB and PUVA therapies. One study (84) investigated the effects of using calcitriol ointment (an endogenously hormonally active derivative of vitamin D3) in combination with BB-UVB phototherapy. Two other studies involved NB-UVB in combination with other topical agents, specifically, 5% oleic acid cream emollient (94) and bergamoli oil (95), a photosensitizing agent.
Table 5: Phototherapy Alone and in Combination Therapy with Topical Agents for Moderate-To-Severe Plaque Psoriasis.
| Study | Comparison of Clinical Outcomes of Phototherapy vs. Combination Phototherapy and Topical Agents | |||
|---|---|---|---|---|
| Brands et al., (96) 1999; N = 53 | NB-UVB vs. NB-UVB + Calcipotriol Ointment | |||
| Treat Till Clear | Mean PASI Pre/Post (range); Mean % PASI Δ |
Mean Number of Treatments to Clearance or Stability |
Mean Cumulative UVB Dose (J/cm2) to Clearance or Stability | Durability |
| NB-UVB (3x wkly) | 12.5 (3.4 – 35.1)/3.1 (0.7–24.0) p < .001 |
31.7 | 36.71 | NR |
|
|
75.5% |
|
|
|
| NB-UVB (3x wkly) + CO (2x daily) | 13.2(3.5 – 27.3)/3.0(7 – 19.2) p < .001 |
31.0 | 39.88 | NR |
| 79.3% | ||||
| NSD | NSD | NSD | ||
| Rim et al., (97) 2001; N = 28 | NB-UVB vs. NB-UVB + Calcipotriol Ointment | |||
| Treat Till Clear | > 95% Clearance (Grade 1V) | Mean Number Treatments to Grade IV Clearance (Trunk/ Extremities) | Mean Cumulative UVB Dose (J/cm2) to Grade IV Clearance (Trunk/ Extremities) | Remission |
| NB-UVB (3x wkly) | 11/18 (61%) | 15.7 ± 4.1 / 18.5 ± 4.8 | 10.6 ± 3.4 / 14.2 ± 4.8 | NR |
|
NB-UVB (3x wkly) + CO (2x daily) |
9/10 (90%) |
14.3 ± 5.8 / 16.0 ± 4.3 |
9.9 ± 5.9 / 11.7 ± 4.7 |
NR |
| p < .05 | NSD | NSD | ||
| Woo et al., (82) 2003; N = 50 | NB-UVB + Control Emollient vs. NB-UVB + Calcipotriol Cream | |||
| 20 treatment sessions | Mean PASI Pre / Post (20th session) | Mean Number Treatments | Mean Cumulative UVB Dose (J/cm2) | Remission |
| NB-UVB (3x wkly) + control emollient (2x daily) | 14.1 / 2.3 p < .01 | 20.4 | 21.1 | NR |
| NB-UVB (3x wkly) + CC (2x daily) | 12.4 / 1.3 p < .01 Mean Difference = -2.0 (95% CI; -5.9, -1.9) | 18.7 | 16.2 Mean Diff = 4.9 (95% CI; 0.96, 8.8) | NR |
| NSD | NSD | p < .02 | ||
| Ramsay et al., (83) 2000; N = 54 | BB-UVB + Placebo cream vs. BB-UVB + Calcipotriol Ointment | |||
| 12 wk treatment | PASI-80 [Marked Improvement or Clearance] | Median Number of Treatments (range) to PASI-80 | Median Cumulative UVB Dose (J/cm2) (range) to PASI-80 | Relapse Rates (4, 8, 12 wks) |
| BB-UVB (3x wkly) + vehicle cream 2x daily | 58/79 (73.4%) [77.2%] | 19 (8 – 35) | 5.4 (0.3 – 81.8) | IA |
| BB-UVB(2x wkly) + CO 2x daily) | 61/80 (76.2%) [72.5%] OR = 0.79 (95% Cl; 0.43, 1.43) | 12 (7 – 24) RR = 2.59 (95% Cl; 1.71, 3.92) | 1.6 (0.3 – 10.6) RR = 3.87 (95% Cl; 2.51,5.95) | IA |
| NSD | p < .001 | p < .001 | NSD | |
| Ring et al., (84) 2001; N = 104 | BB-UVB + Placebo Ointment vs. BB-UVB + Calcitriol Ointment | |||
| 8 wks treatment (16 sessions) | Overall Global Improvement (C/AC) at week 8 [Mean % PASI Δ] | Median Number of Teatment Days (range) | Mean Cumulative UVB Dose (J/cm2) (end point undefined) | Remission |
| BB-UVB (2x wkly) + vehicle ointment | 11/53 (21%) [43%] | 6 - 70 | 8.96 | NR |
| BB-UVB + CAO (2x daily) | 22/49 (45%) [65%] | 8 - 63 | 5.92 | NR |
| p = .0014 | NSD | NSD | ||
| Roussaki-Schulze et al., (77) 2005; N = 45 | NB-UVB + Calcipotriol Ointment vs. UVA1 + Calcipotriol Ointment vs. Calcipotriol Ointment | |||
| 12 wk treatment or max 24 sessions | Mean PASI at 3 months | Median Number of Treatments (range) | Mean Cumulative UVB Dose (J/cm2) to 3 months | Remission |
| NB-UVB (2x wkly) + CO (2x daily) | PASI < 50 n=3 PASI >50 n=10 PASI-100 n=2 |
NR | 19.27 ± 5.00 | NR |
| UVA1 (2x wkly) + CO (2x daily) | PASI <50 n=3 PASI >50 n=9 PASI-100 n=3 |
NR | 23.05 ± 4.26 | NR |
| CO (2x daily) | PASI <50 n=9 PASI >50 n =2 PASI-100 n=4 | NR | NA | NR |
| p = .027 | NSD | |||
| Torras et al., 2004 (85); N = 120 | Oral-PUVA + Calcipotriol Cream vs. Oral-PUVA + Placebo Cream | |||
| 10 wk treatment (30 sessions) | Mean PASI (95% CI) [Mean % PASI Δ (95% CI)] | Mean Number of Days to: PASI-75, PASI-90 | Mean Cumulative UV Dose (J/cm2) (± SD) to PASI-90 | Remission |
| Oral PUVA (3x wkly) + CC | 2.65 (1.22, 4.08) [-87.5 (-97.3, -77.6] | 42, 56 | 87.6 ± 48 | NR |
| Oral PUVA(3x wkly) + placebo cream | 7.03 (5.56, 8.50) [-49.6 (-59.7,-39.5] | 77, 79 | 118 ± 40.4 | NR |
| p < .0001 | p < .001, p = .004 | p = .03 | ||
| Martin-Ezquerra et al., 2007 (94); N = 44 | NB-UVB + Oleic Acid Cream vs. NB-UVB | |||
| Treat Till Clear | Clinical Response PASI-80 | Mean Number Treatments ± SD to PASI-80 | Mean Cumulative UVB Dose (J/cm2) ± SD to PASI-80 | Remission |
| NB-UVB + oleic acid cream | NR | 19.1± 9.15 | 18.16 ± 16.57 | NR |
| NB-UVB | NR | 24 ± 11.46 | 29.68 ± 24.43 | NR |
| NSD | NSD | |||
| Valkova et al., 2007 (95); N=193 | BB-UVB vs. BB-UVB + Bergamot oil | |||
| Treat till Clear | Proportion Clear Mean pre/ post PASI ± SD |
Mean Nmber of Sessions for Clearance ± SD (range) | Mean Cumulative UVB Dose (J/cm2) ± SD to Clearance | Mean Duration (months) Remission ± SD (range) |
| BB-UVB (3x wkly) Treatment fill clear |
72% (73/102) | 15.9 ± 0.4 (12 - 22) |
3.8 ± 0.2 | 6.8 ± 0.3 (2 – 12) |
| 8.9 ± 0.3 / 1.1 ± 0.1 p = .001 |
||||
| BB-UVB (3x wkly) + Bergamot oil | 58% (53/91) 10.3 ± 0.7 0.8 ± 0.2 p = .001 | 14.8 ± 0.7 (10 - 17) | 2.9 ± 0.2 | 7.3 ± 0.8 (2 – 11) |
| NSD | p = .04 | p = .004 | NSD | |
IA, Inadequately assessed; NR, Not reported; NSD, Not statistically different
2A. Effectiveness of Calcipotriol in Combination with Phototherapy
The addition of calcipotriol either as a cream or ointment to NB-UVB (82;96;97) did not improve clinical response in two of the three trials and mean cumulative UV dose was not decreased for the combination therapy in two of the three studies. The clinical response in the NB-UVB monotherapy treatment arm, however, was already significantly improved in both studies without the addition of the topical agent. The PASI symptom scores were reported to be reduced from baseline after treatment from 12.5 to 3.1 (96) and from 14.1 to 2.3 (82). Reductions in the number of treatments in groups with added topical agent were not compared because the trials involved a fixed treatment schedule. The duration of the remission was not reported in any of the studies.
In two studies (83;84) calcipotriol or calcitriol was used alongside BB-UVB. Clinical response in the calcipotriol study (PASI-80) was not significantly different at 12 weeks and in the study with calcitriol, the overall global rating of improvement (clear/almost clear) at 8 weeks was significantly higher with the BB-UVB calcitriol combination therapy. The mean cumulative UV dose compared to the BB-UVB monotherapy, however, was significantly lower (5.4 vs. 1.6 J/cm2, p < .001) with the calcipotriol combination therapy group but not with the calcitriol combination therapy (8.96 vs. 5.92 J/cm2).
Two studies examined the effects of adding calcipotriol to UVA1 as a combination therapy, one to UVA1 (77) and one to oral-PUVA (85). In both studies, the clinical response to combination therapy was significantly higher. In the UVA1 and calcipotriol ointment combination therapy, the number of treatments to clinical response was not reported and the cumulative UV dose was not reduced in the combination therapy. In the study involving oral-PUVA and calcipotriol cream combination therapy, the mean number of days to clinical response and mean cumulative dose were significantly reduced in the combination therapy group.
2B. Effectiveness of Oleic Acid and Bergamot Oil in Combination with Phototherapy
Two other studies employed the topical agents oleic acid (94) and bergamot oil (95) in combination with phototherapy. Oleic acid, an emollient in combination with NB-UVB, was found to not decrease the number of treatments or the cumulative UV dose compared to NB-UVB monotherapy. The addition of bergamot oil, to BB-UVB did not increase clinical response compared to BB-UVB montherapy but did significantly decrease the mean number of treatments (15.9 to 14.8) and the mean cumulative dose (3.8 to 2.9 J/cm2) although the absolute decreases were limited. The lack of improvement in clinical response in the combination therapy is somewhat countered by the high clinical response in the monotherapy arm with an improvement in mean pre to post PASI scores from 8.9 to 1.1.
2C. Effectiveness of Balneotherapy (Spa Saline Baths) in Combination with Phototherapy
Four studies examined the effects of balneotherapy, spa salt baths on the treatment effectiveness of phototherapy (see Table 6). (78;81;98;99) Two of the studies (98;98) involved similar protocols but varied the concentration of spa salt bath. In both studies, the clinical response at 6 weeks, reported by both physician and patient self ratings of PASI score improvements, were significantly greater in the combination salt spa bath and UVB therapy than the UVB monotherapy group. The greater clinical response in the combination therapy group, however, did not persist to 3 month follow-up. Cumulative UV dose was not significantly reduced in the combination therapy group at either spa bath salt concentration. Remission at 3 and 6 months was significantly better in the combination therapy group with low concentration spa salt bath and inadequately reported for the high concentration bath.
Table 6: Impact of Balneotherapy On Phototherapy Treatment Effectiveness for Moderate-To-Severe Plaque Psoriasis.
| Study | Comparison of Clinical Outcomes of Phototherapy vs. Combination Balneotherapy and Phototherapy | ||||
|---|---|---|---|---|---|
| Brockow et al., 2007 (99); N = 164 | UVB (BB or selective) vs. UVB (BB or selective) + Low Saline Spa Bath | ||||
| 6 wk treatment (18 sessions) | PASI-50Median % PASI Δ (25th -75th percentile) at 6 weeks | Mean Number of Treatments to Clearance | Mean Cumulative UVB Dose (J/cm2) | Remission (S-PASI < 5) at 3 and 6 Months | |
| UVB (BB or selective) (3x wkly) | 32/64 (50%) 53% (15 – 74%) | NR | 4.3 ± 2.4 | 5/53 (9%) 4/53 (8%) | |
| UVB (BB or selective) (3x wkly) + low concentration saline spa bath) | 58/79 (73%) 66% (52 – 86%) | NR | 4.74 ± 2.1 | 22/70 (31%) 12/70 (17%) | |
| p= .01, p= .004 | NSD | p= .004, p= .018 | |||
| Brockow et al., 2007 (99); N = 160 | UVB (BB or selective) vs. UVB (BB or selective) + High Saline Spa Bath | ||||
| 6 wk Treatment (18 sessions) | PASI-50 S-PASI-50 | Mean number of Treatments to Clearance | Mean cumulative UVB (selective, BB) Dose (J/cm2) | Remission | |
| UVB (BB or selective) (3x wkly) | 38/71 (54%) 46/67 (69%) | NR | ± 0.8 20.7 ± 5.9 | IA | |
| UVB (BB or selective) (3x wkly) + high concentration saline spa bath) | 68/79 (86%) 65/77 (84%) | NR | 0.8 19.1 ± 7.8 | IA | |
| p < .001, p< .03 | NSD | ||||
| Leaute-Labreze et al, 2001, (78); N = 71 | Thermal Saline Water vs. NB-UVB vs. Thermal Saline Water + NB-UVB | ||||
| 3 wk treatment (15 sessions) | Mean % PASI Δ at day 21 | Mean Number of Treatments to Clearance | Mean Cumulative UVB dose (J/cm2) | Remission | |
| Thermal saline water (5 days/wk) | 29% | NR | NA | NR | |
| NB-UVB (5 days/wk) | 64% | NR | 12.5 | NR | |
| Thermal saline water + NB-UVB (5 days/wk) | 55% | NR | 11.8 | NR | |
| p< .001 | NSD | NSD | |||
| Schiener et al., 2007 (81); N = 1,241 ITT (N=1159) PPA (N=553) | UVB (BB/SEL/NB) + salt bath vs. Bath-PUVA vs. UVB (BB/SEL/NB) vs. UVB (BB/SEL/NB) + prior Tap Water | ||||
| 8 wk treatment (32 sessions) | ITT (N=1159) PASI-50S-PASI-50PASI-75PPA (N=553)PASI-50 | Mean number of Treatments to Clearance | Mean Cumulative UV Dose (J/cm2) ± SD [BB or SEL UVB] NB-UVBUVA | Remission | |
| UVB (broadband, selective or NB) (4x wkly) + prior salt water bath N=299 | 224 (74.9%) 217 (75.9%) 150 (51%) 128 (84.8%) |
NR | ± 4.0 (n = 233) 31.5 ± 15.2 (n = 54) NA |
NR | |
| Bath-PUVA (4x wkly) N=305 | 239 (78.4%) 237 (81.2%) 186 (62.6%) 148 (91.9%) |
NR | NA NA 63.6 ± 36.9 (n = 305) |
NR | |
| UVB (broadband, selective or NB) (4x wkly) + prior tap water bathN=285 | 173(60.7%) 174(65.4%) 96 (34.9%) 108 (81.2%) |
NR | 5.6 ± 6.2 (n = 210) 29.3 ± 16.2 (n = 55) NA |
NR | |
| UVB (broadband, selective or NB)(4x wkly) N=270 | 117 (43.3%) 134 (51.9%) 62 (23.4%) 65 (60.2%) |
NR | 5.4 ± 7.3 (n=204) 33.3 ± 21.4 (n = 51) |
NR | |
| ITT-PASI-50 | SW-UVB v TW-UVB p < .001 Bath-PUVA v TW-UVB p < .001 Bath-PUVA v SW-UVB p= .34 |
NSD | |||
NA, Not applicable; NR, Not reported; NSD, Not statistically significant
The Leaute-Labreze et al. study (78) was a 3-arm trial and the only trial to include a comparative arm without phototherapy. The clinical response, defined as the mean percentage PASI reduction, was significantly higher lower (p < .001) in the thermal saline group (29%) than in the NB-UVB (64%) or combination NB-UVB plus thermal saline bath therapy (55%) groups. Mean cumulative UV dose, as in former studies, was unaffected by prior thermal saline baths.
The Schiener et al. study (81) was a 4-arm trial with 1,241 subjects and the largest trial on phototherapy identified in the evidence review. Overall, the clinical response measured by PASI-50 and PASI-75 were significantly different among the treatment groups of: NB-UVB, NB-UVB and prior tap water (TW-UVB), NB-UVB and prior salt (SW-UVB), and bath-PUVA. In pair wise combinations, the clinical response was greater in the SW-UVB group compared to the TW-UVB (p < .001) group and in the bath-PUVA group compared to the TW-UVB (p < .001) group. The clinical response was similar in the bath-PUVA and the SW-UVB (p = .34) groups. The mean cumulative dose was highest for the bath-PUVA (63.9 J/cm2) group and lowest in the BB-NVB (5.2, 5.4, 5.6, J/cm2) treatment group.
The primary analysis was performed on the intention to treat (ITT) study group of 1,159 subjects and an additional analysis was conducted on the per-protocol treatment (PPT) subgroup consisting of 553 subjects. The PPT analysis included a restricted group that adhered completely to the study protocol. The improvement in clinical response with PASI-50 scores at 8-weeks were significantly increased in the PPT analysis over the ITT group in each treatment group. For the four treatment groups, increases in PASI-50 scores in the PPT analyses over the ITT, were 10% (74.9 to 84.8) in the combination UVB and prior salt bath group, 13.5% (78.4 to 91.9) in the bath-PUVA group, 20.5% (60.7 to 81.2) in the combination UVB and prior tap water group and 16.9% (43.3 to 60.2) in the UVB monotherapy group.
3. Systemic Agents as Additive Interventions to Phototherapy
Two small studies (79;86) examined the effects of adding systemic agents to NB-UVB on clinical response (see Table 7). One study (86) involved randomizing 24 patients to NB-UVB or combination therapy with NB-UVB and methotrexate. Methotrexate significantly boosted the clinical response with almost all (10/11) patients reaching clearance defined by PASI-90 compared to 38.5% (5/13) of the patients in the NB-UVB group only. Time to clearance was also significantly (p < .0001) shorter in the combination therapy group. The mean cumulative dose was significantly (p = .0026) reduced by almost half (59.22 to 26.92 J/cm2) in the combination therapy compared to the NB-UVB monotherapy group.
Table 7: Impact of Systemic Agents Added to Phototherapy for Management of Moderate-To-Severe Plaque Psoriasis.
| Study | Comparison of Clinical Outcomes of Phototherapy vs. Systemic Agents and Phototherapy | ||||
|---|---|---|---|---|---|
| Asawanonda et al., 2006 (86); N = 24 | NB-UVB + Methotrexate vs. NB-UVB | ||||
| 24 wk treatment | Clearance (PASI-90) Median PASI Score Post Treatment | Median Time to Clearance (weeks) | Mean Cumulative UVB Dose ± SD to Clearance (range) | Median Time to Relapse (weeks) | |
| NB-UVB (3x wkly) for 24-wks + Methotrexate for 3 wks prior to UVB | 10/11 (90.9%) 0.15 | 4 | 26.92 ± 15.54 (6.95- 54.15) | IA | |
|
NB-UVB (3x wkly)+ placebo for a 24-week treatment |
5/13 (38.5%) 5.6 (1.1, 9.7) 3.15 |
At 24-wks (more than half still not cleared or lost to follow-up) |
59.25 ±16.71 (32.52-73.33) |
IA |
|
|
|
p = .01, p < .001 |
p < .0001 |
p = .0026 |
IA |
|
| Ortonne et al., 2005 (79); N = 60 | Alefacept + 6 wks UVB (NB/BB) vs. Alefacept + 12 wks UVB (NB/BB) vs. Alefacept | ||||
| PASI-50At 4-weeks in Treatment | PASI-50At 2-weeks Post Treatment | Mean Cumulative UVB Dose (J/cm2) to PASI-75 BB, NB | Remission | ||
| Alefacept 12-wk IM 15 mg + UVB (NB at French site or BB at US site) 3X wkly for 6-week treatment | NB-UVB: 90% (9/10) BB-UVB: 22% (2/9) | NB-UVB: 80% (8/10) BB-UVB: 100% (5/5) | 3.57, 12.54 | NR | |
|
Alefacept 12-wk IM 15 mg + UVB (NB or BB) 3X wkly for 12-week treatment |
NB-UVB: 78% (7/9) BB-UVB: 18% (2/11) |
NB-UVB: 90% (9/10) BB-UVB: 75% (6/8) |
6.65, 16.04 |
NR |
|
|
Alefacept 12-wk IM 15 mg |
France: 45% (5/11) United States: 0 (0/10) |
France: 88% (7/8) United States: 0 (0/2) |
NA |
NR |
|
NA, Not applicable; NR Not, reported
The second study (79) was a pilot study conducted at two sites evaluating the ability of NB-UVB and BB-UVB to boost the clinical response to alefacept in patients with chronic plaque psoriasis. Patients were randomized to 3-arms comprised of either:
a) monotherapy with a 12-week course of alefacept as an intramuscular injection,
b) combination therapy with a 6-week course of alefacept, followed by a 6-week course of UVB treatment
c) combination therapy with a 6-week course of alefacept followed by, a 12-week course of UVB treatment
For the latter two arms, either NB-UVB or BB-UVB was used, depending on the study location. Only trends were examined and no significance testing was performed. Compared to alefacept alone, there was a strong early boost in clinical response measured by PASI-50 at 4-weeks into treatment with NB-UVB and alefacept but not with BB-UVB and alefacept. However, at 2- weeks following treatment the clinical response appeared similar in all study groups.
4. Combination Treatment with Photochemotherapy and Excimer Laser
One study examined the effects of treating targeted lesions with the excimer laser after undergoing bath or oral-PUVA (see Table 8). (100) Although the clinical response of complete clearance, defined as PASI-90, was similar in two groups, the mean number of treatments (p < .05) and cumulative UV dose (p < .01) to achieve the clinical response was significantly lower in the PUVA and excimer laser group compared to the PUVA monotherapy group. The relapse rate at 3 months was not significantly different between the two groups.
Table 8: Impact of The Excimer Laser as an Additional Light Source on Photochemotherapy Management of Moderate-to-Severe Plaque Psoriasis.
| Study | Comparison of Clinical Outcomes of PUVA vs. Combination Excimer Laser and PUVA Therapy | |||
|---|---|---|---|---|
| Trott et al., 2008 (100); N = 272 | Oral or Bath-PUVA + Excimer Laser vs. Oral or Bath-PUVA | |||
| 6 wk Treatment (24 treatments) | Complete Clearance (PASI-90) | Mean Number of Treatments ± SD to Clearance (PASI-90) | Mean Cumulative UV (A+B) Dose (J/cm2) to Clearance (range) | Relapse by 3 months |
| Oral or Bath-PUVA (4x wkly) | 67.3% (76/113) | 26 ± 7 | 53.2 ± 26.3 (14.4 – 156.5) | 18 |
| Oral or Bath-PUVA (4x wkly)+ Excimer UVB 308-nm laser 6 hrs post PUVA for up to 4 sessions | 63.6% (91/143) | 15 ± 6 | 22.9 ± 5.8(8.3 – 98.9) [+ UVB laser 1.87 ± .492] | 13 |
| NS | p< .05 | p< .01 | NS | |
5. Concurrent Audiotape Sessions for Stress Reduction and Phototherapy
The impact of a combination therapy involving a psychological intervention, either with phototherapy or chemophototherapy, was compared to phototherapy as a monotherapy and evaluated after 40 treatment sessions (see Table 9). The psychological intervention consisted of an audiotape session on mindfulness therapy and stress reduction techniques and was conducted during the phototherapy sessions. (101) Stress and anxiety as assessed by formal measures, the symptom checklist (SCL-90-R) and the State-Trait Anxiety Inventory (STAI), were not reduced after treatment sessions.
Table 9: Effectiveness of Concurrent Mindfulness and Stress Reduction Audiotape Sessions With Phototherapy or Photochemotherapy For Moderate-To-Severe Plaque Psoriasis.
| Study | Comparison of Clinical Outcomes of Phototherapy vs. Combination Meditation Auditory Tape and Phototherapy | |||
|---|---|---|---|---|
| Kabat-Zinn et al., (102) 1998; N = 37 | UVB or PUVA + Meditation Auditory Tape vs. UVB or PUVA | |||
| 40 treatment sessions | Clearance (< 5% Original Surface Area) | Median Time to Clearance (days) | Mean Cumulative UV Dose (J/cm2) | Remission |
| UVB or PUVA (3x wkly for 13 weeks for 40 treatment sessions) | UVB Gp = 4/10 PUVA Gp = 5/8 | UVB Gp = 113 PUVA Gp = 85 | NR | NR |
| UVB or PUVA for 40 treatment sessions with simultaneous mediation auditory tape | UVB Gp = 5/11 PUVA Gp = 5/8 | UVB Gp = 83 PUVA Gp =48.5 | NR | NR |
| NSD | p = .013 | |||
The assessment of clinical response was defined uniquely for this study and represented changes in skin morphology (scaliness, erythema and thickness) in targeted skin lesions representing the most extensive and severe plaques. Photographs of lesions were rated independently by two dermatologists. The addition of the auditory tape to UV sessions did not influence clearance rates for psoriasis but did significantly decrease the median time to clearance in the combination treatment groups for both PUVA (85 versus 48.5 days) and UVB (113 versus 85 days) compared to the monotherapy group.
6. Impact of Setting on Phototherapy Treatment Effectiveness of Psoriasis
6A. Comparisons Between Inpatient Dithranol, Outpatient Dithranol or Outpatient Phototherapy Treatment Programs
Two studies evaluated the impact of setting on the effectiveness of phototherapy for psoriasis. Swinkels et al. evaluated the impact of inpatient versus outpatient treatments (80), while Koek et al. compared clinic versus home-based phototherapy. (103) The results of these studies are summarized in Tables 10A and 10B.
In Swinkels et al. (80), the effectiveness and side effects of an dithranol inpatient treatment program for a 8-week maximum duration was compared to two different outpatient programs (Table 10). The outpatient programs involved assignment to a short-contact outpatient program involving first week daily visits with instructions on dithranol application at home, followed by a maximum of 12 weeks at home self-treatment. The other outpatient program involved assignment to an outpatient, clinic-based phototherapy, either with NB-UVB or BB-UVB, 3x weekly for a maximum of 12 weeks. The assignment of patients to the study arms, was not strictly random. Patients with no contraindications were randomized to either of the three arms. Patients who had a contraindication to phototherapy, or who refused this treatment were randomized to either inpatient or outpatient dithranol treatment, and those who refused inpatient treatment were randomized to phototherapy or outpatient dithranol. The authors stated that there was a 40% contraindication and non-response rate to phototherapy.
Table 10: Comparison of Inpatient Dithranol, Outpatient Dithranol or Phototherapy Treatment Of Moderate-To-Severe Plaque Psoriasis.
| Study | Comparison of Clinical Outcomes Between Clinic-Based and Home-Based Phototherapy | |||
|---|---|---|---|---|
| Swinkels et al, (80) 2004; N=238 | In Patient Dithranol vs. Short Contact Outpatient Stay Dithranol vs. UVB | |||
| Mean % PASI Δ (± SD)ClearancePASI-90 | Mean Number of Days ± SD to Clearance | Mean Cumulative UV Dose (J/cm2) | Median Days in Remission (95% CI)Relapse Rate at 1 Year | |
| UVB (NB or BB) (3x wkly) maximum 12 weeks | 73.9 ± 28.5 56% (43/78) | 75 ± 17 | NR | 328 (245, 440) 58% (21/36) |
| In-patient Dithranol (IPD) 8-week maximum stay | 85.1± 20.4 87% (52/60) | 37 ± 14 | NA | 277 (225, 342) 70% (32/46) |
| Short contact outpatient Dithranol (SCO) 12- week maximum | 75.2 ± 24.8 59% (59/100) | 72 ± 17 | NA | 584 (347, 981) 38% (18/47) |
| IPD vs. SCO, p = .001 IPD vs. UVB, p = .001 SCO vs. UVB, NSD | IPD vs. SCO, p < .001 IPD vs. UVB, p < .001 SCO vs. UVB, NSD | SCO vs. IPD, p = .003 UVB vs. SCO, NSD UVB vs. IPD, NSD | ||
The clinical response, defined as PASI-90 clearance, was significantly higher in the inpatient dithranol treated group compared with the outpatient dithranol (p = .001) and phototherapy (p = .001) programs. Clinical response did not differ between the two outpatient programs. The mean number of days until clearance was also significantly shorter in the inpatient group compared to the outpatient dithranol (p < .001) or phototherapy (p < .001) treatment programs. The durability of the clinical response was also significantly different in the three groups. The relapse rate at one year follow-up was significantly (p = .003) higher in the inpatient group (70%) than either the outpatient dithranol (38%) or phototherapy (58%) programs of the outpatient treatment groups.
6B. Comparison Between Outpatient Clinic and Home-Based Phototherapy
The study by Koek et al. (103), known as the PLUTO study, comparing outpatient clinic-based versus home-based NB-UVB phototherapy was designed as a pragmatic inferiority trial with clinical trial protocols fully published prior to study recruitment. (104) The main finding was that treatment effectiveness of home-based phototherapy based on physician (PASI-50, PASI-90) and patient (SPASI-50, SPASI-90) psoriasis symptom reduction scores was not inferior to outpatient clinic based phototherapy (see Table 11). The use of adjuvant drugs, both vitamin D derivatives and topical steroids were significantly higher in the clinic-based phototherapy setting. The mean number of irradiations and mean cumulative dose was higher but not clinically or statistically higher in the home setting. Overall, the risk of short term cutaneous complications such as burning, erythema or blistering was not significantly different in the two settings.
Table 11: Outpatient Clinic Versus Home-Based Phototherapy Treatment for Plaque Psoriasis.
| Study | Results Comparison | |||
|---|---|---|---|---|
| Koek et al., (103) 2009; N = 196 | Home-Based NB-UVB vs. Outpatient Clinic NB-UVB | |||
| Home-Based Phototherapy | Outpatient Clinic Phototherapy | Difference (95% CI) |
Significance | |
| PASI-50 | 70.3% (64/91) | 72.6% (61/84) | -2.3 (-15.7, 11.1) | NSD |
| PASI-90 | 19.8% (18/91) | 19.0% (16/84) | 0.8 (-10.9, 12.5) | NSD |
| SPASI-50 | 81.9% (77/94) | 79.1% (72/91) | 2.8 (-8.6, 14.2) | NSD |
| SPASI-90 | 43.6% (41/91) | 29.7% (27/91) | 13.9 (0.002, 27.8) | NSD |
| Adjuvant drugs used during phototherapy | ||||
| Topical steroids | 31.5% (29/94) | 52.2% (48/91) | -20.7 (-35.0, -6.4) | SD |
| Vitamin D derivatives | 19.6% (18/94) | 40.2% (37/91) | -20.6 (-33.8, -7.4) | SD |
| Mean number irradiations | 34.4 | 28.6 | 5.8 (2.7,9.0) | NSD |
| Mean cumulative dose (J/cm2) | 51.5 | 46.1 | 5.4 (5.2,16) | NSD |
| Proportion side effects (per irradiation) | ||||
| Severe erythema | 5.5 | 3.6 | 1.9 (-1.1, 4.9) | NSD |
| Blistering | 0.3 | 0.6 | -0.3 (-0.9, 0.3) | NSD |
| Burning sensation | 7.1 | 10.0 | -2.9 (-7.1, 1.2) | NSD |
| Mild erythema | 28.8 | 28.6 | 0.3 (-7.4, 8.0) | NSD |
| Psoriasis Disability Index | 32.8→20.9 | 34.3→22.0 | NSD | |
| Patient satisfaction (very or satisfied) | ||||
| Rate of improvement | 75.6% (68/90) | 71.6% (63/88) | 4% | NSD |
| Nursing care and supervision | 81.1% (73/90) | 89.8% (78/88) | 8.8% | p = .02 |
| Final treatment result | 81.1% (73/90) | 78.4% (69/88) | 2.7% | NSD |
| Global Treatment rating (excellent) | 42% (38/90) | 23% (20/88) | 19% | p = .001 |
| Preference for future home therapy | 92% (83/90) | 60% (53/88) | 32% | p = .001 |
NSD, Not statistically different
The impact on quality of life measured by SF36 (not reported) and disability measured by the Psoriasis Disability Index did not differ between the two settings. Patients in both settings were largely satisfied with the final results of their treatments and their rate of improvement, although satisfaction with nursing care and supervision was higher in the clinic setting. The perceived burden to the patient associated with the treatment (treatment procedures, time lost) was significantly higher in the clinic setting (p < .001). The overall global rating of excellent for phototherapy treatment was almost twice as high in the home versus the clinic setting (42% versus 23%, p = .001). The majority of patients at both sites preferred that future phototherapy be home phototherapy, although significantly more patients in the home setting preferred that future home therapy (92% versus 60%, p = .001).
Quality of the Evidence
Table 12 summarizes the quality of evidence for phototherapy management of moderate-to-severe psoriasis according to the GRADE criteria. The tables were grouped according to the four broad classifications of the study research objectives. The initial grade table on the effectiveness of phototherapy was based on a consideration from three levels of evidence:
Table 12: GRADE Quality of Evidence for Phototherapy Management of Moderate-to-Severe Psoriasis.
| Outcome | Study Design | Quality | Consistency | Directness | Other Issues | Overall Quality |
|---|---|---|---|---|---|---|
| Effectiveness of Phototherapy | ||||||
| Symptom reduction | RCT | Diverse range of evidence – moderate to high | Results across studies were consistent with increased clearance with PT | Range of patients were specified and appropriate, mostly with similar inclusion criteria | Evidence of effectiveness was consistent across diverse data sources that included:
|
Moderate |
| Comparative Effectiveness of Ultraviolet Irradiation Sources | ||||||
| Symptom reduction | RCT | No serious limitations | Generally consistent order of effectiveness: PUVA > NB-UVB > BB-UVB | Range of patients specified and appropriate | Limited number of studies | High |
| Number of treatments | RCT | |||||
| Cumulative irradiation dose | RCT | |||||
| Comparative Effectiveness of Phototherapy Combination Therapies | ||||||
| Symptom reduction | RCT | No serious limitations | Results across studies were consistent | Range of patients specified and appropriate | High | |
| Number of treatments | RCT | |||||
| Cumulative irradiation dose | RCT | |||||
| Effectiveness of Home Versus Outpatient Clinic Phototherapy | ||||||
| Symptom reduction | RCT | High - Well designed and full protocol published prior to study publication | Only one RCT trial but effects similar to cohort studies | Range of patients specified and appropriate | Limited number of trials and generalizability from other country may be an issue | Moderate |
| Number of treatments | RCT | |||||
| Cumulative irradiation dose | RCT | |||||
Early technical studies (55;56) demonstrating responses of individual plaques to specific ultraviolet action spectra;
A within person RCT trial (105) demonstrating significant clinical response in treated plaques compared to covered and untreated plaques; and
And a between person RCT trial (78) demonstrating a significant clinical response with phototherapy over a spa saline bath control arm.
In addition, more than half the patients achieved complete clearance in the UVB phototherapy monotherapy arms in each of the RCT studies reporting complete clinical response. (89;90;97)
Discussion
The RCT trials identified in this review had varying study objectives examining the short-term effectiveness of phototherapy. None of the trials were designed to evaluate the longer-term effectiveness of phototherapy and duration of remission after phototherapy treatment, even the effectiveness just 6 months or one year post-treatment was infrequently or poorly evaluated. The reporting of side-effects, even among the short term study periods, was also generally poor and there were no long-term cohort studies evaluating the cancer risk of cumulative UVB irradiation. This was particularly applicable to NBUVB, which has not been in clinical use long enough to allow for the longer term, large cohort follow-up needed to evaluate carcinogenic risks. This particular souce is, however, thought to pose lower risks related to cumulative use as it relies on lower doses of irradiation than PUVA.
Since the previous evidence review on psoriasis management (24), additional RCT trials were identified that have contributed evidence on the comparative effectiveness of photochemotherapy and phototherapy. These trials have confirmed clinical impressions that PUVA has a greater clinical effectiveness and can achieve clearance with fewer treatments than with UVB phototherapy. It was also clear that PUVA requires a much higher cumulative irradiation dose to achieve that clinical response. Within the UVB spectrum, NB-UVB was shown to have a higher clinical effectiveness than BB-UVB.
A strong limitation in the evidence base remains with the lack of comparator or head-to-head trials evaluating phototherapy against other treatment alternatives for moderate-to-severe psoriasis. There was still limited evidence to guide treatment decisions between phototherapy and the alternative traditional systemic therapies or the new biological immune suppressants. This absence is particularly important for the newer biological agents, which were initially introduced as arthritis treatments. The high costs associated with the continuous use of these agents warrants a close review of their safety, effectiveness, and cost-effectiveness.
Information on the effectiveness of management strategies involving combination or rotational approaches intended to reduce toxicity and side effects from long-term use was also limited. Several of the RCT studies were focused on evaluating the effects of topical agents, particularly calcipotriol, a vitamin D derivative, to reduce cumulative UV exposure. Although the studies were generally not suggestive of a reduction of cumulative irradiation with NB-UVB or BB-UVB, the one study (85) evaluating effects on PUVA did find a reduction of cumulative UVA irradiation.
Only one study (102) evaluated self-management techniques designed to help patients cope with their disease and to minimize stress or other conditions that could exacerbate their symptoms. That study evaluated the effects of a psychological intervention via audio tape sessions on mindfulness and stress reduction techniques conducted simultaneously with phototherapy and did not find a reduction in the number of treatments needed to achieve psoriasis clearance.
The areas of future research recommended in the earlier evidence-based review on psoriasis management review are still relevant. Trials focusing on comparative effectiveness between classes of treatment, such as methotrexate versus phototherapy, are still needed to refine treatment algorithms and improve overall psoriasis management. The need for better assessment of cost-effectiveness of therapies that considers systemic drug costs and costs of surveillance, as well as drug efficacy were also noted. Trials evaluating the effectiveness and cost-effectiveness of phototherapy provided in different settings are also needed. Although two trials were identified in the review comparing the effects of different settings on the effectiveness of phototherapy, the comparisons were different. Swinkels et al. (80) compared inpatient versus outpatient settings for treatments with phototherapy and dithranol. Although inpatient treatment with dithranol was more effective than outpatient treatment with either phototherapy or dithranol, inpatient treatments for psoriasis has been a declining practice and is usually reserved for the most serious cases. (106)
The trend that has been increasing is the provision of home-based phototherapy. The RCT trial (103) identified in this review that compared home-based to clinic phototherapy adds further weight to the observational studies (107-109) evaluating home-based phototherapies. The results in this trial were counter to the range of professional guidelines advising against home phototherapy for reasons of safety and inferior effectiveness. (58; 110) The Dutch RCT pragmatic trial comparing safety and effectiveness of home-based versus hospital clinic-based phototherapy found that home phototherapy was neither less safe, nor less effective than clinic-provided phototherapy. In addition, patients found that the burden of participating in the treatment was far less with home therapy and they were as satisfied with home phototherapy as those with clinic treatment. The majority of patients in both arms of the trial also preferred any future therapy to be home phototherapy.
Conclusion
Many of the evidence gaps identified in the 2000 NIHR evidence-based review of psoriasis management persisted in the updated MAS evidence-based review of phototherapy for psoriasis. In particular, the lack of evidence on comparative effectiveness or cost-effectiveness between the major treatment options for moderate-to-severe psoriasis remained. The evidence of effectiveness and safety of longer term strategies for disease management has also not been addressed. Evidence for the effectiveness or cost-effectiveness of phototherapy delivered in various settings, including home–based phototherapy, is emerging but is limited. In addition, because all available psoriasis treatments are palliative rather than curative, and because psoriasis has a high prevalence, chronicity and cost, strategies for disease control and improvements in self-efficacy employed in other chronic disease management programs should be investigated.
Overall, the conclusion of the MAS evidence-based review is that phototherapy and photochemotherapy are important therapies for the management of moderate-to-severe psoriasis and should be offered as therapeutic options for psoriasis in dermatology practices. The conclusions of the RCT trial evidence on ultraviolet phototherapy are summarized below in Table 13.
Table 13. Summary of RCT Evidence for Ultraviolet Phototherapy Management of Moderate-To-Severe Plaque Psoriasis.
| Conclusion | Evidence Level |
|---|---|
| Phototherapy is an effective treatment for moderate-to-severe plaque psoriasis | Moderate quality and adequate study evidence |
| Narrow band PT is more effective than broadband PT for moderate-to-severe plaque psoriasis | High quality but limited study evidence |
| Oral-PUVA has a greater clinical response, requires fewer treatments and has a greater cumulative UV irradiation dose than UVB to achieve treatment effects for moderate-to-severe plaque psoriasis | High quality and adequate study evidence |
| Spa salt water baths prior to phototherapy increases the short-term clinical response of moderate-to-severe plaque psoriasis but does not decrease cumulative UV irradiation dose | High quality and adequate study evidence |
| Addition of topical agents (vitamin D3 calcipotriol) to NB-UVB did not increase mean clinical response or decrease treatments or cumulative UV irradiation dose | High quality and adequate study evidence |
| Methotrexate prior to NB-UVB in high need psoriasis patients significantly increases clinical response, decreases number of treatment sessions, and decreases cumulative UV irradiation dose | High quality but limited study evidence |
| Phototherapy following alefacept increases the early clinical response in moderate-to-severe plaque psoriasis | Inadequate study evidence |
| Effectiveness and safety of home NB-UVB phototherapy is not inferior to NB-UVB phototherapy provided in a clinic to patients with psoriasis. Treatment burden is lower and patient satisfaction higher with home therapy and patients in both groups prefer future phototherapy treatments at home | High quality study but limited study evidence |
Existing Guidelines
Recommendations by the Canadian Psoriasis Expert Panel for the management of psoriasis are that moderate-to-severe psoriasis should be managed with topical medications and, if they are ineffective, phototherapy should be considered, followed by systemic medications. (6) The Panel members also noted the need for safer and more effective therapies and that there be greater recognition of the importance of health-related quality of life in psoriasis management.
Guideline recommendations were also developed by the American Academy of Dermatology (AAD) for the management of psoriasis and psoriatic arthritis in accordance with the AAD’s evidence-based clinical practice guidelines. (37) Monotherapy with topical agents was stated to be appropriate for patients needing localized therapy but not practical for those who are candidates for phototherapy and/or systemic treatments. The systemic treatments considered were: methotrexate, cyclosporin, narrowband UVB, broadband UVB, PUVA, oral retinoids and newer biological agents.
In the AAD guidelines, UVB was reported to be safe and cost-effective with NB-UVB being more effective than BB-UVB and up to 20 to 25 treatments needed for significant improvement. It was also recommended that phototherapy could be offered in-office or at home, the latter reducing inconvenience to patients. PUVA therapy was reported to be very effective and offers the potential for long remission. However long-term PUVA treatment in Caucasians was noted to be associated with an increased risk of squamous cell carcinoma and possibly malignant melanoma. PUVA also induces photo aging and other skin changes including lentigines (sun spot) and oral-PUVA is contraindicated in pregnancy.
Ontario Health System Impact Analysis
A 2006 survey of ultraviolet phototherapy services in Canada identified 26 phototherapy clinics in Ontario for a census-based population of 12,541,400. (111) At that time, there were 177 dermatologists across 50 geographic regions, 28% (14/50) of which provided phototherapy. The majority of these services were located in densely populated areas; for those living in rural communities, access was limited. (111) The inconvenience of multiple weekly visits for optimal treatment effects poses a burden to those with travel difficulties related to health, job, or family-related responsibilities.
At present, active dermatologists in Ontario have practices spanning generalist, medical, surgical and cosmetic sub-specialties. Some Ontario hospitals, however, have divested their phototherapy services due to the high cost of maintaining the necessary equipment (Personal Communication, Expert, April 2009). In some cases, arrangements have been made with private clinics to continue these services but the number of phototherapy clinics and their capacities in the province is currently unknown.
Physician OHIP billing for phototherapy services (G470 ultraviolet light therapy code) are only allowed for services provided in community-based outpatient clinics, which totalled 117,216 billings in 2007. Assuming about 66 treatments per patient, per year, this represents approximately 1,800 patients in the province being treated in private clinics. The number of patients being treated in hospitals is difficult to estimate, as physician costs are not billed directly to OHIP in this setting. Phototherapy units and services provided in hospitals are instead funded by hospital global budgets.
Technological advances have, however, enabled changes in phototherapy treatment regimens from lengthy hospital inpatient stays to outpatient clinic visits and, more recently, to an at-home basis. (103;107-109;112) Home phototherapy combined with telemedicine follow-up may provide an alternative strategy to improved access and follow-up care, particularly for those with geographic or mobility barriers. (113). Although alternate care models and settings could potentially increase service options and access, the broader consequences of the varying cost structures and incentives that either increase or decrease phototherapy services are unknown. (114)
Economic Analysis of Phototherapy for Psoriasis
Disclaimer: The Medical Advisory Secretariat uses a standardized costing methodology for all of its economic analyses of technologies. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative cost data are used for all in-hospital stay costs for the designated International Classification of Diseases-10 (ICD-10) diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may need to be made to ensure the relevant case mix group is reflective of the diagnosis and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the secretariat normally defaults to considering direct treatment costs only.
Nonhospital: These include physician services costs obtained from the Ontario Schedule of Benefits for physician fees, laboratory fees from the Ontario Laboratory Schedule of Fees, device costs from the perspective of local health care institutions, and drug costs from the Ontario Drug Benefit formulary list price.
Discounting: For all cost-effectiveness analyses, a discount rate of 5% is used as per the Canadian Agency for Drugs and Technologies in Health.
Downstream costs: All costs reported are based on assumptions of utilization, care patterns, funding, and other factors. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature. In cases where a deviation from this standard is used, an explanation has been given as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied for the purpose of developing implementation plans for the technology.
Literature Review
The focus of the current economic analysis was to characterize the costs associated with the provision of NB-UVB phototherapy for plaque-type, moderate-to-severe psoriasis in different clinical settings, including home therapy by the patient. A literature review was conducted and no cost-effectiveness (cost-utility) economic analyses were published in this area.
Hospital, Clinic and Home Costs of Phototherapy
Costs for NB-UVB phototherapy were based on consultations with equipment manufacturers and dermatologists. Device costs applicable to the provision of NB-UVB phototherapy in hospitals, private clinics, and in patients’ homes were estimated. These costs included capital costs of purchasing NB-UVB devices (amortized over 15-20 years), maintenance and bulb replacement costs, physician costs, and medication and laboratory costs. Physician costs (office visits) associated with psoriasis treatment were estimated for all settings and taken from the cost comparison study done by Mikhael et al. (115) An additional physician fee of $7.85 per phototherapy treatment was added to the physician costs in the private clinic setting as outlined in the OHIP Schedule of Benefits. (116) “Other” costs were also included from the Mikhael et al. study, such as laboratory and drug costs (including dispensing fees) for psoriasis-related medication.
The cost of certain non-phototherapy psoriasis treatments were also included in the economic analysis to provide a range of treatment costs with which to compare NB-UVB phototherapy in different clinical settings. The costs for following additional psoriasis treatment options were taken from Mikhael et al. systemic agents methotrexate, acitretin, and cyclosporin; and biologics etanercept (25mg/week) and alefacept (15mg/week for 12 weeks). Note that all costs used in the Mikhael et al. study were related to the treatment of moderate psoriasis, including physician and other costs associated with NB-UVB phototherapy treatment.
Estimation of NB-UVB Phototherapy Costs
Device costs for NB-UVB phototherapy were estimated for two types of phototherapy units: a “booth unit” consisting of 48 bulbs used in hospitals and clinics, and a “panel unit” consisting of 10 bulbs for home use. (117) According to consultations with manufacturers and dermatologists, both units were capable of treating moderate-to-severe psoriasis and used the same series of six-foot, 100W (full body) NB-UVB bulbs. The device costs of the booth and panel units were estimated at approximately $18,600 and $2,900, respectively. Simple amortization over 15 and 20 years implied yearly costs of approximately $2,500 and $150, respectively. The replacement cost for each 6-foot, 100W bulb was about $120. Through consultations with experts, each booth unit would need to replace all bulbs every 0.67 years, on average; all bulbs for panel units at home would need to be replaced every 10 years. In terms of the total number of 100W bulb replacements needed annually, each booth unit would require 72 bulbs and each panel unit would require 1 bulb. The resulting total annual cost of maintenance is about $8,640 and $120 for booth and panel units, respectively.
Certain assumptions were made regarding the number of NB-UVB phototherapy treatments performed per year in hospital and private clinic settings. Each hospital or clinic providing phototherapy services was assumed to have two booth units, resulting in an average annual cost of $19,800. According to experts, the number of psoriasis treatments that could be supplied by each booth per year was approximately 3,075 (i.e. about 15 treatments per day for 205 operating days per year, per unit). As every moderate-to-severe psoriasis patient would undergo approximately 66 treatments per year, the total number of psoriasis patients treated by a hospital or clinic with two NB-UVB booths was estimated to be 90 to 100 patients. The results of the annual cost estimation per patient for NB-UVB phototherapy by clinical setting, including home phototherapy, are summarized in Table 14.
Table 14. Average Annual Costs Per Patient of NB-UVB Phototherapy by Type of Setting.
| Setting | Unit of measure | Description | |
|---|---|---|---|
|
Hospital (NB-UVB booth) |
Treatments per year | Number of treatments per year | 6,150 |
| Patients per year | Patients per year | 93.2 | |
| Cost per year | Maintenance (72 replacement bulbs) | $17,280 | |
| Device cost (15 year capital amortization) | $2,480 | ||
| Cost per patient, per year | Device cost | $27 | |
| Physician cost | $80 | ||
| Other cost (maintenance only) | $185 | ||
| Total cost per patient per year | $292 | ||
| Private clinic (NB-UVB booth) | Treatments per year | Treatments per year | 6,150 |
| Patients per year | Patients per year | 93.2 | |
| Cost per year | Maintenance (48 replacement bulbs) | $17,280 | |
| Device cost (15 year capital amortization) | $2,480 | ||
| Cost per patient, per year | Device cost | $27 | |
| Physician cost | $598 | ||
| Other cost (maintenance only) | $185 | ||
| Total cost per patient per year | $810 | ||
| Home (NB-UVB panel) | Treatments per year | Treatments per year | 66 |
| Patients per year | Patients per year | 1.0 | |
| Cost per year | Maintenance (1 replacement bulb) | $120 | |
| Device cost (20 year capital amortization) | $145 | ||
| Cost per patient, per year | Device cost | $145 | |
| Physician cost | $100 | ||
| Other cost (maintenance only) | $120 | ||
| Total cost per patient per year | $365 | ||
Annual Costs per Patient
The average annual cost per patient for NB-UVB phototherapy and other selected treatments for moderate psoriasis are shown in Figure 2. The annual costs per patient for treatments with the biologics etanercept and alefacept were $20,300 and $18,700, respectively. NB-UVB phototherapy provided in a hospital setting was found to have the lowest average annual cost per patient, with phototherapy provided in private clinics differing from hospital costs only in terms of additional physician costs. NB-UVB home phototherapy was found to be relatively more expensive as the equipment and maintenance costs are attributable to only one patient. It is important to note that in addition to varying costs for all treatments for moderate psoriasis, the clinical effect of the treatments may also differ for certain patients and severity of disease.
Figure 2: Average annual cost per patient of NB-UVB phototherapy (PT) and other selected treatments for moderate psoriasis.
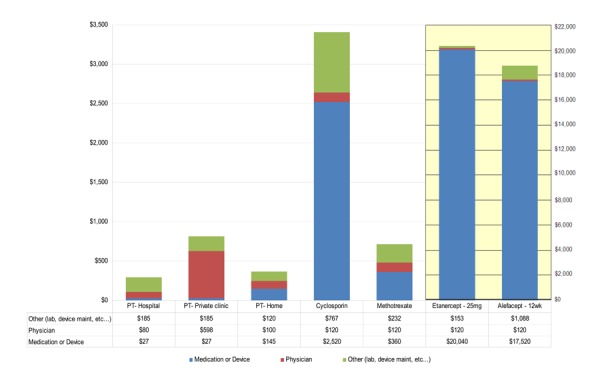
Cases of Psoriasis in Ontario
The prevalence of psoriasis was estimated to be approximately 2% of the population of age 18 or older, of which about 85% have plaque-type psoriasis. (5) Of this population, approximately 20-30% is considered to have a condition that is moderate-to-severe in disease severity; an estimate of 25% was used in the current economic analysis. (4) According to consultations with dermatologists, about 21% of these patients use NB-UVB phototherapy for treatment. Using these rates and the population estimate of Ontario in 2006 (age 18 or older), the number of cases of moderate-to-severe psoriasis (plaque-type) was found to be in the range of 29,500 to 44,200 cases. The corresponding number of cases using NB-UVB phototherapy was estimated to be between 6,200 and 9,300 cases; an estimate of 7,700 cases was used to calculate associated costs for Ontario.
Estimated Total Costs for Ontario
To estimate the cost of NB-UVB phototherapy for Ontario, the average annual costs of phototherapy treatment in hospitals, private clinics, and home settings were used. Note that the resulting costs were specific to moderate-to-severe psoriasis (plaque-type) patients and presented a total estimated cost of providing NB-UVB phototherapy services to this population group (age 18 or older). The total annual costs of NB-UVB phototherapy were estimated to be: $2.3 million in a hospital setting, $6.3 million in a private clinic setting, and $2.8 million for home phototherapy.
Discussion
In Ontario, the cost of providing phototherapy services to patients with moderate-to-severe, plaque-type psoriasis in hospital settings and at home were comparable. The annual cost per patient was estimated to be $292 and $365 for hospital and home settings, respectively; the total costs to Ontario were approximately $2.3 and $2.8 million annually, respectively. Costs for phototherapy services provided in private clinics were greater ($810 per patient annually; total of $6.3 million annually) and differed from the same services provided in the hospital setting only in terms of additional physician costs associated with phototherapy OHIP fees.
NB-UVB phototherapy services provided in a hospital setting were paid for by hospitals directly. Phototherapy services in private clinics and home settings were paid for by the clinics and patients, respectively, except for physician services covered by OHIP. The MOHLTC perspective for NB-UVB phototherapy currently provided coverage of physician services and medication costs, if accessed through the Ontario Drug Benefit program. Indirect funding was provided to hospitals as part of global budgeting and resource allocation. Home therapy services for NB-UVB phototherapy were not covered by the MOHLTC, although in some cases coverage for home-based phototherapy is provided by third-party insurers.
Appendix 1: Search Strategy
Phototherapy– Final Search Strategy
Search date: March 31, 2009
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1996 to March Week 3 2009>
Search Strategy:
exp Psoriasis/ (8500)
(pustulosis or psoriasis or Psoriasiform or Psoriatic).ti,ab. (10377)
1 or 2 (11432)
exp Phototherapy/ (11527)
(ultraviolet or UVB or NBUVB or BBUVB or UVA or PUVA or Goeckerman or PUVA or photochemotherapy or photoradiation or phototherap* or photo-therap*).ti,ab. (19679)
(light adj therap*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (515)
(Panasol or Houva or Handisol or Skinstation or xtremeclear or SPR Phototherapy or Cleartouch or Mistral or lumera or Foldalite or Dermalight or SolarC Systems).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (17)
or/4-7 (28588)
3 and 8 (1266)
limit 9 to (english language and humans and yr=“1999 - 2009”) (893)
limit 10 to (controlled clinical trial or meta analysis or randomized controlled trial) (119)
exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ (36362)
(health technology adj2 assess$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (668)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ab. (69384)
exp Random Allocation/ or random$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (388421)
exp Double-Blind Method/ (54853)
exp Control Groups/ (850)
exp Placebos/ (9571)
(RCT or placebo? or sham?).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (97831)
or/11-19 (500173)
10 and 20 (172)
Database: EMBASE <1980 to 2009 Week 13>
Search Strategy:
exp Psoriasis/ (22289)
(pustulosis or psoriasis or Psoriasiform or Psoriatic).ti,ab. (20445)
2 or 1 (27021)
exp Phototherapy/ (24700)
(ultraviolet or UVB or NBUVB or BBUVB or UVA or PUVA or Goeckerman or PUVA or photochemotherapy or photoradiation or phototherap* or photo-therap*).ti,ab. (34282)
(light adj therap*).mp. (817)
(Panasol or Houva or Handisol or Skinstation or xtremeclear or SPR Phototherapy or Cleartouch or Mistral or lumera or Foldalite or Dermalight or SolarC Systems).mp. (29)
or/4-7 (52910)
3 and 8 (4313)
limit 9 to (human and english language and yr=“1999 - 2009”) (1635)
Randomized Controlled Trial/ (167319)
exp Randomization/ (26682)
exp RANDOM SAMPLE/ (1450)
exp Biomedical Technology Assessment/ or exp Evidence Based Medicine/ (301360)
(health technology adj2 assess$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (675)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (66029)
Double Blind Procedure/ (71914)
exp Triple Blind Procedure/ (13)
exp Control Group/ (3162)
exp PLACEBO/ or placebo$.mp. or sham$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (215875)
(random$ or RCT).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (435309)
(control$ adj2 clinical trial$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (285975)
or/11-22 (805006)
10 and 23 (400)
Appendix 2: CINAHL Search Strategy
| # | Query | Limiters/Expanders | Last Run Via | Results |
|---|---|---|---|---|
| S14 | (S11 or S12) and (S10 and S13) | Search modes - Boolean/Phrase | Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
11 |
| S13 | S11 or S12 | Search modes - Boolean/Phrase | Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
95261 |
| S12 | random* or sham*or rct* or health technology N2 assess* or meta analy* or metaanaly* or pooled analysis or (systematic* N2 review*) or published studies or medline or embase or data synthesis or data extraction or cochrane or control* N2 clinical trial* | Limiters - Published Date from: 199901-200912; Language: English Search modes - Boolean/Phrase |
Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
90198 |
| S11 | (MH “Random Assignment”) or (MH “Random Sample+”) or (MH “Meta Analysis”) or (MH “Systematic Review”) or (MH “Double-Blind Studies”) or (MH “Single-Blind Studies”) or (MH “Triple-Blind Studies”) or (MH “Placebos”) or (MH “Control (Research)”) | Limiters - Published Date from: 199901-20911; Language: English Search modes - Boolean/Phrase |
Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
51153 |
| S10 | S3 and S9 | Limiters - Published Date from: 199901-20911; Language: English Search modes - Boolean/Phrase |
Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
96 |
| S9 | (S4 or S5 or S6 or S7) | Search modes - Boolean/Phrase | Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
2339 |
| S8 | (Panasol or Houva or Handisol or Skinstation or xtremeclear or SPR Phototherapy or Cleartouch or Mistral or lumera or Foldalite or Dermalight or SolarC Systems) and (S4 or S5 or S6 or S7) | Search modes - Boolean/Phrase | Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
1 |
| S7 | Panasol or Houva or Handisol or Skinstation or xtremeclear or SPR Phototherapy or Cleartouch or Mistral or lumera or Foldalite or Dermalight or SolarC Systems | Search modes - Boolean/Phrase | Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
1 |
| S6 | light N2 therap* | Search modes - Boolean/Phrase | Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
227 |
| S5 | ultraviolet or UVB or NBUVB or BBUVB or UVA or PUVA or Goeckerman or PUVA or photochemotherapy or photoradiation or phototherap* or photo-therap* | Search modes - Boolean/Phrase | Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
2160 |
| S4 | (MH “Phototherapy+”) | Search modes - Boolean/Phrase | Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
1025 |
| S3 | S1 or S2 | Search modes - Boolean/Phrase | Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
1520 |
| S2 | pustulosis or psoriasis or Psoriasiform or Psoriatic | Search modes - Boolean/Phrase | Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
1520 |
| S1 | (MH “Psoriasis+”) | Search modes - Boolean/Phrase | Interface - EBSCOhost Search Screen - Advanced Search Database - CINAHL;Pre-CINAHL |
1272 |
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Ultraviolet phototherapy management of moderate-to-severe plaque psoriasis: an evidence-based analysis. Ontario Health Technology Assessment Series, 2009;9(27).
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4435-0669-4
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practising medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
List of Tables
| Table ES1: RCT Evidence for Ultraviolet Phototherapy Treatment of Moderate-To-Severe Plaque Psoriasis |
| Table 1. Level of Evidence Summary for Studies Included in MAS Review |
| Table 2. Ultraviolet Phototherapy Management of Psoriasis: RCT Evidence (1999 to May 2009) |
| Table 3. Effectiveness of Different UVB Wavelengths for Moderate-To-Severe Plaque Psoriasis |
| Table 4. Treatment Comparisons of UVB Phototherapy and UVA Photochemotherapy for Moderate-To-Severe Plaque Psoriasis |
| Table 5. Phototherapy Alone and in Combination Therapy with Topical Agents for Moderate-To-Severe Plaque Psoriasis |
| Table 6. Impact of Balneotherapy On Phototherapy Treatment Effectiveness for Moderate-To-Severe Plaque Psoriasis |
| Table 7. Impact of Systemic Agents Added to Phototherapy for Management of Moderate-To-Severe Plaque Psoriasis |
| Table 8. Impact of The Excimer Laser as an Additional Light Source on Photochemotherapy Management of Moderate-to-Severe Plaque Psoriasis |
| Table 9. Effectiveness of Concurrent Mindfulness and Stress Reduction Audiotape Sessions With Phototherapy or Photochemotherapy For Moderate-To-Severe Plaque Psoriasis |
| Table 10. Comparison of Inpatient Dithranol, Outpatient Dithranol or Phototherapy Treatment Of Moderate-To-Severe Plaque Psoriasis |
| Table 11. Outpatient Clinic Versus Home-Based Phototherapy Treatment for Plaque Psoriasis |
| Table 12. GRADE Quality of Evidence for Phototherapy Management of Moderate-to-Severe Psoriasis |
| Table 13. Summary of RCT Evidence for Ultraviolet Phototherapy Management of Moderate-To-Severe Plaque Psoriasis |
| Table 14. Average Annual Costs Per Patient of NB-UVB Phototherapy by Type of Setting |
List of Abbreviations
- BB-UVB
Broad band ultraviolet B radiation
- BSI
Body surface involvement
- J
Joule
- MED
Minimum erythematic dose
- MOP
Methoxypsoralen
- MTX
Methotrexate
- NB-UVB
Narrow band ultraviolet B radiation
- OR
Odds ratio
- PASI
Psoriasis Area and Severity Index
- PUVA
Psoralens ultraviolet A radiation
- QOL
Quality of life
- RCT
Randomized controlled trial
- UVB
Ultraviolet B radiation
- UVA
Ultraviolet A radiation
References
- 1.Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Maradit KH. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J Am Acad Dermatol. 2009;60(3):394–401. doi: 10.1016/j.jaad.2008.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25(6):535–46. doi: 10.1016/j.clindermatol.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–20. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 4.Finlay AY, Ortonne JP. Patient satisfaction with psoriasis therapies: an update and introduction to biologic therapy. J Cutan Med Surg. 2004;8(5):310–20. doi: 10.1007/s10227-005-0030-6. [DOI] [PubMed] [Google Scholar]
- 5.Naldi L, Griffiths CEM. Traditional therapies in the management of moderate to severe chronic plaque psoriasis: An assessment of the benefits and risks. Br J Dermatol. 2005;152(4):597–615. doi: 10.1111/j.1365-2133.2005.06563.x. [DOI] [PubMed] [Google Scholar]
- 6.Guenther L, Langley RG, Shear NH, Bissonnette R, Ho V, Lynde C, et al. Integrating biologic agents into management of moderate-to-severe psoriasis: a consensus of the Canadian Psoriasis Expert Panel February 27, 2004. J Cutan Med Surg. 2004;8(5):321–37. doi: 10.1007/s10227-005-0035-1. [DOI] [PubMed] [Google Scholar]
- 7.Nevitt GJ, Hutchinson PE. Psoriasis in the community: prevalence, severity and patients’ beliefs and attitudes towards the disease. Br J Dermatol. 1996;135(4):533–7. [PubMed] [Google Scholar]
- 8.Ferrandiz C, Pujol RM, Garcia-Patos V, Bordas X, Smandia JA. Psoriasis of early and late onset: a clinical and epidemiologic study from Spain. J Am Acad Dermatol. 2002;46(6):867–73. doi: 10.1067/mjd.2002.120470. [DOI] [PubMed] [Google Scholar]
- 9.Youn JI, Park BS, Park SB, Kim SD, Suh DH. Characterization of early and late onset psoriasis in the Korean population. J Dermatol. 1999;26(10):647–52. doi: 10.1111/j.1346-8138.1999.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 10.Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985;13(3):450–6. doi: 10.1016/s0190-9622(85)70188-0. [DOI] [PubMed] [Google Scholar]
- 11.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–9. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 12.Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol. 2004;51(5):704–8. doi: 10.1016/j.jaad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60(2):218–24. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell LM, Sedlack R, Beard CM, Perry HO, Michet CJ, Kurland LT. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127(8):1184–7. [PubMed] [Google Scholar]
- 15.Huerta C, Rivero E, Rodriguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143(12):1559–65. doi: 10.1001/archderm.143.12.1559. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–71. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 17.Nestle FO, Kaplan DH, Barker J, Psoriasis N. Engl J Med. 2009;361(5):496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 18.Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol. 2007;157(1):68–73. doi: 10.1111/j.1365-2133.2007.07986.x. [DOI] [PubMed] [Google Scholar]
- 19.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32(6):982–6. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- 20.Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298(7):321–8. doi: 10.1007/s00403-006-0703-z. [DOI] [PubMed] [Google Scholar]
- 21.Mrowietz U, Elder JT, Barker J. The importance of disease associations and concomitant therapy for the long-term management of psoriasis patients. Arch Dermatol Res. 2006;298(7):309–19. doi: 10.1007/s00403-006-0707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boehncke S, Thaci D, Beschmann H, Ludwig RJ, Ackermann H, Badenhoop K, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157(6):1249–51. doi: 10.1111/j.1365-2133.2007.08190.x. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb AB, Mease PJ, Jackson JM, Eisen D, Xia HA, Asare C, et al. Clinical characteristics of psoriatic arthritis and psoriasis in dermatologists’ offices. J Dermatolog Treat. 2006;17(5):279–87. doi: 10.1080/09546630600823369. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths CE, Clark CM, Chalmers RJ, Li Wan PA, Williams HC. A systematic review of treatments for severe psoriasis. Health Technol Assess. 2000;4(40):1–125. doi: 10.3310/hta4400. [DOI] [PubMed] [Google Scholar]
- 25.Gelfand JM, Gladman DD, Mease PJ, Smith N, Margolis DJ, Nijsten T, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53(4):573. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 26.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55(5):829–35. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig RJ, Herzog C, Rostock A, Ochsendorf FR, Zollner TM, Thaci D, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156(2):271–6. doi: 10.1111/j.1365-2133.2006.07562.x. [DOI] [PubMed] [Google Scholar]
- 28.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 29.Mallbris L, Akre O, Granath F, Yin L, Lindelof B, Ekbom A, et al. Increased risk for cardiovascular mortality in psoriasis inpatients but not in outpatients. Eur J Epidemiol. 2004;19(3):225–30. doi: 10.1023/b:ejep.0000020447.59150.f9. [DOI] [PubMed] [Google Scholar]
- 30.Barker JN. Genetic aspects of psoriasis. Clin Exp Dermatol. 2001;26(4):321–5. doi: 10.1046/j.1365-2230.2001.00830.x. [DOI] [PubMed] [Google Scholar]
- 31.Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152(5):861–7. doi: 10.1111/j.1365-2133.2005.06502.x. [DOI] [PubMed] [Google Scholar]
- 32.Berth-Jones J, Grotzinger K, Rainville C, Pham B, Huang J, Daly S, et al. A study examining inter- and intrarater reliability of three scales for measuring severity of psoriasis: Psoriasis Area and Severity Index, Physician’s Global Assessment and Lattice System Physician’s Global Assessment. Br J Dermatol. 2006;155(4):707–13. doi: 10.1111/j.1365-2133.2006.07389.x. [DOI] [PubMed] [Google Scholar]
- 33.Garduno J, Bhosle MJ, Balkrishnan R, Feldman SR. Measures used in specifying psoriasis lesion(s), global disease and quality of life: A systematic review. J Dermatolog Treat. 2007;18(4):223–42. doi: 10.1080/09546630701271807. [DOI] [PubMed] [Google Scholar]
- 34.Katz KA. Psoriasis Area and Severity Index 50 as an endpoint in psoriasis trials: an unconvincing proposal. J Am Acad Dermatol. 2005;53(3):547–51. doi: 10.1016/j.jaad.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137(3):280–4. [PubMed] [Google Scholar]
- 36.Pariser DM, Bagel J, Gelfand JM, Korman NJ, Ritchlin CT, Strober BE, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143(2):239–42. doi: 10.1001/archderm.143.2.239. [DOI] [PubMed] [Google Scholar]
- 37.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2009;60(4):643–59. doi: 10.1016/j.jaad.2008.12.032. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. [DOI] [PubMed] [Google Scholar]
- 38.Yamauchi PS, Rizk D, Kormeili T, Patnaik R, Lowe NJ. Current systemic therapies for psoriasis: where are we now. J Am Acad Dermatol. 2003;49((2 Suppl)):S66–S77. doi: 10.1016/mjd.2003.550. [DOI] [PubMed] [Google Scholar]
- 39.Stern RS, Lunder EJ. Risk of squamous cell carcinoma and methoxsalen (Psoralen) and UV-A radiation (PUVA) Arch Dermatol. 1998;134:1582–5. doi: 10.1001/archderm.134.12.1582. [DOI] [PubMed] [Google Scholar]
- 40.Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature. Int J Dermatol. 2005;44(5):355–60. doi: 10.1111/j.1365-4632.2004.02186.x. [Review] [33 refs] [DOI] [PubMed] [Google Scholar]
- 41.Pasker-de Jong PC, Wielink G, van, d V, Van Der Wilt GJ. Treatment with UV-B for psoriasis and nonmelanoma skin cancer: a systematic review of the literature. Arch Dermatol. 1999;135(7):834–40. doi: 10.1001/archderm.135.7.834. [DOI] [PubMed] [Google Scholar]
- 42.Richardson SK, Gelfand JM. Update on the natural history and systemic treatment of psoriasis. Adv Dermatol. 2008;24:171–96. doi: 10.1016/j.yadr.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. J Am Acad Dermatol. 2008;58(5):826–50. doi: 10.1016/j.jaad.2008.02.039. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. [DOI] [PubMed] [Google Scholar]
- 44.Gottlieb A, Korman NJ, Gordon KB, Feldman SR, Lebwohl M, Koo JY, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. J Am Acad Dermatol. 2008;58(5):851–64. doi: 10.1016/j.jaad.2008.02.040. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. [DOI] [PubMed] [Google Scholar]
- 45.Schmitt J, Zhang Z, Wozel G, Meurer M, Kirch W. Efficacy and tolerability of biologic and nonbiologic systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. Br J Dermatol. 2008;159(3):513–26. doi: 10.1111/j.1365-2133.2008.08732.x. [DOI] [PubMed] [Google Scholar]
- 46.Salonen SH EUROPSO Patient Survey Study Group. The EUROPSO psoriasis patient study:treatment history and satisfaction reported by 17,990 members of European psoriasis patient associations. Poster presented at Spring symposium of the European Academy of Dermatology and Venereology, February 27 - March 1, 2003. Malta. 2009. [Google Scholar]
- 47.Poulin Y, Papp K, Wasel N, Andrew N, Fraquelli E, Chan D. Canadian plaque psoriasis patients are unaware of their treatment options and are dissatisfied with their previous and current treatments. Paris, France. Poster Presentation at the European Academy of Dermatology & Venereology 17th Annual Congress. September 17 - 21, 2008. 1 2009. [Google Scholar]
- 48.Nijsten T, Margolis DJ, Feldman SR, Rolstad T, Stern RS. Traditional systemic treatments have not fully met the needs of psoriasis patients: results from a national survey. J Am Acad Dermatol. 2005;52((3:Pt 1)):t–44. doi: 10.1016/j.jaad.2004.10.862. [DOI] [PubMed] [Google Scholar]
- 49.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. 2004;195(3):298–308. doi: 10.1016/j.taap.2003.08.019. Toxicol Appl Pharmacol. [DOI] [PubMed] [Google Scholar]
- 50.Zanolli M. Phototherapy treatment of psoriasis today. J Am Acad Dermatol. 2003;49((2 Suppl)):S78–S86. doi: 10.1016/s0190-9622(03)01139-3. [DOI] [PubMed] [Google Scholar]
- 51.Coven TR, Walters IB, Cardinale I, Krueger JG. PUVA-induced lymphocyte apoptosis: mechanism of action in psoriasis. Photodermatol Photoimmunol Photomed. 1999;15(1):22–7. doi: 10.1111/j.1600-0781.1999.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 52.Goeckerman W.H. Treatment of psoriasis. Northwest Med. 1925;24:229–31. [Google Scholar]
- 53.Muller SA, Perry HO. The Goeckerman treatment in psoriasis: six decades of experience at the Mayo Clinic. Cutis. 1984;34(3):265–8. 270. [PubMed] [Google Scholar]
- 54.Lebwohl M, Ali S. Treatment of psoriasis. Part 1. Topical therapy and phototherapy. J Am Acad Dermatol. 2001;45(4):487–98. doi: 10.1067/mjd.2001.117046. [DOI] [PubMed] [Google Scholar]
- 55.Fisher T. Uv-light treatment of psoriasis. Acta Derm Venereol. 1976;56:473–9. [Google Scholar]
- 56.Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76(5):359–62. doi: 10.1111/1523-1747.ep12520022. [DOI] [PubMed] [Google Scholar]
- 57.British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996) Br J Dermatol. 1997;137(3):327–30. [PubMed] [Google Scholar]
- 58.Ibbotson SH, Bilsland D, Cox NH, Dawe RS, Diffey B, Edwards C, et al. An update and guidance on narrowband ultraviolet B phototherapy: A British Photodermatology Group Workshop Report. Br J Dermatol. 2004;151(2):283–97. doi: 10.1111/j.1365-2133.2004.06128.x. [DOI] [PubMed] [Google Scholar]
- 59.Menter A, Griffiths CE. Current and future management of psoriasis. Lancet. 2007;370(9583):272–84. doi: 10.1016/S0140-6736(07)61129-5. [DOI] [PubMed] [Google Scholar]
- 60.Laube S, George SA. Adverse effects with PUVA and UVB phototherapy. J Dermatolog Treat. 2001;12(2):101–5. doi: 10.1080/095466301317085390. [DOI] [PubMed] [Google Scholar]
- 61.Pearce DJ, Higgins KB, Stealey KH, Balkrishnan R, Crane MM, Camacho F, et al. Adverse events from systemic therapies for psoriasis are common in clinical practice. J Dermatolog Treat. 2006;17(5):288–93. doi: 10.1080/09546630600920041. [DOI] [PubMed] [Google Scholar]
- 62.Martin JA, Laube S, Edwards C, Gambles B, Anstey AV. Rate of acute adverse events for narrow-band UVB and Psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23(2-3):68–72. doi: 10.1111/j.1600-0781.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 63.Patel RV, Clark LN, Lebwohl M, Weinberg JM. Treatments for psoriasis and the risk of malignancy. J Am Acad Dermatol. 2009;60(6):1001–17. doi: 10.1016/j.jaad.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 64.Stern RS, Laird N, Melski J, Parrish JA, Fitzpatrick TB, Bleich HL. Cutaneous squamous-cell carcinoma in patients treated with PUVA. N Engl J Med. 1984;310(18):1156–61. doi: 10.1056/NEJM198405033101805. [DOI] [PubMed] [Google Scholar]
- 65.Stern RS, Zierler S, Parrish JA. Skin carcinoma in patients with psoriasis treated with topical tar and artificial ultraviolet radiation. Lancet. 1980;1(8171):732–5. doi: 10.1016/s0140-6736(80)91231-3. [DOI] [PubMed] [Google Scholar]
- 66.Stern RS, Liebman EJ, Vakeva L. Oral psoralen and ultraviolet-A light (PUVA) treatment of psoriasis and persistent risk of nonmelanoma skin cancer. J Natl Cancer Inst. 1998;90(17):1278–84. doi: 10.1093/jnci/90.17.1278. UVA Follow-up Study. [DOI] [PubMed] [Google Scholar]
- 67.Henseler T, Christophers E, Honigsmann H, Wolff K. Skin tumors in the European PUVA Study. Eight-year follow-up of 1,643 patients treated with PUVA for psoriasis. J Am Acad Dermatol. 1987;16(1 Pt 1):108–16. [PubMed] [Google Scholar]
- 68.Larko O, Swanbeck G. Is UVB treatment of psoriasis safe? A study of extensively UVB-treated psoriasis patients compared with a matched control group. Acta Derm Venereol. 1982;62(6):507–12. [PubMed] [Google Scholar]
- 69.Pittelkow MR, Perry HO, Muller SA, Maughan WZ, O’Brien PC. Skin cancer in patients with psoriasis treated with coal tar. A 25-year follow-up study. Arch Dermatol. 1981;117(8):465–8. [PubMed] [Google Scholar]
- 70.Stern RS. Genital tumors among men with psoriasis exposed to psoralens and ultraviolet A radiation (PUVA) and ultraviolet B radiation. The Photochemotherapy Follow-up Study. N Engl J Med. 1990;322(16):1093–7. doi: 10.1056/NEJM199004193221601. [DOI] [PubMed] [Google Scholar]
- 71.Hannuksela-Svahn A, Pukkala E, Koulu L, Jansen CT, Karvonen J. Cancer incidence among Finnish psoriasis patients treated with 8- methoxypsoralen bath PUVA. J Am Acad Dermatol. 1999;40(5 I):694–6. doi: 10.1016/s0190-9622(99)70148-9. [DOI] [PubMed] [Google Scholar]
- 72.Weischer M, Blum A, Eberhard F, Rocken M, Berneburg M. No evidence for increased skin cancer risk in psoriasis patients treated with broadband or narrowband UVB phototherapy: a first retrospective study. Acta Derm Venereol. 2004;84(5):370–4. doi: 10.1080/00015550410026948. [DOI] [PubMed] [Google Scholar]
- 73.Man I, Crombie IK, Dawe RS, Ibbotson SH, Ferguson J. The photocarcinogenic risk of narrowband UVB (TL-01) phototherapy: early follow-up data. Br J Dermatol. 2005;152(4):755–7. doi: 10.1111/j.1365-2133.2005.06537.x. [DOI] [PubMed] [Google Scholar]
- 74.Black RJ, Gavin AT. Photocarcinogenic risk of narrowband ultraviolet B (TL-01) phototherapy: early follow-up data. Br J Dermatol. 2006;154(3):566–7. doi: 10.1111/j.1365-2133.2005.07085.x. [DOI] [PubMed] [Google Scholar]
- 75.Diffey BL, Farr PM. The challenge of follow-up in narrowband ultraviolet B phototherapy. Br J Dermatol. 2007;157(2):344–9. doi: 10.1111/j.1365-2133.2007.07980.x. [DOI] [PubMed] [Google Scholar]
- 76.Grundmann-Kollmann M, Ludwig R, Zollner TM, Ochsendorf F, Thaci D, Boehncke WH, et al. Narrowband UVB and cream psoralen-UVA combination therapy for plaque-type psoriasis. J Am Acad Dermatol. 2004;50(5):734–9. doi: 10.1016/s0190-9622(03)00792-8. [DOI] [PubMed] [Google Scholar]
- 77.Roussaki-Schulze AV, Kouskoukis C, Klimi E, Zafiriou E, Galanos A, Rallis E. Calcipotriol monotherapy versus calcipotriol plus UVA1 versus calcipotriol plus narrow-band UVB in the treatment of psoriasis. Drugs Under Experimental & Clinical Research. 2005;31(5-6):169–74. [PubMed] [Google Scholar]
- 78.Leaute-Labreze C, Saillour F, Chene G, Cazenave C, Luxey-Bellocq ML, Sanciaume C, et al. Saline spa water or combined water and UV-B for psoriasis vs conventional UV-B: lessons from the Salies de Bearn randomized study. Arch Dermatol. 2001;137(8):1035–9. [PubMed] [Google Scholar]
- 79.Ortonne JP, Khemis A, Koo JY, Choi J. An open-label study of alefacept plus ultraviolet B light as combination therapy for chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2005;19(5):556–63. doi: 10.1111/j.1468-3083.2005.01247.x. [DOI] [PubMed] [Google Scholar]
- 80.Sminkels OQ, Prins M, Veeniiuis RT, de BT, Gerritsen MJ, Van Der Wilt GJ, et al. Effectiveness and side effects of UVB-phototherapy, dithranol inpatient therapy and a care instruction programme of short contact dithranol in moderate to severe psoriasis. Eur J Dermatol. 2004;14(3):159–65. [PubMed] [Google Scholar]
- 81.Schiener R, Brockow T, Franke A, Salzer B, Peter RU, Resch KL. Bath PUVA and saltwater baths followed by UV-B phototherapy as treatments for psoriasis: a randomized controlled trial. Arch Dermatol. 2007;143(5):586–96. doi: 10.1001/archderm.143.5.586. [DOI] [PubMed] [Google Scholar]
- 82.Woo WK, McKenna KE. Combination TL01 ultraviolet B phototherapy and topical calcipotriol for psoriasis: a prospective randomized placebo-controlled clinical trial. Br J Dermatol. 2003;149(1):146–50. doi: 10.1046/j.1365-2133.2003.05380.x. [DOI] [PubMed] [Google Scholar]
- 83.Ramsay CA, Schwartz BE, Lowson D, Papp K, Bolduc A, Gilbert M. Calcipotriol cream combined with twice weekly broad-band UVB phototherapy: a safe, effective and UVB-sparing antipsoriatric combination treatment. Dermatology. 2000;200(1):17–24. doi: 10.1159/000018309. The Canadian Calcipotriol and UVB Study Group. [DOI] [PubMed] [Google Scholar]
- 84.Ring J, Kowalzick L, Christophers E, Schill WB, Schopf E, Stander M, et al. Calcitriol 3 microg g-1 ointment in combination with ultraviolet B phototherapy for the treatment of plaque psoriasis: results of a comparative study. Br J Dermatol. 2001;144(3):495–9. doi: 10.1046/j.1365-2133.2001.04074.x. [DOI] [PubMed] [Google Scholar]
- 85.Torras H, Aliaga A, Lopez-Estebaranz JL, Hernandez I, Gardeazabal J, Quintanilla E, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15(2):98–103. doi: 10.1080/09546630410023322. [DOI] [PubMed] [Google Scholar]
- 86.Asawanonda P, Nateetongrungsak Y. Methotrexate plus narrowband UVB phototherapy versus narrowband UVB phototherapy alone in the treatment of plaque-type psoriasis: a randomized, placebo-controlled study. J Am Acad Dermatol. 2006;54(6):1013–8. doi: 10.1016/j.jaad.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Goodman C. Literature searching and evidence interpretation for assessing health care practices. 1993 doi: 10.1017/s0266462300008321. [DOI] [PubMed] [Google Scholar]
- 88.Yuehua Y, Khalaf A, Xiaoxiang Z, Xinggang W. Narrow-band ultraviolet B and conventional UVB phototherapy in psoriasis: a randomised controlled trial. Am J Appl Sci. 2008;5(8):905–8. [Google Scholar]
- 89.Kirke SM, Lowder S, Lloyd JJ, Diffey BL, Matthews JN, Farr PM. A randomized comparison of selective broadband UVB and narrowband UVB in the treatment of psoriasis. J Invest Dermatol. 2007;127(7):1641–6. doi: 10.1038/sj.jid.5700767. [DOI] [PubMed] [Google Scholar]
- 90.Gordon PM, Diffey BL, Matthews JNS, Farr PM. A randomized comparison of narrow-band TL-01 phototherapy and PUVA photochemotherapy for psoriasis. J Am Acad Dermatol. 1999;41(5 I):728–32. doi: 10.1016/s0190-9622(99)70008-3. [DOI] [PubMed] [Google Scholar]
- 91.Markham T, Rogers S, Collins P. Narrowband UV-B (TL-01) phototherapy vs oral 8-methoxypsoralen psoralen-UV-A for the treatment of chronic plaque psoriasis. Arch Dermatol. 2003;139(3):325–8. doi: 10.1001/archderm.139.3.325. [DOI] [PubMed] [Google Scholar]
- 92.Yones SS, Palmer RA, Garibaldinos TT, Hawk JL. Randomized double-blind trial of the treatment of chronic plaque psoriasis: efficacy of psoralen-UV-A therapy vs narrowband UV-B therapy. Arch Dermatol. 2006;142(7):836–42. doi: 10.1001/archderm.142.7.836. [DOI] [PubMed] [Google Scholar]
- 93.Khurshid K, Haroon TS, Hussain I, Pal SS, Jahangir M, Zaman T. Psoralen-ultraviolet A therapy vs. psoralen-ultraviolet B therapy in the treatment of plaque-type psoriasis: our experience with fitzpatrick skin type IV. Int J Dermatol. 2000;39(11):865–7. doi: 10.1046/j.1365-4362.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- 94.Martin-Ezquerra G, Sanchez-Regana M, Umbert-Millet P. Optimization of narrow-band uvb with a 5% oleic acid cream in the treatment of psoriasis. J Drugs Dermatol. 2007;6(3):290–2. [PubMed] [Google Scholar]
- 95.Valkova S. UVB phototherapeutic modalities. Comparison of two treatments for chronic plaque psoriasis. Acta Dermatovenerol Alp Panonica Adriat. 2007;16(1):26–30. [PubMed] [Google Scholar]
- 96.Brands S, Brakman M, Bos JD, de Rie MA. No additional effect of calcipotriol ointment on low-dose narrowband UVB phototherapy in psoriasis. J Am Acad Dermatol. 1999;41(6):991–5. doi: 10.1016/s0190-9622(99)70259-8. [DOI] [PubMed] [Google Scholar]
- 97.Rim JH, Choe YB, Youn JI. Positive effect of using calcipotriol ointment with narrow-band ultraviolet B phototherapy in psoriatic patients. Photodermatol Photoimmunol Photomed. 2002;18(3):131–4. doi: 10.1034/j.1600-0781.2002.180305.x. [DOI] [PubMed] [Google Scholar]
- 98.Brockow T, Schiener R, Franke A, Resch KL, Peter RU. A pragmatic randomized controlled trial on the effectiveness of low concentrated saline spa water baths followed by ultraviolet B (UVB) compared to UVB only in moderate to severe psoriasis. J Eur Acad Dermatol Venereol. 2007;21(8):1027–37. doi: 10.1111/j.1468-3083.2007.02152.x. [DOI] [PubMed] [Google Scholar]
- 99.Brockow T, Schiener R, Franke A, Resch KL, Peter RU. A pragmatic randomized controlled trial on the effectiveness of highly concentrated saline spa water baths followed by UVB compared to UVB only in moderate to severe psoriasis. J Altern Complement Med. 2007;13(7):725–32. doi: 10.1089/acm.2007.7099. [DOI] [PubMed] [Google Scholar]
- 100.Trott J, Gerber W, Hammes S, Ockenfels HM. The effectiveness of PUVA treatment in severe psoriasis is significantly increased by additional UV 308-nm excimer laser sessions. Eur J Dermatol. 2008;18(1):55–60. doi: 10.1684/ejd.2008.0311. [DOI] [PubMed] [Google Scholar]
- 101.Kabat-Zinn J. Participatory medicine. J Eur Acad Dermatol Venereol. 2000;14(4):239–40. doi: 10.1046/j.1468-3083.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- 102.Kabat-Zinn J, Wheeler E, Light T, Skillings A, Scharf MJ, Cropley TG, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA) Psychosom Med. 1998;60(5):625–32. doi: 10.1097/00006842-199809000-00020. [DOI] [PubMed] [Google Scholar]
- 103.Koek MB, Buskens E, van WH, Steegmans PH, Bruijnzeel-Koomen CA, Sigurdsson V. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study) BMJ. 2009. pp. 338–b1542. [DOI] [PMC free article] [PubMed]
- 104.Koek MB, Buskens E, Steegmans PH, van WH, Bruijnzeel-Koomen CA, Sigurdsson V. UVB phototherapy in an outpatient setting or at home: a pragmatic randomised single-blind trial designed to settle the discussion. BMC Medical Research Methodology. 2006;6(39) doi: 10.1186/1471-2288-6-39. The PLUTO study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dawe RS, Cameron H, Yule S, Man I, Ibbotson SH, Ferguson J. UV-B phototherapy clears psoriasis through local effects. Arch Dermatol. 2002;138(8):1071–6. doi: 10.1001/archderm.138.8.1071. [DOI] [PubMed] [Google Scholar]
- 106.Stern RS. Inpatient hospital care for psoriasis: a vanishing practice in the United States. J Am Acad Dermatol. 2003;49(3):445–50. doi: 10.1067/s0190-9622(03)00858-2. [DOI] [PubMed] [Google Scholar]
- 107.Yelverton CB, Yentzer BA, Clark A, Pearce DJ, Balkrishnan R, Camacho FT, et al. Home narrowband UV-B phototherapy in combination with low-dose acitretin in patients with moderate to severe psoriasis. Arch Dermatol. 2008;144(9):1224–5. doi: 10.1001/archderm.144.9.1224. [DOI] [PubMed] [Google Scholar]
- 108.Haykal KA, DesGroseilliers JP. Are narrow-band ultraviolet B home units a viable option for continuous or maintenance therapy of photoresponsive diseases? J Cutan Med Surg. 2006;10(5):234–40. doi: 10.2310/7750.2006.00052. [DOI] [PubMed] [Google Scholar]
- 109.Cameron H, Yule S, Moseley H, Dawe RS, Ferguson J. Taking treatment to the patient: development of a home TL-01 ultraviolet B phototherapy service. Br J Dermatol. 2002;147(5):957–65. doi: 10.1046/j.1365-2133.2002.04860.x. [DOI] [PubMed] [Google Scholar]
- 110.Koek MB, Buskens E, Bruijnzeel-Koomen CA, Sigurdsson V. Home ultraviolet B phototherapy for psoriasis: discrepancy between literature, guidelines, general opinions and actual use. Br J Dermatol. 2006;154(4):701–11. doi: 10.1111/j.1365-2133.2006.07136.x. Results of a literature review, a web search, and a questionnaire among dermatologists. [DOI] [PubMed] [Google Scholar]
- 111.Mittmann N, Koo M, Chan B, Hales B, Chau D. Availability of phototherapy services in Canada [Abstract] Can J Clin Pharmacol. 2006;13(1:e144) [Google Scholar]
- 112.Abel E. Selection of the right approach treatment settings. Clin Dermatol. 1997;15:823–30. doi: 10.1016/s0738-081x(97)00011-4. [DOI] [PubMed] [Google Scholar]
- 113.Kanthraj GR. Classification and design of teledermatology practice: what dermatoses? Which technology to apply? J Eur Acad Dermatol Venereol. 2009;23(8):865–75. doi: 10.1111/j.1468-3083.2009.03136.x. [DOI] [PubMed] [Google Scholar]
- 114.Simpson GL, Yelverton CB, Rittenberg S, Feldman SR. Do utilization management controls for phototherapy increase the prescription of biologics. J Dermatolog Treat. 2006;17(6):359–61. doi: 10.1080/09546630601028786. [DOI] [PubMed] [Google Scholar]
- 115.Mikhael D, Babcock K, DesGroseilliers JP. Cost comparison of psoriasis treatments. J Cutan Med Surg 2009. 10.2310/7750.2009.0014. [DOI] [PubMed]
- 116.Ontario Ministry of Health and Long-Term Care. Ontario Health Insurance (OHIP) schedule of benefits and fees. [[updated 2008 Jun 3; cited 2009 Jun 24]]. [Internet] Available from: http://www.health.gov.on.ca/english/providers/program/ohip/sob/sob_mn.html .
- 117.Solarc Systems Inc. SolRx 1000 Series Home Phototherapy Full Body Panel. [[updated 2009; cited 2009 Jun 24]]. [Internet] Available from: http://www.solarcsystems.com/home_phototherapy_panel_1.html .


